Research Article - Clinical Practice (2019) Volume 16, Issue 3
Pattern of apoptotic endothelial cell-derived micro vesicles in patients with different phenotypes of chronic heart failure
- Corresponding Author:
- Alexander E Berezin
Consultant of Therapeutic Unit, Internal Medicine Department
State Medical University of Zaporozhye, 26
Mayakovsky av., Zaporozhye, UA-69035, Ukraine
E-mail: aeberezin@gmail.com; dr_berezin@mail.ru
Abstract
Background: The aim of the study was to evaluate the associations between the signature of apoptotic endothelial cellderived microvesicles (MVs) and biomarkers of fibrosis, inflammation and cardiac remodeling in patients with different phenotypes of chronic Heart Failure (HF). Methods: The study cohort consisted of 388 prospectively involved subjects with established chronic HF. The phenotype of HF was determined according to left ventricular ejection fraction (LVEF) value per contemporary clinical guideline. HFrEF (LVEF ≤ 40%), HFmrEF (41-49%) and HFpEF (LVEF ≥ 50%) were determined. All biomarkers were measured at baseline. Results: The number of circulating CD31+/annexin V+MVs in HFpEF patients was significantly different from both HFrEF and HFmrEF individuals, but it was similar in HFrEF and HFmrEF patients. The number of circulating CD144+/ annexin V+MVs in HFrEF patients was significantly higher to HFmrEF and HFpEF. We determined that a combination of a number of circulating CD31+/annexin V+MVs and galectin-3 (AUC=0.68; 95% CI=0.61-0.77; P=0.001) was the best predictor of HFpEF. The predictive values of sST2 (AUC=0.65; 95% CI=0.60-0.69), number of circulating CD31+/annexin V+MVs (AUC=0.63; 95% CI=0.58-0.69) alone and their combination (AUC=0.65; 95% CI=0.59-0.70) for HFmrEF did not distinguished significantly (P=0.48). The double combinations of number of circulating CD144+/annexin V+MVs and sST2 (AUC=0.70; 95% CI=0.66-0.75) or number of circulating CD144+/annexin V+MVs and galectin-3 (AUC=0.71; 95% CI=0.650.76) were the best prognosticators for HFrEF. Conclusion: we found that the number of circulating CD31+/annexin V+MVs may improve a prediction of galectin-3 for HFpEF, and that number of circulating CD144+/annexin V+MVs is able to increase predictive capabilities of sST2 and galectin-3 for HFrEF.
Keywords
Chronic heart failure, biomarkers, endothelial cell-derived microvesicles
Abbreviations
ACEI: Angiotensin-Converting Enzyme Inhibitors; ARBs: Angiotensin Receptor Blockers; AUC: Area Under Curve; BMI: Body Mass Index ; BNP: Brain Natriuretic Peptide; CV: Cardiovascular; GDF-15: Growth-Differential Factor-15; GFR: Glomerular Filtration Rate; HDL-C: High Density Lipoprotein Cholesterol; HF: Heart Failure; HFmrEF: Chronic Hf with Mid-Range Ejection Fraction; HFpEF: Chronic HF with Preserved Ejection Fraction; HFrEF: Chronic HF with Reduced Ejection Fraction; hs-CRP: High Sensitive C-Reactive Protein; LDL-C: Low-Density Lipoprotein Cholesterol; LVEF: Left Ventricular Ejection Fraction; MPs: Endothelial Cell-Derived Micro Vesicles; MVs: Micro Vesicles
Introduction
Heart Failure (HF) remains a global health problem with an increased risk of premature death and extremely high economic and social burden [1,2]. The pathogenesis of HF appears to be complex and sophisticated with multiple molecular mechanisms that lead to cardiac and vascular remodeling and possible to the progression of the disease [3,4]. Several animal and clinical studies have shown the importance of microvascular inflammation, endothelial dysfunction, vascular fibrosis, and remodeling in HF developing [5-8], but only a few small clinical studies have reported on the effect of impaired vascular repair in manifestation and progression of HF [9,10]. Indeed, the weak ability to circulate and resident precursors of endothelial cells different origin to migration, proliferation, differentiation, and survival known as progenitor endothelial cell dysfunction may play a pivotal role in vascular remodeling [11,12]. Moreover, numerous factors corresponding to HF severity, such as some hormones (angiotensin-II, aldosterone, endothelial-1), pro-inflammatory cytokines (tumor necrosis factor-alpha, interleukin-[IL]-1, IL-2beta, IL-6), chemokines, components of oxidative stress (free radicals, superoxides, and oxidized lipids) may be triggers of an apoptosis of both immature and mature endothelial cells and thereby negatively influence on vascular function [13,14]. Additionally, apoptoticmodified endothelial cells release microvesicles (MVs) that are not just cargo of several active molecules, peptides, growth factors, and microRNAs participating in cell-to-cell cooperation, but they are able to directly injury endothelium and sub-intima layer inducing microvascular inflammation and extracellular matrix accumulation [15,16]. All these processes lead to vascular remodeling with worsening of endothelial function. Nevertheless, there is a large body of evidence that the altered vascular repair may link HF progression with several etiology factors and various comorbidities including ischemia, hypertension, diabetes mellitus, hypothyroidism, and that impaired immune phenotypes of endothelial cell-derived MVs could be biomarkers of these interrelations [17]. In this way, elevated levels of apoptotic endothelial cell-derived MVs have been reported as a high likely predictor of adverse clinical outcomes in predominantly HF patients with reduced left ventricular ejection fraction (HFrEF) [18]. Although there are biomarkers of biomechanical stress (natriuretic peptides), fibrosis (galectin-3, soluble ST2), inflammation (C-reactive protein), remodeling (growthdifferentiation factor-15) with established predictive value in HF patients, the results of clinical studies regarding their prognostication in different phenotypes of HF are controversial [19]. Moreover, there is limiting evidence of corresponding of these biomarkers with vascular remodeling in HF with preserved (HFpEF) and mid-range (HFmrEF) ejection fraction. The aim of the present study was to evaluate the associations between the signature of apoptotic endothelial cell-derived MVs and biomarkers of fibrosis, inflammation and cardiac remodeling in patients with different phenotypes of chronic HF.
Methods
Study population
The study cohort consisted of 388 subjects with chronic HF who were prospectively involved between April 2010 and October 2017. All these patients were treated in Zaporozhye Regional Hospital, City Hospital #6 (Zaporozhye), City Hospital #10, Zaporozhye Regional Center of Cardiovascular Diseases with primary diagnosis chronic HF, which was defined according to contemporary criteria provided by actual clinical recommendation [1]. Phenotypes of chronic HF were determined according to this recommendation as HF with reduced ejection fraction [HFrEF] (n=85; LVEF ≤ 40%), HF with mid-range ejection fraction [HFmrEF] (n=125; LVEF=41-49%) and HF with preserved ejection fraction [HFpEF] (n=178; LVEF ≥ 50%). The criteria of non-inclusion in the study were estimated Glomerular Filtration Rate (GFR) <35 mL/min/m2; implanted pacemaker/defibrillator/cardioverter; acute infections; active inflammation; valvular heart disease; pregnancy; ischemic stroke; intracranial hemorrhage; surgery; trauma, autoimmune disease, and malignancy prior to the study entry.
T2DM was diagnosed with revised criteria provided by the American Diabetes Association when source documents were reviewed [20]. Patients with T2DM were treated with metformin in individually adjusted daily doses under continuous control of fasting glycemia, the daily profile of glucose concentration and glycated hemoglobin level (HbAc1). Rarely sitagliptin was added to the treatment scheme. No insulin given patients with T2DM were selected in the study.
All subjects who were treated with antihypertensive drugs and/or who have demonstrated elevated office blood pressure (>140/90 mm Hg) are considered as hypertensive individuals. Dyslipidemia was checked and determined according to NCEP Adult Treatment Panel III (National Cholesterol Education Program) [21].
Demographic data and anthropometric measurements
Age, gender, height, weight, body mass, body mass index, waist circumference, and waist-tohip ratio past medical and medication history were collected at baseline. Anthropometric data were measured by professional health attendants with the participants standing without shoes and heavy outer garments with a wall-mounted stadiometer (OMRON, Japan). Body mass index (BMI) was calculated by staff person as weight (Kg) divided by height squared (m2). Waist and hip circumference were measured in a standing position per protocol [22,23].
Smoking status
Current smoking was defined as consumption of one cigarette daily for three months [24].
Cardiac ultrasound and doppler
Transthoracic echocardiography was performed on ACUSON ultrasound system (SIEMENS, Germany) in В-mode regimen and Tissue Doppler Imaging (TDI) regimen. Left Ventricular Ejection Fraction (LVEF) was measured by modified Simpson’s method [25].
Glomerular filtration rate measurement
Calculation of Glomerular Filtration Rate (GFR) was calculated by CKD-EPI formula [26].
Blood sampling
Blood samples were drawn in the morning following overnight fasting (at 7-8 a.m.) into barcoded silicone test tubes (Thermo Fisher Scientific, Waltham, MA, USA) wherein 2 mL of 5% Trilon B solution was added. Then samples were centrifuged upon permanent cooling at 6,000 rpm for 3 minutes and then plasma was collected to be immediately refrigerated. Each aliquot was stored at a temperature 70ºС.
N-terminal pro-brain natriuretic peptide (NT-pro-BNP) level was measured by immunoelectrochemoluminescent assay using sets by R and D Systems (USA) on Elecsys 1010 analyzer (Roche, Mannheim, Germany). Galectin-3 was measured using an ELISA kit (BG Medicine, Germany) and obtained with Elecsys 1010 analyzer (Roche, Mannheim, Germany). Soluble ST2 was measured by commercial ELISA kit “Presage ST2 Assay” (Critical Diagnostics, San Diego, USA) according to the manufacturers’ recommendations. Growth-Differential Factor-15 (GDF-15) was determined by ELISA kit (LifeSpan BioSciences, USA) on Elecsys 1010 analyzer (Roche, Mannheim, Germany). High-sensitivity C-reactive protein (C-RP) levels were measured by the nephelometric technique with a commercial kit (Eagle Biosciences, Nashua, NH, USA) and obtained with “AU640 Analyzer” (Olympus Diagnostic Systems Group, Japan). High-performance liquid chromatography method was performed to determine hemoglobin A1c (HbA1c) in 5% Trilon B anticoagulated blood samples.
Concentrations of total cholesterol (TC), cholesterol of High-Density Lipoproteins (HDL-C), Low-Density Lipoproteins (LDL-C) and triglycerides (TG) were measured by the direct enzymatic method with commercial kits (DIALAB, Neudorf, Austria) using automatic analyzer Roche P800 (F Hoffmann-La Roche AG, Basel, Switzerland).
Blood preparation, labeling, and measurement of endothelial cellderived micro vesicles
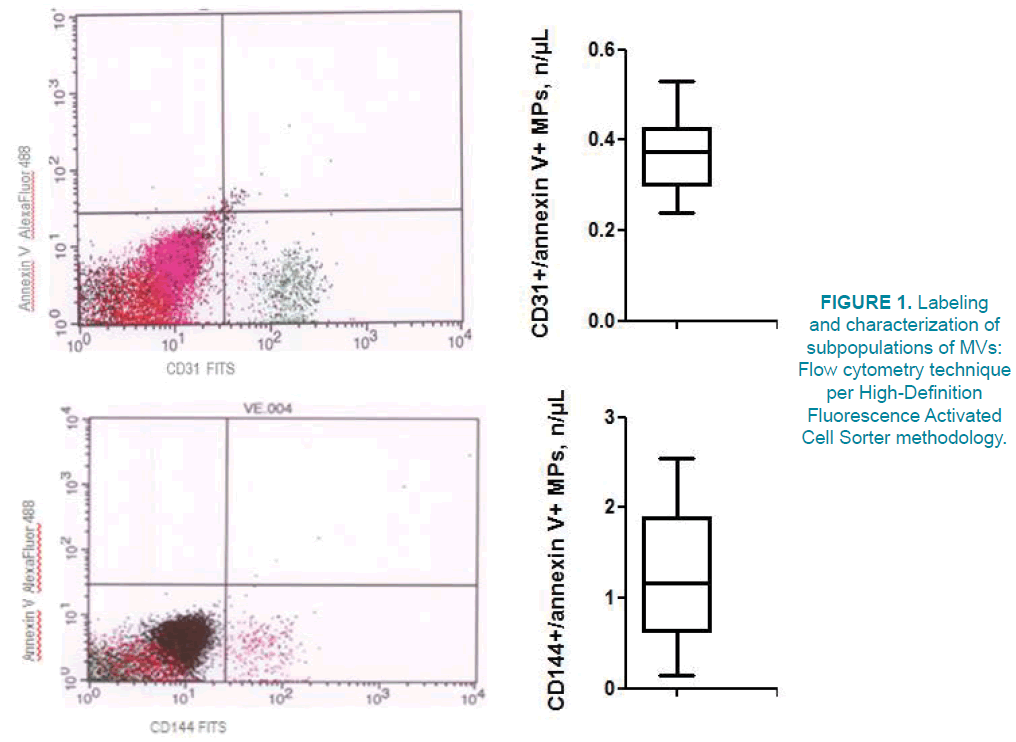
Circulating micro vesicles (MVs) were isolated from 5 mL of venous citrated blood drawn from the fistula-free arm per protocol that was previously described [27]. Flow cytometry technique per High-Definition Fluorescence Activated Cell Sorter methodology was used to label and characterize subpopulations of MVs immediately after blood sampling without freeze [28].
The methodology of measure of MVs includes size-calibration procedure with fluorescent beads (sizes ranging from 0.1 to 1.0 μm). In the study, we used forward angle light scattering from polystyrene microsphere (0.5- 0.9 μm) for preliminary standardization. MVs’ gate was defined less than a 0.4 μm polystyrene microsphere extending down to the noise threshold level that is equivalent to cell-derived MPs less 1 μm diameter. In order to separate true events from background noise, we defined MPs as particles that were less than 1.0 μm in diameter and expressed cell-specific markers. Per protocol CD31 and CD144 antigens were determined as essential markers of endothelial cell, and Annexin V was a marker of apoptosis (FIGURE 1). Consequently, CD31+/annexin V+ and CD144+/annexin V+MVs were defined as apoptotic endothelial cell-derived MVs [29].
Statistical Analysis
Data were analyzed using SPSS 20.0 (SPSS, IBM Corporation, NY, USA) and Prism v.6 (GraphPad Software Inc., La Jolla, CA, USA). The data were expressed as mean (М) and error of the mean ( ±m ) or a 95% Confidence Interval (CI); the Median (Ме) and the Interquartile Range (IQR). Categorical variables were reported as numerous (n) and percentages (%). Shapiro-Wilk test and Kolmogorov- Smirnov test were used to assay the normality of continuous variables. To compare the main parameters of patients’ groups (subject to the type of distribution of the parameters analyzed), one-tailed Student t-test or Mann-Whitney U-test were used. The two-tailed version of Wilcoxon test was used for paired comparison of parameter values inside the group. Categorical variables between groups were compared with Chi2 test (χ2) and Fisher F exact test.
The potential factors that may be associated with HFrEF, HFmrEF, HFpEF were identified first with the univariate analysis (ANOVA), and then the independent predictors for each phenotype of HF were searched with the multivariate one-step backward linear regression analysis, initially including variables for which a P value<0.1 was achieved from the univariate analysis. Pearson’s correlation coefficient was calculated for all regression models. The Odds Ratio (OR) and 95% CI were calculated for factors independently predicted HFpEF, HFmrEF, HFrEF in the Cox regression model. C-statistic was used for each model that is able to associate with HFpEF, HFmrEF, and HFrEF. AUC (Area Under Curve) and 95% CI were calculated. A calculated difference of P<0.05 was considered significant.
Results
The characteristics of the study population are summarized in TABLE 1. The entire group consisted of predominantly male patients (51.8%) with a mean age of 56.13 years old with symptomatic mild-to-severe chronic HF due to myocardial infarction (56.7%) and dilated cardiomyopathy (13.9%). The most important comorbidities in HF individuals were dyslipidemia (70.6%), hypertension (57.5%), obesity (37.8%) and T2DM (11.9%) (TABLE 2). T2DM was diagnosed significantly higher in HFrEF patients than in HFpEF and HFmrEF individuals. There were no significant differences between patients with HFrEF, HFmrEF, and HFpEF in age, sex, NYHA functional classes, the frequency of hypertension, dyslipidemia, obesity, an adherence to smoking, Body Mass Index (BMI), systolic and diastolic blood pressure, and heart rate. In contrast, previous myocardial infarction and dilated cardiomyopathy have determined frequently in HFrEF patients than in HFpEF, while proportions of patients with both these diseases did not differ in HFpEF and HFmrEF.
| Variables | Entire patient group (n=388) | Subjects with HFrEF (n=85) | Subjects with HFmrEF (n=125) | Subjects with HFpEF (n=178) | P value between HF cohorts |
|---|---|---|---|---|---|
| Age, years | 56.13  ±  7.80 | 57.50  ±  6.70 | 56.51 ± 6.44 | 54.79 ± 6.62 | 0.78 |
| Male, n (%) | 201 (51.8%) | 49 (57.6%) | 77 (61.6%) | 75 (42.7%) | 0.24 |
| II NYHA class, n (%) | 174 (44.8%) | 29 (34.1%) | 51 (40.8%) | 94 (52.8%) | 0.16 |
| III NYHA class, n (%) | 146 (37.6%) | 36 (42.4%) | 52 (41.6%) | 58 (32.6%) | 0.48 |
| IV NYHA class, n (%) | 68 (17.5%) | 20 (23.5%) | 22 (17.6%) | 26 (14.6%) | 0.05 |
| Previous MI, n (%) | 220 (56.7%) | 66 (77.6%) | 62 (49.6%) | 92 (51.7%) | 0,046 |
| Dilated cardiomyopathy, n (%) | 54 (13.9%) | 19 (22.4%) | 17 (13.6%) | 18 (14.4%) | 0,042 |
| Hypertension, n (%) | 223 (57.5%) | 53 (62.4%) | 68 (54.4%) | 102 (57.3%) | 0.88 |
| Dyslipidemia, n (%) | 288 (70.6%) | 68 (80.0%) | 94 (75.2%) | 126 (70.8%) | 0.05 |
| T2DM, n (%) | 46 (11.9%) | 15 (17.6%) | 8 (6,4%) | 23 (12.9%) | 0.04 |
| Obesity, n (%) | 62 (37.8%) | 26 (30.5%) | 29 (23.5%) | 36 (20.2%) | 0.05 |
| Adherence to smoke, n (%) | 35 (21.3%) | 18 (21.2%) | 25 (20.0%) | 34 (19.1%) | 0.98 |
| BMI, kg/m2 | 24.5 (21.2-28.9) | 22.5 (20.6-26.2) | 24.3 (20.5-30.1) | 25.1 (20.7-33.6) | 0.72 |
| Systolic BP, mm Hg | 132  ±  9 | 130 ± 7 | 131 ± 8 | 133 ± 6 | 0.88 |
| Diastolic BP, mm Hg | 77 ± 6 | 76 ± 5 | 78 ± 6 | 78 ± 5 | 0.92 |
| Heart rate, beat per min. | 72.35  ±  6.95 | 76.20 ± 5.11 | 75.70 ± 6.20 | 66.70 ± 5.24 | 0.12 |
| LVEF, % | 45.5 (30.4-55.3) | 36.50 (30.7-39.1) | 44.3 (40.8-48.2) | 55.1 (50.9-58.4) | 0.038 |
Abbreviations: NYHA-New York Heart Association; T2DM-Type two diabetes mellitus, MI-myocardial infarction; LVEF-left ventricular ejection fraction
TABLE 1. General characteristics of participants in the study.
| Variables | Entire patient cohort (n=388) | Subjects with HFrEF (n=85) | Subjects with HFmrEF (n=125) | Subjects with HFpEF (n=178) | P value between HF cohorts |
|---|---|---|---|---|---|
| GFR, mL/ min/1.73 m2 | 82.3 (68.7-102.6) | 79.6 (63.1-92.3) | 85.4 (78.5-100.9) | 88.2 (77.1-102.1) | 0.056 |
| Hemoglobin, g/L | 135.4 (128.5-142.1) | 128.1 (124.2-133.1) | 128.9 (125.3-134.0) | 138.5 (126.2-141.8) | 0.16 |
| Fasting glucose, mmol/L | 5.17 (3.5-9.6) | 4.98 (3.8-8.1) | 5.10 (3.8-6.9) | 5.27 (3.6-7.3) | 0.28 |
| HbA1c, % | 6.8 (4.1-9.5) | 6.4 (4.6-8.0) | 6.5 (4.6-8.5) | 6.9 (4.3-7.2) | 0.22 |
| Creatinine, µmol/L | 72.3 (58.7-92.6) | 82.1 (64.9-90.5) | 79.6 (66.1-91.0) | 67.7 (59.1-84.1) | 0.26 |
| Total cholesterol, mmol/L | 5.1 (3.9-6.1) | 5.3 (4.6-6.0) | 5.3 (4.4-6.1) | 5.0 (3.5-5.9) | 0.94 |
| HDL Cholesterol, mmol/L | 0.92 (0.88-1.13) | 0.97 (0.92-1.08) | 0.96 (0.90-1.10) | 0.88 (0.83-1.03) | 0.72 |
| LDL Cholesterol, mmol/L | 3.23 (3.11-4.40) | 3.71 (3.50-4.20) | 3.69 (3.50-4.17) | 3.50 (3.10-3.96) | 0.06 |
| Uric acid, µmol/L | 345 (253-420) | 357 (253-412) | 344 (257-409) | 311 (206-369) | 0.88 |
| NT-pro-BNP, pg/mL | 2336.2 (988.5-3552.8) | 2774.5 (1520.4-3870.2) | 2701.2 (1590.1-3540.5) | 2130.8 (954.5-3056.2) | 0.02 |
| sST2, ng/mL | 36.8 (23.9-55.8) | 41.4 (25.9-66.7) | 38.5 (24.3-58.1) | 34.8 (22.1-54.7) | 0.01 |
| Galectin-3, µg/L | 18.9 (14.2-23.1) | 19.3 (15.8-23.9) | 18.5 (14.1-21.2) | 16.9 (13.7-19.2) | 0.01 |
| GDF-15, pg / mL | 712 (538-940) | 845 (651-1023) | 755 (580-936) | 637 (487-796) | 0.01 |
| hs-CRP, mg/L | 7.1 (5.2-9.2) | 7.05 (6.1-8.1) | 7.11 (6.3-8.1) | 7.14 (6.22-8.3) | 0.26 |
Abbreviations: GFR:Glomerular Filtration Rate; BMP:Brain Natriuretic Peptide; hs-CRP: High Sensitive C-Reactive Protein; GDF-15: Growth-Differential Factor-15; MVs–Micro Vesicles; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; HbA1c:glycated Haemoglobin
TABLE 2. The biomarkers in the patient study population.
Three cohorts of HF patients did not distinguish each other in levels of basic biomarkers, such as GFR, hemoglobin, fasting glucose, HbA1c, creatinine, uric acid, lipids, and hs-CRP. However, there were found significantly higher levels of NT-pro-BNP, sST2, galectin-3, GDF-15 in HFrEF patients than in HFmrEF and HFpEF subjects, but the concentrations of these biomarkers were not sufficiently differed in HFpEF and HFmrEF patients.
TABLE 3 is reported the medications that were used in HF patients depending on HF phenotypes. Individuals with HFrEF were treated frequently with ivabradine, mineralocorticoid receptor antagonists, loop diuretics than individuals with HFmrEF and HFpEF. At the same time, dihydropyridine calcium channel blockers as a component of concomitant antihypertensive care were used predominantly in HFpEF and rarely in HFmrEF, but they were not prescribed in HFrEF patients.
| Medicine | Entire patient cohort (n=388) | Subjects with HFrEF (n=85) | Subjects with HFmrEF (n=125) | Subjects with HFpEF (n=178) | P value |
|---|---|---|---|---|---|
| ACE inhibitors or ARBs, n (%) | 388 (100%) | 85 (100%) | 125 (100%) | 178 (100%) | 1 |
| Aspirin, n (%) | 303 (78.1%) | 63 (74.1%) | 96 (76.8%) | 144 (80.8%) | 0.05 |
| Other antiplatelet drugs, n (%) | 85 (21,9%) | 22 (26,9%) | 29 (23,2%) | 34 (19,2%) | 0.05 |
| Beta-adrenoblockers, n (%) | 319 (82.2%) | 68 (80.0%) | 104 (83.2%) | 147 (82.6%) | 0.96 |
| Dihydropyridine calcium channel blockers, n (%) | 64 (16.5%) | 0 (0%) | 15 (12.0%) | 49 (27.5%) | 0.001 |
| Ivabradine, n (%) | 114 (29.4%) | 42 (49.4%) | 31 (24.8%) | 41 (23.0%) | 0.001 |
| Mineralocorticoid receptor antagonists, n (%) | 134 (34.5%) | 82 (96.5%) | 32 (25.6) | 20 (11.2%) | 0.001 |
| Loop diuretics, n (%) | 324 (83.5%) | 85 (100%) | 99 (79.2%) | 140 (78.7%) | 0.001 |
| Statins, n (%) | 288 (70.6%) | 68 (80.0%) | 94 (75.2%) | 126 (70,8%) | 0.05 |
| Metformin, n (%) | 46 (11.9%) | 15 (17.6%) | 8 (6.4%) | 23 (12.9%) | 0.01 |
| Sitagliptin, n (%) | 21 (5.4%) | 4 (4.7%) | 7 (5.6%) | 10 (5.6%) | 0.92 |
Abbreviations: ACE:Angiotensin-Converting Enzyme; ARBs: Angiotensin-2 Receptor Blockers
TABLE 3. The medications in the patient study population.
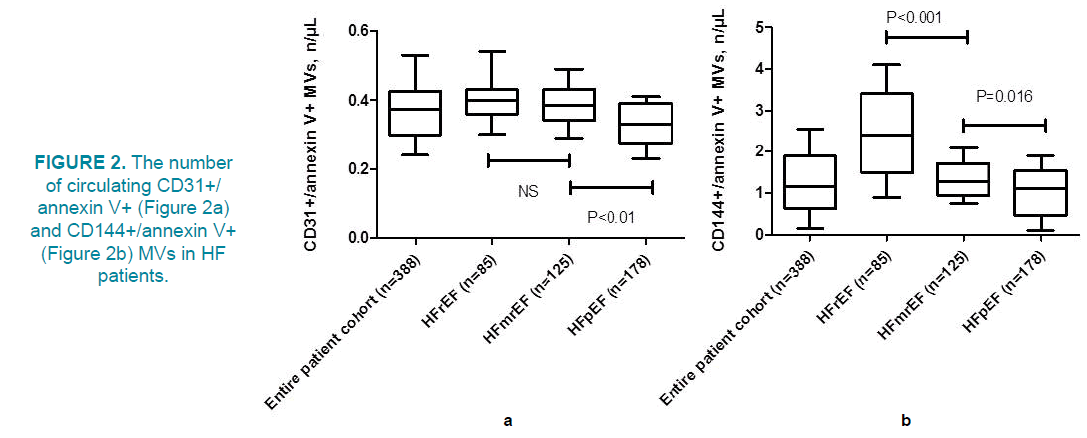
In this study, we have tested a hypothesis that apoptotic endothelial cell-derived MVs could correspond to the biological markers of inflammation, fibrosis and biomechanical stress and that these relations associated with phenotypes of HF. Interestingly, the number of circulating CD31+/annexin V+MVs in HFpEF patients revealed to be significantly different from both HFrEF and HFmrEF individuals (FIGURE 2) Additionally, levels of CD31+/ annexin V+MVs did not differ in patients with HFrEF and HFmrEF. In contrast, a number of circulating CD144+/annexin V+MVs in HFrEF patients was significantly higher to HFmrEF and HFpEF, while this parameter was the lowest in HFpEF (FIGURE 2b). Thus, HFmrEF was similar HFrEF in circulating a number of CD31+/annexin V+MVs, but HFmrEF was determined as interpolating state between HFrEF and HFpEF taking into consideration the level of CD144+/annexin V+MVs.
In entire population of HF patients the univariate linear regression analysis has shown an association between number of CD31+/annexin V+MVs and CD144+/annexin V+MVs and CV risk factors, hemodynamic performances, and various biomarkers. Indeed, the number of circulating CD31+/annexin V+MVs positively associated with levels of NT-proBNP (r=0.46, P=0.001), NYHA functional class of HF (r=0.44, P=0.001), levels of galectin-3 (r=0.44, P=0.002), GDF-15 (r=0.40, P=0.001), T2DM (r=0.38, P=0.001), previous MI (r=0.34, P=0.012), LVEF (r=0.34, P=0.003), levels of hs-CRP (r=0.32, P=0.001), sST2 (r=0.32, P=0.026), serum uric acid (r=0.28, P=0.02), LDL cholesterol (r=0.24, P=0.024). Additionally, the number of circulating CD144+/annexin V+MVs positively associated with levels of galectin-3 (r=0.54, P=0.002), NT-proBNP (r=0.52, P=0.001), GDF-15 (r=0.48, P=0.001), NYHA functional class of HF (r=0.46, P=0.001), T2DM (r=0.34, P=0.001), LVEF (r=0.38, P=0.003), sST2 (r=0.34, P=0.012), previous MI (r=0.36, P=0.002), hs-CRP (r=0.36, P=0.001), serum uric acid (r=0.32, P=0.02), LDL cholesterol (r=0.28, P=0.01). There was found a weak non-significant relation of number of CD31+/ annexin V+MVs and CD144+/annexin V+MVs to age, sex, smoking, some co-existing comorbidities, such as hypertension and obesity, while BMI poorly associated with number of CD31+/annexin V+MVs and CD144+/ annexin V+MVs (r=0.22, P=0.06 and r=0.23, P=0.05 respectively). Consequently, number of CD31+/annexin V+MVs related stronger to HFpEF (r=0.42, P=0.001) than HFrEF (r=0.36, P=0.001) and HFmrEF (r=0.30, P=0.002), but number of CD144+/annexin V+MVs rather associated with HFrEF (r=0.52, P=0.001) and HFmrEF (r=0.49, P=0.002) to HFpEF (r=0.34, P=0.001).
Multivariate linear regression analysis revealed that the number of circulating CD31+/ annexin V+MVs positively associated with HFpEF (r=0.40, P=0.001), levels of galectin-3 (r=0.46, P=0.002), NT-proBNP (r=0.42, P=0.003), and GDF-15 (r=0.36, P=0.001). The number of circulating CD144+/annexin V+MVs positively associated with levels of galectin-3 (r=0.50, P=0.001), HFrEF (r=0.48, P=0.001), HFmrEF (r=0.46, P=0.002), NTproBNP (r=0.46, P=0.001), GDF-15 (r=0.40, P=0.001), and sST2 (r=0.32, P=0.001).
TABLE 4 is reported an impact of various factors on the phenotype of chronic HF. Unadjusted Cox-regression analysis showed that HFpEF was predicted with levels of galectin-3, GDF-15, and number of circulating CD31+/annexin V+EVs, but sST2 and number of circulating CD31+/annexin V+MVs prognosticated HFmrEF. At the same time, the list of independent predictors of HFrEH included previous MI, dilated cardiomyopathy, T2DM, NT-proBNP, galectin-3, GDF-, sST2, and a number of circulating CD144+/annexin V+MVs.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Dependent variable: HFpEF | ||||||
| Hypertension | 1.06 | 1.02-1.17 | 0.044 | 1.03 | 1.00-1.07 | 0.18 |
| T2DM | 1.08 | 1.03-1.19 | 0.042 | 1.04 | 1.00-1.09 | 0.22 |
| NT-proBNP | 1.12 | 1.05-1.25 | 0.001 | 1.04 | 1.00-1.10 | 0.16 |
| Galectin-3 | 1.11 | 1.04-1.22 | 0.002 | 1.06 | 1.02-1.13 | 0.012 |
| GDF-15 | 1.07 | 1.03-1.12 | 0.001 | 1.04 | 1.01-1.06 | 0.048 |
| sST2 | 1.05 | 1.02-1.09 | 0.001 | 1.04 | 1.00-1.09 | 0.24 |
| CD31+/annexin V+EVs | 1.09 | 1.04-1.16 | 0.001 | 1.06 | 1.02-1.11 | 0.02 |
| CD144+/annexin V+EVs | 1.04 | 1.02-1.07 | 0.026 | 1.02 | 0.99-1.04 | 0.66 |
| Dependent variable: HFmrEF | ||||||
| Hypertension | 1.04 | 1.00-1.07 | 0.64 | - | - | - |
| Previous MI | 1.06 | 1.01-1.09 | 0.001 | 1 | 0.96-1.08 | 0.72 |
| Dilated CMP | 1.02 | 0.99-1.03 | 0.7 | - | - | - |
| T2DM | 1.03 | 1.01-1.05 | 0.046 | 1.02 | 1.00-1.04 | 0.42 |
| NT-proBNP | 1.06 | 1.02-1.11 | 0.001 | 1.03 | 1.01-1.06 | 0.05 |
| Galectin-3 | 1.05 | 1.02-1.13 | 0.001 | 1.02 | 1.00-1.03 | 0.66 |
| GDF-15 | 1.04 | 1.00-1.07 | 0.76 | - | - | - |
| sST2 | 1.08 | 1.03-1.15 | 0.001 | 1.06 | 1.02-1.12 | 0.001 |
| CD31+/annexin V+ EVs | 1.08 | 1.04-1.11 | 0.001 | 1.05 | 1.01-1.08 | 0.046 |
| CD144+/annexin V+ EVs | 1.04 | 1.00-1.07 | 0.66 | - | - | - |
| Dependent variable: HFrEF | ||||||
| Previous MI | 1.16 | 1.08-1.27 | 0.001 | 1.1 | 1.06-1.18 | 0.001 |
| Dilated CMP | 1.12 | 1.09-1.18 | 0.001 | 1.07 | 1.04-1.12 | 0.001 |
| T2DM | 1.08 | 1.04-1.13 | 0.003 | 1.07 | 1.02-1.11 | 0.002 |
| NT-proBNP | 1.1 | 1.03-1.16 | 0.001 | 1.04 | 1.02-1.08 | 0.001 |
| Galectin-3 | 1.12 | 1.06-1.20 | 0.001 | 1.09 | 1.05-1.14 | 0.003 |
| GDF-15 | 1.1 | 1.07-1.15 | 0.001 | 1.06 | 1.02-1.10 | 0.001 |
| sST2 | 1.08 | 1.03-1.12 | 0.001 | 1.07 | 1.03-1.11 | 0.002 |
| CD31+/annexin V+ EVs | 1.03 | 1.01-1.06 | 0.022 | 1.02 | 1.00-1.04 | 0.42 |
| CD144+/annexin V+ EVs | 1.09 | 1.05-1.13 | 0.001 | 1.06 | 1.02-1.10 | 0.001 |
TABLE 4. Predictive value of biomarkers on HF phenotype. The unadjusted Cox regression analysis.
When we adjusted results of univariate and multivariate Cox-regression models to etiology factors of HF (previous MI, dilated cardiomyopathy, T2DM), galectin-3, GDF-15, and the number of circulating CD31+/annexin V+EVs were determined as predictors of HFpEF (TABLE 5). Two variables (sST2 and number of circulating CD31+/annexin V+MVs) yielded a discriminative value for HFmrEF. In contrast, HFrEF was predicted with a wide range of biomarkers, such as NT-proBNP, galectin-3, GDF-15, sST2, and a number of circulating CD144+/annexin V+EVs.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Dependent variable: HFpEF | ||||||
| NT-proBNP | 1.12 | 1.05-1.25 | 0.001 | 1.04 | 1.00-1.10 | 0.16 |
| Galectin-3 | 1.11 | 1.04-1.22 | 0.002 | 1.06 | 1.02-1.13 | 0.012 |
| GDF-15 | 1.07 | 1.03-1.12 | 0.001 | 1.04 | 1.01-1.06 | 0.048 |
| sST2 | 1.05 | 1.02-1.09 | 0.001 | 1.04 | 1.00-1.09 | 0.24 |
| CD31+/annexin V+ EVs | 1.09 | 1.04-1.16 | 0.001 | 1.06 | 1.02-1.11 | 0.02 |
| CD144+/annexin V+ EVs | 1.04 | 1.02-1.07 | 0.026 | 1.02 | 0.99-1.04 | 0.66 |
| Dependent variable: HFmrEF | ||||||
| NT-proBNP | 1.06 | 1.02-1.11 | 0.001 | 1.03 | 1.01-1.06 | 0.05 |
| Galectin-3 | 1.05 | 1.02-1.13 | 0.001 | 1.02 | 1.00-1.03 | 0.66 |
| GDF-15 | 1.04 | 1.00-1.07 | 0.76 | - | - | - |
| sST2 | 1.08 | 1.03-1.15 | 0.001 | 1.06 | 1.02-1.12 | 0.001 |
| CD31+/annexin V+ EVs | 1.08 | 1.04-1.11 | 0.001 | 1.05 | 1.01-1.08 | 0.046 |
| CD144+/annexin V+ EVs | 1.04 | 1.00-1.07 | 0.66 | - | - | - |
| Dependent variable: HFrEF | ||||||
| NT-proBNP | 1.1 | 1.03-1.16 | 0.001 | 1.04 | 1.02-1.08 | 0.001 |
| Galectin-3 | 1.12 | 1.06-1.20 | 0.001 | 1.09 | 1.05-1.14 | 0.003 |
| GDF-15 | 1.1 | 1.07-1.15 | 0.001 | 1.06 | 1.02-1.10 | 0.001 |
| sST2 | 1.08 | 1.03-1.12 | 0.001 | 1.07 | 1.03-1.11 | 0.002 |
| CD31+/annexin V+ EVs | 1.03 | 1.01-1.06 | 0.022 | 1.02 | 1.00-1.04 | 0.42 |
| CD144+/annexin V+ EVs | 1.09 | 1.05-1.13 | 0.001 | 1.06 | 1.02-1.10 | 0.001 |
TABLE 5. Predictive value of biomarkers on HF phenotype. The adjusted Cox regression analysis to etiology factors of HF (hypertension, MI, T2DM, dilated cardiomyopathy).
Using C-statistic technique we compared an ability of different biomarkers to associate with HFpEF, HFmrEF and HFrEF. The best predictor of HFpEF appeared to be a combination of number of circulating CD31+/annexin V+EVs and galectin-3 (area under curve (AUC)=0.68; 95% confidence interval (CI)=0.61-0.77; P=0.001), but GDF-15 (AUC=0.61; 95% CI=0.59-0.63), galectin-3 (AUC=0.60; 95% CI=0.56-0.65), and number of circulating CD31+/annexin V+EVs (AUC=0.62; 95% CI=0.57-0.66) alone and combination of GDF-15 and galectin-3 (AUC=0.64; 95% CI=0.57-0.68) were not significantly better than circulating CD31+/annexin V+EVs and GDF- 15 (AUC=0.65; 95% CI=0.60-0.69; P=0.26).
The predictive values of sST2 (AUC=0.65; 95% CI=0.60-0.69), number of circulating CD31+/annexin V+EVs (AUC=0.63; 95% CI=0.58-0.69) alone and their combination (AUC=0.65; 95% CI=0.59-0.70) for HFmrEF did not significantly distinguish each other (P=0.48).
HFrEF was predicted significantly better with double combinations of number of circulating CD144+/annexin V+MVs and sST2 (AUC=0.70; 95% CI=0.66-0.75) or number of circulating CD144+/annexin V+MVs and galectin-3 (AUC=0.71; 95% CI=0.65-0.76) in contrast to NT-proBNP (AUC=0.67; 95% CI=0.62-0.75), galectin-3 (AUC=0.66; 95% CI=0.60-0.73), GDF-15 (AUC=0.65; 95% CI=0.61-0.70), sST2 (AUC=0.63; 95% CI=0.60-0.67), and number of circulating CD144+/annexin V+MVs (AUC=0.66; 95% CI=0.62-0.71) alone (P=0.001). Moreover, triple combination of any biomarkers with obligatory including of number of circulating CD144+/annexin V+MVs was not sufficiently better to any double combination.
Thus, a number of circulating CD31+/ annexin V+EVs improved the discriminative value of galectin-3 to predict HFpEF, but the number of circulating CD144+/annexin V+MVs increased the ability of both sST2 and galectin-3 to prognosticate HFrEF. Interestingly, there was identified a similarity in predictive values of sST2 and number of circulating CD31+/ annexin V+EVs for HFmrEF. Nevertheless, a combination of these biomarkers did not improve their predictive potency in that manner.
Discussion
The main result of the study confirmed our previous hypothesis that apoptotic endothelial cell-derived MVs may associate with specific phenotypes of HF. Indeed, a number of circulating CD31+/annexin V+EVs better corresponded to HFpEF, while the number of circulating CD144+/annexin V+EVs strongly associated with HFrEF. Recent studies have shown that elevated levels of MVs of different origin, such as platelet-derived, monocytederived and endothelial cell-derived MVs, may reflect thrombotic and inflammatory burden in HF patients [30,31]. Moreover, the signature of MVs in HF individuals appeared to be different depending on the phenotypes of HF and comorbidities including T2DM, obesity, hypertension, inflammatory cardiomyopathies, coronary artery disease [32-34]. This fact sufficiently limited an interpretation of data regarding a number of circulating MVs with numerous immune phenotypes in HF, because the signature of platelet-derived and noneapoptotic MVs originated form mononuclear in HF patients was not unique. Moreover, the altered signature of MVs originated from platelets and mononuclear is incapable of predicting future clinical events including lifethreatening outcomes in HF, but describes the risk of a present thrombus, thrombotic complications and coronary occlusion [34,35]. In contrast, endothelial-cell-derived MVs labeled as CD31+ and CD144+ showed a strong correlation with endothelial dysfunction in not just HF patients, but in at high risk of HF individuals [18]. Although CD31+ and CD144+MVs could be present a risk of HF development and progression, independent prognostic information for HF-related outcomes was found for apoptotic endothelial cell-derived MVs with phenotypes CD31+/Annexin V+ and CD144+/Annexin V+ [36,37]. However, the association of a number of CD31+/Annexin V+ and CD144+/Annexin V+MVs predominantly with HFpEF and HFrEF respectively was identified first. Therefore, we established that signature of endothelial cell-derived apoptotic MVs may correspond to levels of biomarkers with known predictive values for HF, i.e. galectin-3, sST2, and NT-proBNP. In fact, there were speculations regarding the ability of these biomarkers to predict the development of HF phenotypes, but the discriminative role of these molecules for HFrEF, HFmrEF, and HFpEF did not determine [38]. Taking into consideration that at least half of all individuals with established chronic HF in worldwide exhibited preserved and mid-range LVEF and that the number of them progressively increases strongly associating with poor clinical outcomes, an identification of an optimal combination of biomarkers for exact prediction of HF phenotypes could improve current risk assessment models [39,40]. Indeed, NT-proBNP, sST2, galectin-3 had different diagnostic and predictive values for use in patients who have HFpEF and HFmrEF [40]. Overall, our results showed that various immune phenotypes of apoptotic endothelial cell-derived MVs correlated to biomarkers of inflammation (hs-CRP, GDF-15), fibrosis (galectin-3, sST2) and biomechanical stress (NT-proBNP) in HFpEF, HFmrEF, and HFrEF, although the strength of relation was variable. Innate molecular mechanisms, which could explain the interrelation between apoptotic endothelial cell-derived MVs and phenotypes of HF, remain to be unclear. Probably, conventional biomarkers (NT-proBNP, hs- CRP, GDF-15, galectin-3, sST2) and CD31+/ Annexin V+ and CD144+/Annexin V+ may reflect similar pathophysiological states (cardiac remodeling, fibrosis, inflammation) [17,18], but this does not explain why a number of circulating CD31+/Annexin V+MVs CD144+/ Annexin V+MVs corresponds to HFpEF and HFrEF respectively. First, it can suggest that apoptotic endothelial cell-derived MVs may be a component of the endogenous vascular repair system, which is actively involved in restoring of cardiac and vasculature structure and function. Moreover, both immune phenotypes of apoptotic endothelial cell-derived MVs may have tissue specificity [11,15], while this assumption requires to be elucidated in details. Secondary, apoptotic endothelial cell-derived MVs are not just cargo for active molecules, regulatory peptides, hormones, growth factors, and other molecules, which are defined as secret, but they may directly impair vascular and cardiac endothelium leading to endothelial dysfunction [27,39]. It has postulated that there was no significant difference between various MVs originated from apoptotic endothelial cells inability to the injury of vasculature [17]. However, the results of the study did not confirm this statement and demonstrate a sufficient difference between circulating CD31+/Annexin V+MVs CD144+/Annexin V+MVs incapability to accompany to HFpEF and HFrEF.
Additionally, received findings revealed that a number of circulating CD31+/annexin V+MVs may improve a prediction of galectin-3 for HFpEF, and that number of circulating CD144+/annexin V+MVs is able to increase predictive capabilities of sST2 and galectin-3 for HFrEF. All these open new perspective in prognostication of HF evolution and shaping more accurate predictive scales based on biomarker measurement. Because data regarding this topic are very limited, large studies are required to establish which endothelial cellderived MPs are of prognostic value in different HF phenotypes.
Study Limitations
This study has some limitations. The first limitation was a small sample size. We used a retrospective design of the study as a part of a large cohort that was investigated with an aim to assay a predictive value of MVs’ signature in HF patients. In this context, we performed a measure of MVs in a central laboratory using a conventional method to minimize the risk of analytical errors. The second limitation was numerous etiology factors of HF in the study. Before receiving final statistics we used the adjusted Cox-regression model to ischemia, diabetes mellitus, hypertension, and cardiomyopathy. The authors suppose that these restrictions might have no significant impact on the study data interpretation.
Conclusion
In the study, we found that a number of circulating CD31+/annexin V+MVs may improve a prediction of galectin-3 for HFpEF, and that number of circulating CD144+/ annexin V+MVs is able to increase predictive capabilities of sST2 and galectin-3 for HFrEF.
Ethical Declaration
All the patients have given their written informed consent for participation in the study. The study was approved by the Local Ethical Committee (IRB #3/2010) of State Medical University of Zaporozhye (Ukraine). The investigators followed strictly all the requirements to clinical trials in conformity with the World Medical Association Declaration of Helsinki, 1964, good clinical practice provided by International Conference on Harmonization, Council of Europe Convention for the Protection of Human Rights and Dignity of the Human Being in view of using achievements in biology and medicine, convention on Human Rights and Biomedicine, including Additional Protocol to the Convention on Human Rights and Biomedicine, concerning Biomedical Research, and legislation of Ukraine.
Acknowledgment
We thank all patients for their participation in the investigation, staff of the Regional Zaporozhye Hospital (Ukraine) and the doctors, nurses, and administrative staff in City hospital #6 (Zaporozhye, Ukraine), Regional Hospital (Zaporozhye, Ukraine), Regional Center of Cardiovascular Diseases (Zaporozhye, Ukraine), Private Hospital “Vita Center” (Zaporozhye, Ukraine), Central Clinical and Immunology Laboratory “Dia-Service” (Zaporozhye, Ukraine), general practices, and site-managed organizations that assisted with the study.
Authors’ Contribution
AB initiated the hypothesis and designed the study protocol, contributed to collect, analyze and interpret the data, performed statistical analysis, wrote the manuscript and approved the final version of the paper. AK enrolled the patients; collected and analyzed the data reviewed the source documents, performed flow cytometry technique and interpreted the obtained results. TS performed echocardiography and interpreter of the obtained results. TB contributed to enrolling the patients in the study and collected the data. All authors read the manuscript before submitting and agree with the final version of the paper.
References
- Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart. Fail. 37(27), 2129-2200 (2016).
- Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 62, e147-e239 (2013).
- Petutschnigg J, Edelmann F. Heart failure with mid-range ejection fraction and with preserved ejection fraction. Herz. 43(5), 392-405 (2018).
- Bartekova M, Radosinska J, Jelemensky M, et al. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 23(5), 733-758 (2018).
- Xu M, Yan L, Xu J, et al. Predictors and prognosis for incident in-hospital heart failure in patients with preserved ejection fraction after first acute myocardial infarction: An observational study. Medicine (Baltimore). 97, e11093 (2018).
- Voors AA, Ouwerkerk W, Zannad F, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur. J. Heart Fail. 19(5), 627-634 (2017).
- Curl CL, Danes VR, Bell JR, et al. Cardiomyocyte functional etiology in heart failure with preserved ejection fraction is distinctive-a new preclinical model. J. Am. Heart Assoc. 7(11), e007451 (2018).
- Vanderlaan RD, Caldarone CA, Backx PH. Heart failure in congenital heart disease: the role of genes and hemodynamics. Pflugers Arch. 466(6), 1025-1035 (2014).
- Berezin AE. Endothelial derived micro particles: Biomarkers for heart failure diagnosis and management. J. Clin. Trial. Cardiol. 2, 1-3 (2015).
- Berezin AE, Kremzer AA. The impaired phenotype of circulating endothelial microparticles in chronic heart failure patients: Relevance to body mass index. Diabetes Metab. Syndr. 9(4), 230-236 (2015).
- Berezin AE. Endothelial progenitor cells dysfunction and impaired tissue reparation: The missed link in diabetes mellitus development. Diabetes Metab. Syndr. 11(3), 215-20 (2017).
- Tsukada S, Masuda H, Jung SY, et al. Impaired development and dysfunction of endothelial progenitor cells in type 2 diabetic mice. Diabetes Metab. 43(2), 154-162 (2017).
- Chong AY, Blann AD, Patel J, et al. Endothelial dysfunction and damage in congestive heart failure: relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation. 110(13), 1794-1798 (2004).
- Berezin A, Zulli A, Kerrigan S, et al. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin. Biochem. 48(9), 562-568 (2015).
- Berezin AE, Kremzer AA, Samura TA, et al. Impaired immune phenotype of circulating endothelial-derived microparticles in patients with metabolic syndrome and diabetes mellitus. J. Endocrinol Invest. 38(8), 865-874 (2015).
- Zhang Z, Yang J, Yan W, et al. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J. Am. Heart Assoc. 5(1), e002856 (2016).
- Eming SA, Martin P2, Tomic CM. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 6(265), 265sr6 (2014).
- Berezin AE. Microparticles in chronic heart failure. Adv. Clin. Chem. 81, 1-41 (2017).
- Chow SL, Maisel AS, Anand I, et al. American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association Circulation. 135(22), e1054-91 (2017).
- Executive summary: Standards of medical care in diabetes-2013. Diabetes Care. 36, S4-10 (2013).
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106(25), 3143-421 (2002).
- World Health Organization. Waist circumference and waist-hip ratio report of a WHO expert consultation. Geneva: World Health Organization (2008).
- Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes. Rev. 13(3):275–86 (2012)
- Lindson HN, Begh R, McDermott MS, et al. The importance of practitioner smoking status: a survey of NHS Stop Smoking Service practitioners. Patient Educ. Couns. 93(1): 139-145 (2013).
- Quiñones MA, Douglas PS, Foster E, et al. American College of Cardiology; American Heart Association; American College of Physicians; American Society of Internal Medicine Task Force on Clinical Competence. American College of Cardiology/American Heart Association clinical competence statement on echocardiography: a report of the American College of Cardiology/American Heart Association/American College of Physicians-American Society of Internal Medicine Task Force on Clinical Competence. Circulation. 107(7), 1068-89 (2003).
- Levey AS, Stevens LA, Schmid CH, et al. for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604-612 (2009).
- Berezin AE, Kremnzer AA, Berezina TA, et al. The signature of circulating microparticles in heart failure patients with metabolic syndrome. J. Circ. Biomark. 5, 1-10 (2016).
- Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 77(6), 502-514 (2010).
- Lacroix R, Judicone C, Mooberry M, et al. The ISTH SSC Workshop. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J. Thromb. Haemost. (2013).
- Chiva BG, Laake K, Myhre P, et al. Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity. PLoS. One. 12(2), e0172558 (2017).
- Gohar A, de Kleijn DPV, Hoes AW, et al. Vascular extracellular vesicles in comorbidities of heart failure with preserved ejection fraction in men and women: The hidden players. A mini review. Vascul. Pharmacol. 111, 1-6 (2018).
- Huang PH, Huang SS, Chen YH, et al. Increased circulating CD31+/annexin V+ apoptotic microparticles and decreased circulating endothelial progenitor cell levels in hypertensive patients with microalbuminuria. J. Hypertens. 28, 1655-1665 (2010).
- Werner N, Wassmann S, Ahlers P, et al. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb. Vasc. Biol. 26(1), 112-116 (2006).
- McClane N, Jeske W, Walenga JM, et al. Identification of novel hemostatic biomarkers of adverse clinical events in patients implanted with a continuous-flow left ventricular assist device. Clin. Appl. Thromb. Hemost. 24(1), 965-972 (2018).
- Chiva BG, Ritschel V, Andersen GØ, et al. Monocyte-derived circulating microparticles (CD14+, CD14+/CD11b+, and CD14+/CD142+) are related to long-term prognosis for cardiovascular mortality in STEMI patients. Int. J. Cardiol. 227, 876-881 (2017).
- Berezin AE, Kremzer AA, Samura TA, et al. Predictive value of apoptotic microparticles to mononuclear progenitor cells ratio in advanced chronic heart failure patients. J. Cardiol. 65(5), 403-411 (2015).
- Sinning JM, Losch J, Walenta K, et al. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 32(16), 2034-2041 (2011).
- Berezin AE. Impaired phenotype of circulating endothelial-derived microparticles: Novel marker of cardiovascular risk. J. Cardiol. Therap. 2(4), 273-278 (2015).
- Thulin Å, Christersson C, Alfredsson J, et al. Circulating cell-derived microparticles as biomarkers in cardiovascular disease. Biomark Med. 10(9), 1009-22 (2016).
- Cui Y, Qi X, Huang A, et al. Differential and predictive value of galectin-3 and soluble suppression of tumorigenicity-2 (sst2) in heart failure with preserved ejection fraction. Med. Sci. Monit. 24, 5139-5146 (2018).