Perspective - Imaging in Medicine (2012) Volume 4, Issue 3
MRI appearances of tuberculous meningitis in HIV-infected children: a paradoxically protective mechanism?
Savvas Andronikou1, Nishentha Govender1, Arhana Ramdass1and Ronald van Toorn2*1 Department of Radiology, Faculty of Health Sciences, University of the Witwatersrand, York Road, Parktown, Johannesburg 2193, South Africa
2 Department of Paediatrics & Child Health, University of Stellenbosch, Fransie van Zyl Drive, Parrow, South Africa
- *Corresponding Author:
- Ronald van Toorn
Department of Paediatrics & Child Health University of Stellenbosch
Fransie van Zyl Drive Parrow, South Africa
Tel.: +27 767 789 129
E-mail: docsav@mweb.co.za
Abstract
Immune suppression predisposes HIV-infected children to opportunistic infections including Mycobacterium tuberculosis. HIV infection increases the risk of progression to active disease and also increases the risk for extrapulmonary involvement, including tuberculous meningitis (TBM). Brain injury in TBM is the consequence of an immune-mediated vasculopathy. HIV-related immune dysfunction prevents the production of thick exudates that cause parenchymal infarctions and cerebrospinal fluid flow obstruction. It could be postulated that HIV may be ‘protective’ from some of the common complications related to TBM. The aim of this paper is to highlight the ‘protective’ features of HIV-related immune suppression observed on MRI in children with TBM and promote the use of MRI for detecting subtle and atypical meningeal enhancement in HIV and TBM coinfected children.
Keywords
atrophy; basal ganglia; basal meningeal enhancement; HIV; hydrocephalus; immune suppression; infarction; milliary TB; MRI; tuberculous meningitis
Background
Tuberculosis (TB) is a leading cause of death among people infected with HIV. In the advanced stages of HIV infection, tuberculosis may have atypical presentations including tuberculous meningitis (TBM) [1]. This is the most severe and deadly form of TB in infants and children [2–6] and this age group is also at a particularly high risk for acquiring the disease. In addition, younger age at presentation is associated with a worse outcome [7].
Diagnosis
The traditional diagnosis of TBM relies on the combination of cerebrospinal fluid (CSF) chemistry, microscopy and culture. Numerous drawbacks, including false-negatives and time for culture, hamper traditional tests and new methods such as gene amplification techniques have been developed to improve speed and accuracy. These, however, are costly and require CSF, sputum or tissue sampling, which in itself is a challenge in pediatric practice. Imaging, therefore, including CT and MRI continues to play a role in the evaluation of patients with suspected TBM for a rapid and early diagnosis, as well as for detection of complications and monitoring disease progression [2,8]. MRI is an excellent imaging tool for evaluating the CNS and is reportedly superior to CT for the detection of meningeal disease and parenchymal abnormalities [7]. However, it has been considered unlikely to make an impact on the diagnosis of TBM worldwide because of limited availability in areas where it is needed most. Technical considerations such as MRIcompatible life-support equipment and the requirement for general anesthesia or sedation in younger children, also hamper efforts to increase its use in pediatric TBM diagnosis [9].
Pathogenesis
Intracranial TB is caused by hematogeneous spread from a primary focus elsewhere in the body, usually the lungs [7]. Features responsible for the clinical manifestations of TBM are the basal inflammatory exudates that cause cranial nerve palsies and obstruct CSF pathways, resulting in hydrocephalus and the obliterative vasculitis, which leads to infarctions [4]. Cell-mediated immunity not only plays a major role in limiting the spread of tubercle bacilli within the body but also results in many of the pathological features of TBM through cellular immune responses to the presence of tubercle bacilli in the meninges. The granulomatous response is the cause of the subsequent pathology in TBM [5]. Two theories exist: that rupture of subependymal or subpial granulomata into the CSF causes the meningitis, or that direct penetration of the walls of meningeal vessels occurs by hematogenous spread [8,10]. The subsequent granulomatous inflammatory response that develops is thick, gelatinous and localized predominantly in the basal cisterns and along the middle and anterior cerebral arteries. This is responsible for the basal leptomeningeal enhancement seen on intravenous contrast-enhanced CT and gadolinium enhanced MRI [11].
As HIV infection primarily impairs the cellmediated immunity, the immune responses to TB bacilli in HIV-infected individuals may be different resulting in different clinical and radiological features [5]. Garg et al. describe ways in which HIV infection may influence the pathological features of TBM. Most importantly, tuberculous exudates in the HIV-infected patients are minimal, thinner and of a serous type, contain fewer lymphocytes, epithelioid cells and Langhans type of giant cells as compared with HIV-negative patients but have larger numbers of acid-fast bacilli in the cerebral parenchyma and meninges of HIV-infected patients. Hydrocephalus is not common even though ventricular dilatation may be present secondary to cerebral atrophy [1,5].
The main features of TBM on imaging are summarized in Table 1 alongside the proposed pathogenetic mechanism.
MRI features of TBM
Basal enhancement
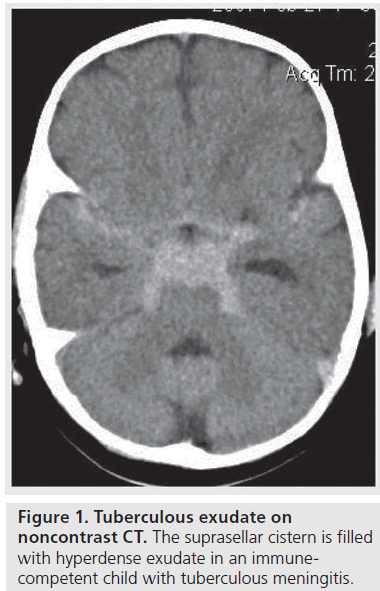
Basal enhancement is the most characteristic and sensitive CT finding of pediatric TBM, reported in up to 93% of patients [11–15]. It is not reported to occur with viral or pyogenic meningitis [9]. The involved meninges and cisterns are iso- or hyper-dense relative to the CSF on unenhanced CT (Figure 1) and these demonstrate enhancement after intravenous contrast administration [9].
Pathological enhancement on CT is irregular in outline and different from the sharply defined enhancement of normal vessels in the fissures and cisterns [16]. Przybojewski and colleagues described nine objective criteria for abnormal basal enhancement in TBM, to improve detection as this may not always be obvious on CT [9]. Teoh et al. and Ozates et al. both reported that on CT the most common locations for abnormal enhancement were the Sylvian fissures, around the brain stem and tentorial cisterns [16,17]. The pathological enhancement is thought to occur either due to extravasation of the contrast medium from the vascular endothelium, which is disrupted because of the activity of lymphocytes [18], or as a result of enhancement of vascularized granulation tissue, which has organized and replaced CSF within the cisterns at the base of the brain [15].
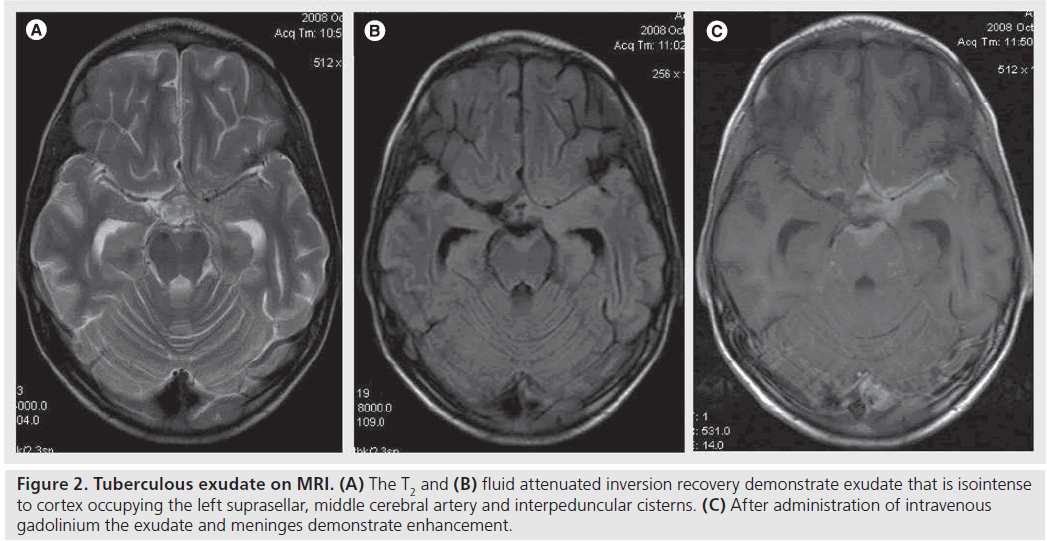
Pienaar et al. showed that the diagnosis of basal meningeal enhancement on contrast-enhanced CT is poorer than MRI with only 70% enhancement in comparison to 97% on contrasted MRI studies [7]. This is probably because MRI has the advantages of greater contrast resolution, and no bone artifacts [7]. Distension of the affected subarachnoid spaces by inflammatory exudate causes some shortening of T1 and T2 relaxation times as compared with normal CSF without prior administration of intravenous contrast agents (Figure 2) [19]. MRI also allows more detailed demonstration of enhancement patterns involving the leptomeninges (pia and arachnoid) and pachymeninges (dural) individually [8,18]. A nodular form of leptomeningeal enhancement in TBM seen on MRI is attributed to the presence of pial tuberculomas [8,18], which occur more commonly in HIV-infected patients [20].
Ozates et al. found that the severity of TBM was not related to the degree of meningeal enhancement and enhancement was found with the same frequency in each clinical stage [17]. This finding is supported by Teoh et al. who also found basal enhancement with the same frequency in each clinical stage [16]. However, follow-up CT has shown new or progressive enhancement in some patients, and no change in others [2]. This may be a reflection of the deficiencies of CT in picking up disease features when performed too early in the disease process and the evolving imaging appearances representing existing pathology, which become visible in the absence of clinical deterioration. This is supported by Teoh et al. who reported some patients with enhancement that appeared to increase during the illness but was not associated with a change in their condition [16]. In other patients, meningeal enhancement persisted after the complete disappearance of clinical symptoms and signs [16]. Ozates et al. reported no enhancement of the meninges in 178 patients on admission who developed equivocal, moderate or marked enhancement of the basal meninges within 1 month after the initiation of antituberculous therapy [17].
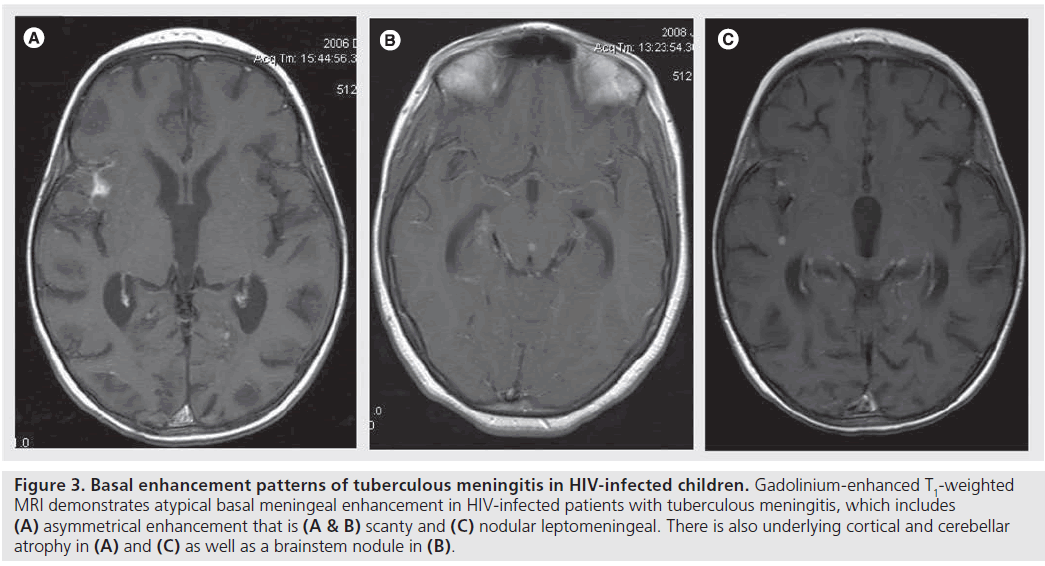
There have been conflicting results regarding the effect of HIV and basal enhancement. Most authors have reported minimal or absent meningeal enhancement on CT or MR images of HIV-infected patients with TBM (Figure 3) [1]. Other studies, however, have not shown any major difference regarding presence of meningeal enhancement between HIV-infected and HIV-uninfected TBM patients [21]. In a study of 25 HIV-infected adults with CNS TB, cisternal/ meningeal enhancement was present in only nine patients (36%) [10]. In a study of adult patients with TBM, Katrak et al. reported prominent meningeal enhancement only in the HIV-negative group. This was in contrast to the HIV-positive group, where meningeal enhancement was infrequent, generally less prominent and seen on follow-up imaging after antituberculous therapy [5]. Pathological correlation showed that the scant (thin, minimal and serous) exudates observed pathologically in the HIV-positive patients were reflected as minimal meningeal enhancement on imaging [5]. The most likely reason was thought to be the severe degree of immunosuppression in HIV-positive patients leading to impairment of the granulomatous response to mycobacteria [5]. A study of children with TBM using MRI also found that intense basal meningeal enhancement occurred less frequently (p = 0.31) in HIV-infected children and also that more HIV-infected children presented with asymmetrical and nodular meningeal enhancement than did the immune-competent children (Figure 3) [20].
Supporting the hypothesis that immune suppression results in less basal enhancement, a study by Srikanth et al. demonstrated that imaging features of TBM in the elderly were also found to be atypical with minimal or absent basal exudates thought to be secondary to agerelated immune senescence [22]. Furthermore, the research group of Schoeman et al. showed that children with TBM who receive steroids have less basal enhancement on CT scans after 1 month of treatment than control patients and concluded that steroids improve survival rate and intellectual outcome in TBM [23]. Enhanced resolution of basal exudate by steroids was shown by serial CT scanning. Corticosteroids did not affect intracranial pressure or the incidence of basal ganglia infarction significantly [23]. The extrapolation is that immune suppression reduces the development of meningeal inflammation and exudate formation in TBM.
Figure 2.Tuberculous exudate on MRI. (A) The T2 and (B) fluid attenuated inversion recovery demonstrate exudate that is isointense to cortex occupying the left suprasellar, middle cerebral artery and interpeduncular cisterns. (C) After administration of intravenous gadolinium the exudate and meninges demonstrate enhancement.
Figure 3.Basal enhancement patterns of tuberculous meningitis in HIV-infected children. Gadolinium-enhanced T1-weighted MRI demonstrates atypical basal meningeal enhancement in HIV-infected patients with tuberculous meningitis, which includes (A) asymmetrical enhancement that is (A & B) scanty and (C) nodular leptomeningeal. There is also underlying cortical and cerebellar atrophy in (A) and (C) as well as a brainstem nodule in (B).
Vasculitic infarcts
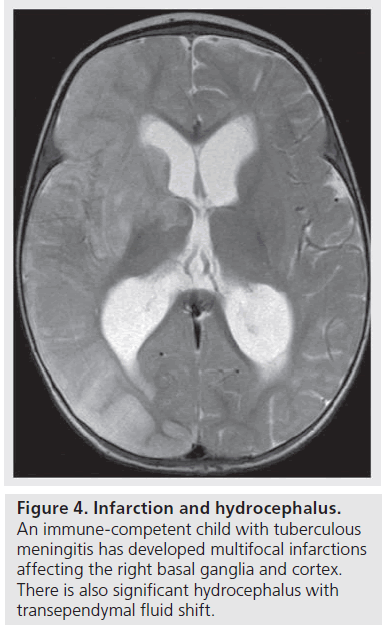
Infarction is a common complication of TBM. TBM-related injury is mediated by the formation of thick gelatinous inflammatory exudates that envelope the vessels at the base of the brain and result in a panarteritis that in turn leads to thrombosis and occlusion causing infarcts. The vessels most commonly involved are the perforating vessels at the base of the brain, the lenticulostriate branches of the middle cerebral artery. The stem and cortical branches of the middle cerebral artery in the Sylvian fissures as well as the supraclinoid internal carotid artery are less commonly involved. This accounts for the predominant distribution of infarctions involving the basal ganglia, anterior limbs of the internal capsules and thalami (Figure 4). A study by Andronikou et al. demonstrated that, in particular, the heads of the caudate nuclei and anteromedial thalami are the most commonly involved [24]. Furthermore, the metabolic needs of the developing basal ganglia in children exceeds those of adults, making them even more susceptible to ischemic injury [1,8,12,24].
Cerebral infarction is a major determinant of outcome in childhood TBM [7]. In general, all infarctions are associated with a poor outcome but bilateral basal ganglia involvement has the worst prognosis with extensive bilateral infarcts resulting in death [15]. The study by Andronikou et al. determined that the highest risk was associated with bilateral basal ganglia and internal capsule infarctions followed by bilateral infarcts in general [24]. Hemispheric infarction was associated with the lowest risk of a poor outcome (Figure 4) [24].
CT may miss acute infarctions, making MRI the modality of choice for detecting acute infarcts in TBM [24]. In the study by Pienaar et al., two of 12 patients with bilateral basal ganglia infarcts were only identified on MRI [7]. Infarction in another important region, the brainstem, was poorly demonstrated on CT (80% not visualized). The majority were acute infarcts showing restricted diffusion on diffusion weighted imaging [7]. However, the unavailability of MRI, long waiting lists and long scanning times limits its usage, especially in resource-poor countries [9].
Garg et al. found that HIV-infected individuals are more likely to present with cerebral infarcts located in the cortex while HIVuninfected patients predominantly presented with basal ganglia infarctions [1]. This finding supports the hypothesis that HIV-related immune suppression reduces the risk of tuberculous exudate-mediated vasculopathy of the perforating branches that supply the basal ganglia. The higher prevalence of cortical infarcts in HIV-infected TBM children are most likely to be related to HIV vasculopathy involving medium and large vessels.
Hydrocephalus
CT and MRI are both capable of demonstrating TBM-related hydrocephalus (Figure 4). Early identification of hydrocephalus is important as effective treatment is available [15,24]. CSF obstruction in TBM is caused by the dense and adhesive basal exudate that fills the basal cisterns and is usually most prevalent in the interpeduncular fossa and suprasellar region as well as the ambient and prepontine cisterns and the spinal subarachnoid space. The resulting bottleneck of the CSF pathway at the level of the tentorial opening results in hydrocephalus, which is of the communicating type in more than 80% of children with TBM [3,8,9,10]. In the other 20% of patients with TBM hydrocephalus, this is of the obstructive, noncommunicating type, which occurs when the exit foramina of the fourth ventricle are blocked by the exudate [3,25].
Figure 4.Basal enhancement patterns of tuberculous meningitis in HIV-infected children. Gadolinium-enhanced T1-weighted MRI demonstrates atypical basal meningeal enhancement in HIV-infected patients with tuberculous meningitis, which includes (A) asymmetrical enhancement that is (A & B) scanty and (C) nodular leptomeningeal. There is also underlying cortical and cerebellar atrophy in (A) and (C) as well as a brainstem nodule in (B).
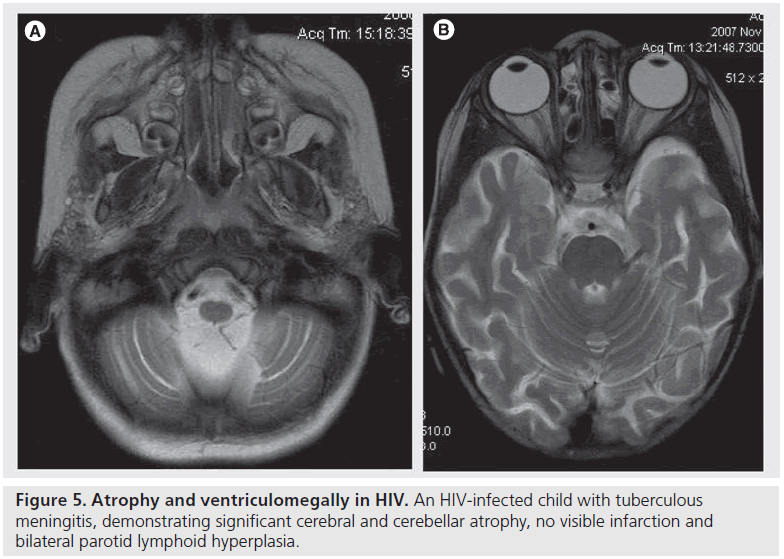
By contrast, HIV-infected children develop cerebral atrophy (68%; Figure 5) and ventriculomegaly (78%) as a result of volume loss rather than hydrocephalus. In children with HIV encephalopathy the amount of atrophy correlates with the viral load and disease severity [26]. Furthermore, in both HIV-infection and TB, cortical atrophy may be secondary to a poor nutritional state (wasting disease) [26]. Obstructive hydrocephalus appears to occur less frequently in HIV-infected children with TBM [1,26]. A report by Katrak et al. indicates that noncommunicating hydrocephalus was significantly more frequent in their HIV-negative group [5]. Marais and colleagues report that noncommunicating hydrocephalus occurs less frequently in the HIV-infected patients [21]. The decreased likelihood of developing hydrocephalus associated with TBM in children who are infected with HIV is probably a consequence of the defective host inflammatory response [21,26]. Any ventricular dilatation seen in HIV-infected patients is therefore most likely to be secondary to cerebral atrophy [1], which protects against the development of raised intracranial pressure.
Immune reconstitution inflammatory syndrome
TB-associated immune reconstitution inflammatory syndrome (TB-IRIS) is a potentially life-threatening complication in HIV-infected children with TB of the CNS. Paradoxical TB-IRIS should be considered when new neurological signs develop shortly after initiation of antiretroviral therapy (ART) in children with CNS TB. Immunological risk factors for TB IRIS include low baseline CD4 count and a stronger CD4 percentage increase with HIV viral load decline [27]. Corticosteroid therapy has been shown to improve outcome in TB-IRIS [28]. TB-IRIS serves as another example of the detrimental effects of an ‘overfunctional’ immune system in HIV-infected children with TBM. Basal enhancement may appear when the patient is initiated on ART, which may give the appearance of worsening on imaging but which may also make the diagnosis of TBM more obvious. The differential diagnosis of paradoxical neurological TB-IRIS includes other opportunistic CNS infections, noncompliance to anti-TB treatment and drug reactions and toxicities [28].
Conclusion
Both the comparative research and opinions reported in the literature concur that HIV-infected children are unable to mount a significant inflammatory response to the arrival of tuberculous bacilli at the meninges. The usual cascade starting with the production of a thick exudate and cisternal granulation tissue, the resultant panvasculitis with infarction and the CSF flow obstruction with development of noncommunicating hydrocephalus do not occur as frequently or with as much severity in children infected with HIV who develop TBM. HIV, which tends to predispose to blood-borne and extrapulmonary disease due to inability to seal off infection in the lungs, appears to protect against the complications of TBM, which determine prognosis and management. The hypothesis that the immune suppression in HIV-infected patients is protective supports the rationale for treating HIV-uninfected children who present with TBM using steroids in addition to antituberculous drugs. Use of corticosteroids in the treatment of HIV-uninfected patients needs more supporting data and clarification, however.
Future perspective
The immune suppression in HIV and resultant dampening of the host response masks imaging findings, such as basal enhancement, and results in atypical appearances. This supports the use of MRI for early and accurate diagnosis of TBM even though MRI may be difficult to access in certain geographic locations.
MRI CSF flow analysis may become useful for prediciting impending hydrocephalus in TBM, as velocities and direction of CSF flow change with improving levels of immune competence and atrophy in HIV-infected children.
The novel ability to detect white matter fiber number and integrity using diffusion tensor imaging and white matter volume using segmentation techniques will allow prediction of white matter volume gain in HIV-infected patients receiving highly active ART successfully and will allow calculation of contributing factors to hydrocephalus and intracranial pressure.
CSF spectroscopy has been used in vitro and in vivo and shows some promise for diagnosis of TBM. This would conceivably replace CSF sampling through lumbar puncture.
The future may lie within a full spectrum of physiological imaging using MRI. Radiologists dream of a world where rapid and accurate imaging diagnosis can replace CSF pressure sampling, intracranial pressure monitoring and CSF culture as the gold standards of care. The prediction of the likelihood for developing complications of TBM as host immunity increases may become a critical analytical tool when dealing with combined HIV and TB CNS infection.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
* of interest
* of considerable interest
- Garg RK, Sinha, MK. Tuberculous meningitis in patients infected with human immunodeficiency virus. J. Neurol. 258, 3–13 (2011).
- Andronikou S, Wieselthaler N, Smith B et al. Value of early follow-up CT in paediatric tuberculous meningitis. Pediatr. Radiol. 35, 1092–1099 (2005).
- Bruwer GE, van der Westhuizen S, Lombard CJ, Schoeman JF. Can CT predict the level of CSF block in tuberculous hydrocephalus? Childs Nerv. Syst. 20, 183–187 (2004).
- van Well GT, Paes BF, Terwee CB et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics 123, E1–E8 (2009).
- Katrak SM, Shembalkar PK, Bijwe SR, Bhandarkar LD. The clinical, radiological and pathological profile of tuberculous meningitis in patients with and without human immunodeficiency virus infection. J. Neurol. Sci. 181, 118–126 (2000).
- Thwaites GE, Bang ND, Dung NH et al. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with tuberculous meningitis. J. Infect. Dis. 192, 2134–2141 (2005).
- Pienaar M, Andronikou S, van Toorn R. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Childs Nerv. Syst. 25, 941–947 (2009). & Supports of the use of MRI in tuberculous meningitis (TBM).
- Janse van Rensburg P, Andronikou S, van Toorn R, Pienaar M. Magnetic resonance imaging of miliary tuberculosis of the central nervous system in children with tuberculous meningitis. Pediatr. Radiol. 38, 1306–1313 (2008). & Presents evidence for the development of TBM from hematogenous spread to the meninges and the concept of milliary nodules.
- Przybojewski S, Andronikou S, Wilmshurst J. Objective CT criteria to determine the presence of abnormal basal enhancement in children with suspected tuberculous meningitis. Pediatr. Radiol. 36, 687–696 (2006).
- Whiteman M, Espinoza L, Post MJ, Bell MD, Falcone S. Central nervous system tuberculosis in HIV-infected patients: clinical and radiographic findings. Am. J. Neuroradiol. 16, 1319–1327 (1995).
- Jamieson DH. Imaging intracranial tuberculosis in childhood. Pediatr. Radiol. 25, 165–170 (1995).
- Andronikou S, Smith B, Hatherhill M, Douis H, Wilmshurst J. Definitive neuroradiological diagnostic features of tuberculous meningitis in children. Pediatr. Radiol. 34, 876–885 (2004).
- Kumar R, Singh SN, Kohli N. A diagnostic rule for tuberculous meningitis. Arch. Dis. Child. 81, 221–224 (1999).
- Farinha NJ, Razali KA, Holzel H, Morgan G, Novelli VM. Tuberculosis of the central nervous system in children: a 20-year survey. J. Infect. 41, 61–68 (2000).
- Andronikou S, Wieselthaler N. Imaging for tuberculosis in children. In: Tuberculosis a Comprehensive Clinical Reference. Schaaf S, Zumla A (Eds). Saunders Elsevier, Oxford, UK, 262–295 (2009). & Reference source for imaging TBM in children.
- Teoh R, Humphries MJ, Hoare RD, O’Mahony G. Clinical correlation of CT changes in 64 Chinese patients with tuberculous meningitis. J. Neurol. 236, 48–51 (1989).
- Ozates M, Kemaloglu S, Gurkan F, Ozkan U, Hosoglu S, Simsek MM. CT of the brain in tuberculous meningitis. A review of 289 patients. Acta Radiol. 41, 13–17 (2000).
- Oztoprak I, Gumus C, Oztoprak B, Engin A. Contrast medium-enhanced MRI findings and changes over time in stage I tuberculous meningitis. Clin. Radiol. 62, 1206–1215 (2007).
- Morgado C, Ruivo N. Imaging meningoencephalic tuberculosis. Eur. J. Radiol. 55, 188–192 (2005).
- Dekker G, Andronikou S, van Toorn R, Scheepers S, Brandt A, Ackermann C. MRI findings in children with tuberculous meningitis: a comparison of HIV-infected and non-infected patients. Childs Nerv. Syst. 27, 1943–1949 (2011). & Directly tests the hypothesis of HIV affecting imaging findings on MRI in children with TBM.
- Marais S, Pepper DJ, Marais BJ, Torok ME. HIV-associated tuberculous meningitis – diagnostic and therapeutic challenges. Tuberculosis (Edinb.) 90, 367–374 (2010). & Directly related to the concept of HIV being protective against the effects of TBM.
- Srikanth SG, Taly AB, Nagarajan K, Jayakumar PN, Patil S. Clinicoradiological features of tuberculous meningitis in patients over 50 years of age. J. Neurol. Neurosurg. Psych. 78, 536–538 (2007).
- Schoeman JF, van Zyl LE, Laubscher JA, Donald PR. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics 99, 226–231 (1997).
- Andronikou S, Wilmshurst J, Hatherill M, van Toorn R. Distribution of brain infarction in children with tuberculous meningitis and correlation with outcome score at 6 months. Pediatr. Radiol. 36, 1289–1294 (2006).
- Figaji AA, Fieggen AG, Peter JC. Endoscopic third ventriculostomy in tuberculous meningitis. Childs Nerv. Syst. 19, 217–224 (2003).
- George R, Andronikou S, du Plessis J, du Plessis AM, van Toorn R, Maydell A. Central nervous system manifestations of HIV infection in children. Pediatr. Radiol. 39, 575–585 (2009).
- Valin N, Pacanowski J, Denoeud L et al. Risk factors for ‘unmasking immune reconstitution inflammatory syndrome’ presentation of tuberculosis following combination antiretroviral therapy initiation in HIVinfected patients. AIDS 24, 1519–1525 (2010).
- Meintjes G, Wilkinson RJ, Morroni C et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosisassociated immune reconstitution inflammatory syndrome. AIDS 24, 2381–2390 (2010). & Clinical application for the concept of TBM features resulting as an immune response from the host in HIV.