Review Article - Interventional Cardiology (2022) Volume 14, Issue 0
Mitochondrion as one of the key targets in viral infection
- Corresponding Author:
- Anastasia V. Poznyak Institute for Atherosclerosis Research, Osennyaya 4-1-207, 121609 Moscow, Russia, E-mail: tehhy_85@mail.ru;
Alexander N. Orekhov Institute for Atherosclerosis Research, Osennyaya 4-1-207, 121609 Moscow, Russia, E-mail: a.h.orexob@gmail.com
Received date: 12-Dec-2022, Manuscript No. FMIC-22-83176; Editor assigned: 14-Dec-2022, PreQC No. FMIC-22-83176 (PQ); Reviewed date: 28-Dec-2022, QC No. FMIC-22-83176;Revised date: 04-Jan-2023, Manuscript No. FMIC-22-83176 (R);Published date: 16-Jan-2023, DOI: 10.37532/1755-5310.2022.14(S14). 328
Abstract
Viruses are able to affect cellular organelles to increase the productivity of reproduction, maintain tire viability of visions and evade the antiviral immune response. Understanding the mechanisms and knowledge of the proteins involved in the interaction of viruses with cellular structures allows for a better understanding of the pathogenesis of the virus and is the basis for the creation of antiviral drugs. Mitochondria is an important strategic target for viruses, capturing which viruses are able to change cell metabolism, regulate the cell cycle and inhibit the antiviral immune response. Using the example of SARS-CoV-2, a model of the viral effect on the mitochondria is shown: SARS-CoV-2 shifts metabolism to glycolysis and inhibits interferon production, which, as a result leads to increased inflammation, which is a characteristic feature of COVID-19. Based on the described model, we have discussed possible therapeutic strategies to combat COVID-19.
Keywords
Mitochondria • Sars-CoV-2 • MAVS • Glycolysis
Introduction
Despite the significant progress of medicine in the fight against viral infections, the discovery of new emergent viruses poses a high threat to people around the world. The emergence and spread of SARS-CoV-2 in the human population have led to the development of the COVID-19 pandemic, which has been going on for more than 2 years. During the first year of the pandemic, COVID-19 became the most common infectious disease, which infected more than 37 million people [1], and at the moment more than 342 million, of which more than 5.5 million deaths were recorded [2]. The difficulty in combating COVID-19 is due to several factors: Firstly, the high variability of the virus leads to the emergence of new, sometimes more pathogenic strains of SARS-CoV-2 [3]; secondly, the airborne transmission pathway is highly contagious, which contributes to the better spread of infection; thirdly, the peak viral load of SARS-CoV-2 occurs in the first days after the onset of the disease’s symptoms, which also contributes to the spread of the virus [4]; fourth, SARS-CoV-2 has complex pathogenesis peculiar only to it, many characteristics of which remain unknown, which complicates the treatment of COVID-19.
The mild form of COVID-19 can be transmitted in the form of a catarrhal disease characteristic of many respiratory viruses, the medium-severe and severe forms of COVID-19 are often associated with the occurrence of a cytokine storm, which is a strong self-reproducing inflammatory reaction that can lead to multiple organ failure and death [5]. Understanding the mechanisms underlying the development of cytokine storm in COVID-19 at the cellular and molecular level can help in the development of effective antiviral and anti-inflammatory drugs aimed, among other things, at the treatment of patients with severe COVID-19. Initiation of reactions leading to the development of inflammation begins at the level of interaction of SARS- CoV-2 proteins with proteins of cellular organelles, including mitochondrial proteins. This review explains how a virus can adjust the functioning of a cell to its needs by targeting mitochondria. Using the example of SARS-CoV-2, the model of this interaction leading to the development of inflammation has been shown, and therapeutic approaches for the treatment of COVID-19 have been also proposed based on this model.
Literature Review
Mitochondrial structure, function and homeostasis
Mitochondria play a crucial role in the production of metabolic energy in eukaryotic cells. They initiate the production of most of the useful energy released during the breakdown of carbohydrates and fatty acids. The resulting energy is accumulated in the form of ATP molecules, whose formation is the result of the oxidative phosphorylation process [6].
A feature of the mitochondrial structure is the presence of its own circular DNA molecule, which encodes tRNA, rRNA, and some mitochondrial proteins [7-9]. At the same time, mitochondria include both proteins encoded by their mitochondrial genome and proteins encoded by the nuclear genome and imported from the cytosol [6]. Mitochondria are two membrane organelles: They are composed of an Outer Membrane (OM) and an Inner Membrane (IM), which are separated by an intermembrane space. The inner membrane forms numerous folds (cristae) that limit the internal environment of the organelle, called the matrix [10,11].
Each of the designated components plays different functional roles, with the matrix and IM being the most important working compartments of mitochondria [11]. Pyruvate, formed as a result of glucose oxidation, and fatty acids enter the matrix from the cytoplasm. Here, these metabolites enter the Krebs cycle, as a result of which their complete oxidation to CO2 molecules occurs, as well as the reduction of the electron carriers NAD+ and FAD to NADH and FADH2 , respectively. Then these reduced molecules are transferred to IM, where, during the process of oxidative phosphorylation, the energy accumulated as a result of electron transfer reactions is converted into potential energy of the proton gradient, which triggers the work of ATP synthase, and, accordingly, the formation of ATP [6]. IM, unlike OM, is the practically impermeable membrane for ions and small molecules, which is an important condition for maintaining the proton gradient [6,11].
In addition to the fact that the main role of mitochondria is associated with obtaining energy as a result of cellular respiration, these organelles are also involved in many other physiological processes, such as programmed cell death, innate immunity, autophagy, redox signaling, calcium homeostasis, and reprogramming of stem cells [12,13]. To effectively perform the required functions, depending on the state and type of cells in which they are located, mitochondria undergo several processes: Fusion, division, transport, and mitophagy [14]. The processes of fusion and division regulate the number and size of mitochondria: When division from one larger organelle, two smaller ones are formed, while fusion, on the contrary, from two smaller mitochondria, one large one is formed [13]. Mitochondrial transport is necessary for the movement of mitochondria within the cell in the area where the greatest energy consumption occurs at the moment. This is especially relevant for highly polarized cells, for example, neurons, in which a high energy supply is required at the site of nerve impulse generation [15]. The process of mitophagy is the control of the quality of mitochondria the destruction of defective and old dysfunctional mitochondria, the accumulation of which may be one of the reasons for the development of various chronic diseases [16].
Involvement of cellular structures in the maintenance of the virus vital activity
The topic of this article is related to the effect of the virus on mitochondria, however, when viruses infect cells, they affect many other organelles, thus, trying to achieve the maximum exploitation of the infected cell. Understanding the mechanisms underlying the interaction of the virus with the cellular structures of the host is the key to the development of effective antiviral drugs.
Nucleus: The replication of many viruses depends on the host’s nuclear proteins; therefore, penetration into the cell nucleus is a prerequisite for the reproduction of such viruses [17]. Some of them, like the murine leukemia retrovirus, take advantage of the opportunity to penetrate the nucleus during the disintegration of the nuclear envelope during mitosis [18]. Another strategy for the virus penetration into the cell nucleus is a receptor-mediated interaction of the Nuclear Localization Signal (NLS) sequence, which was found in various viral proteins with the nuclear pore receptors-importins, as a result of which viral particles penetrate the nucleus. After successfully penetrating the nucleus, viruses begin to use various nuclear components, such as the nucleolus, to enhance their replication [17]. Nucleolin, fibrillarin, and B23 (nucleophosmin) are proteins of the nucleoli that perform several important functions for protein synthesis, such as the posttranscriptional process, ribosome assembly, and others [19]. There is evidence that nucleolar proteins are involved in the replication and translation of various viral proteins [17]. Also, some viruses, such as herpes simplex virus and adenovirus, were involved in the movement and destruction of nucleolar proteins. The likely reason for this strategy is the ability to suppress the synthesis of cellular proteins by inhibiting the production of ribosomes, in which the proteins of the nucleolus are directly involved. For other viruses, such as polioviruses and rhinoviruses, their proteins had been shown to block nuclear transport, which leads to the accumulation of nucleolin in the cytoplasm. Nucleolin interacts with a sequence of Internal Ribosome Entry Sites (IRES) located at the upper 5’-end of the viral genome to stimulate its translation [20]. On the other hand, an excess of nucleolin in the cytoplasm and its deficiency in the nucleus leads to inhibition of the transcription of cellular proteins, including those responsible for the antiviral response [21].
Endoplasmic reticulum: It was noticed that viral infection contributes to changes in the structure of the Endoplasmic Reticulum (ER) in various types of cells [22]. These changes in the ER structure are intended for the formation of viroplasm which is the site in the cytoplasm where the virus replicates and the viral particles are assembled. In the viroplasm, viral DNA and RNA are protected from the effects of cellular nucleases [23]. Viruses cause a change in the metabolism of fatty acids, which leads to a strong change in the morphology of the ER membranes. The final variant of such changes may be the formation of Double-Membrane Vesicles (DMV), where the replication of RNA viruses is maintained [24]. An additional function of the ER in the life cycle of the virus consists of post-translational modifications of viral proteins. For inclusion in the virion and proper folding, some antigens undergo N-glycosylation in the ER [25]. In addition, it is considered that the degree of glycosylation can change the antigenicity of viral proteins [22].
Golgi apparatus: Like ER in viral infection, a modification of the Golgi apparatus structure is often observed, which is usually expressed in the form of fragmentation of an entire organelle into separate components [22]. An interesting modification was discovered upon infection of tobacco with the turnip mosaic virus: Infection with this virus caused the fusion of the ER, the Golgi apparatus, and chloroplasts [26]. Fragmented Golgi membrane compartments can form vesicles which are then used by RNA viruses as virosomes to replicate their genomes [27]. In addition, fragmentation of the Golgi apparatus leads to disruption of the export of external membrane proteins in the composition of vesicles, including MHC-I molecules, which, as a result, suppresses the antiviral immune response [28]. Additionally, viruses use the Golgi apparatus for various post-translational modifications of their proteins [22].
Lysosomes and peroxisomes: It is reported that viruses are able to use lysosomal enzymes to improve the replication process and the release of viral particles from the cell [29]. Additionally, there is a hypothesis that viruses use lysosomal enzymes to enhance glycolysis, which is the preferred method of obtaining energy in infected cells [22]. For the H5N1 influenza virus, it was shown that its enzyme neuraminidase caused deglycosylation of lysosomal membrane proteins, which led to the release of lysosomal hydrolytic enzymes into the cytoplasm and subsequent cell death [30]. The destruction of lysosomes leading to cell death had also been shown for some other viruses, such as adenovirus, HIV, and human papillomavirus [31]. In addition to lysosomes, viruses can initiate the destruction of peroxisomes. This possibility was demonstrated on cell cultures A549 and HEK293T for West Nile virus and Dengue virus [32]. In the infected cells, a decrease in the concentration of catalase, which is an antioxidant enzyme, was noted, which led to inhibition of the triggering of the antiviral response. Additionally, it was discovered that viruses could exploit the metabolic function of peroxisomes: This is how rotavirus uses myristoyl-CoA, an intermediate product of (3-oxidation of fatty acids, to modify its own proteins VP2 and VP6 [33].
The impact of viruses on mitochondria and processes involving mitochondria
Changes in mitochondrial dynamics: As described above, mitochondria are dynamic cellular structures and for the proper functioning of the cell, equilibrium maintenance of the processes of fusion, division, mitochondrial transport, as well as mitophagy is required. Viruses can change the course of these processes to maintain their life cycle. Thus, it had been shown that the Hepatitis C Virus (HCV) was able to block cellular apoptosis for stable virus persistence through increased mitochondrial division and subsequent mitophagy [34]. The key target in these processes for HCV is the protein Drpl, which is directly involved in the mitochondrial division: HCV triggers the phosphorylation reaction of Drpl, which increases the activity of this protein [35]. Additionally, HCV can induce the expression of Parkin and PINK1, which are key enzymes that ensure the process of mitophagy [34]. For the classical swine fever virus, it had been shown that some of its proteins could cause degradation of mitochondrial fusion proteins: MFN1 and MFN2 [36]. Strengthening of mitochondrial division and mitophagy to ensure viral persistence had also been shown for hepatitis В virus [37]. On the other hand, viruses such as Dengue virus and SARS-CoV virus, on the contrary, inhibit the division of mitochondria and promote their fusion, which is associated with triggering eluding reactions from the host immune response [38,39].
Regulation of mitochondrial-mediated apoptosis: One of the important functions of mitochondria is the induction of internal apoptosis. After the apoptotic impulse occurs, the permeability of the outer mitochondrial membrane increases with the involvement of Bcl-2 protein channels, which leads to the release of apoptosis proteins (Cytochrome c and APAF1) into the cytoplasm. After the interaction of these apoptotic proteins, procaspase-9 is activated, which then cleaves caspase-3 and caspase-7, inducing further reactions leading to cell death [34]. As was briefly noted in the previous section, some viruses, such as HCV, can block cellular apoptosis through a change in mitochondrial dynamics by activating mitophagy and mitochondrial division proteins. This strategy is aimed at increasing the yield of viral particles formed as a result of the life cycle of the virus, and can also be used for the persistence of the virus in the latent phase. Similarly, viruses do not allow the cell to self-destruct until they use their resources in full to generate maximum offspring. The reverse strategy is aimed at activating apoptosis by the virus. This mechanism determines the cytopathic effect of the virus-when the virus has used the cellular resources to the maximum and the cell can no longer serve as an effective factory for the production of new virions, the virus initiates cell destruction. Thus, nonstructural NS4A and NS4B proteins of HCV cause mitochondrial damage and, as a consequence, cytochrome c is released into the cytoplasm, which initiates the launch of internal apoptosis [40], [41]. Hepatitis B Virus (HBV) and Dengue virus can initiate mitochondrial apoptosis through interaction with the tumor suppressor protein p53, which leads to aggregation of mitochondria near the nucleus and their subsequent destruction [42,43]. Thus, viruses can have exactly opposite strategies for affecting cells depending on their main task. The opposite strategies described above are summarized in Table 1.
| Parameter | Mitochondrial dynamics | Cell apoptosis | ||
|---|---|---|---|---|
| The result of the impact | Increased mitochondrial fission and mitophagy | Increased mitochondrial fusion, decreased fission | Activation of apoptosis | Inhibition of apoptosis |
| Viral aim | Inhibition of apoptosis | Escaping the immune response | Exhaustion of cellular resources | Virion production growth |
| Being in the latent phase | ||||
Table 1: The opposite viral strategies for influence on mitochondrial dynamics and cell apoptosis.
Modulation of the antiviral immune response: Mitochondrial proteins are known to be involved in the activation of the innate antiviral response. Initiation of this response occurs when RIG-I-Like Receptors (RLRs), which in addition to RIG-I include MDA5 and LGP2, bind to RNA molecules of RNA viruses during their replication [44]. After binding to viral nucleic acid, RLRs undergo conformational changes, which lead to the exposure of the caspase activation domain CARD, which interacts with the CARD domain of the Mitochondrial Antiviral Signaling protein (MAVS) [44]. The MAVS protein is located on the outer membrane of mitochondria and can enter into further reactions with a large number of proteins, which eventually lead to the activation of transcription of interferons and proinflammatory cytokines [44]. Viruses in the course of evolution have developed several mechanisms that counteract the RLR signaling pathway to evade the host’s immune response. Since the MAVS protein is one of the key links in this antiviral pathway, it serves as a good target for viral counteraction of the host immune response. Some viruses, such as HCV, HBV, and Newcastle disease virus, inhibit the action of MAVS by disrupting its conformational structure [34]. HCV NS3/4A protein can cleave MAVS at the Cys-508 position, which leads to the displacement of the N-terminal fragment of MAVS beyond the mitochondria, resulting in the loss of the enzymatic activity of MAVS [45]. HBx protein of HBV causes degradation of MAVS by ubiquitination at the Lys (136) position [46]. The Newcastle disease virus protein V also causes the cleavage of MAVS by recruiting ubiquitin ligase E3 RNF5 [47]. Another way of viral inhibition of the MAVS action is to block the binding of MAVS to RIG-1 and MDA5. A similar type of inhibition has been demonstrated for Dengue virus [48] and Zika virus [49]. The third way to inhibit the action of MAVS is to suppress the expression of MAVS by inducing microRNAs that inhibit the transcription of a given gene. An example of a virus using a similar strategy of suppressing immunity is the Vesicular Stomatitis Virus (VSV) [50].
Regulation of metabolism: Various viruses can alter the energy metabolism of cells to ensure better survival and reproducibility. The general rule that can be observed in viral infections is the shift of the energy metabolism of an infected cell from oxidative phosphorylation to glycolysis. During one cycle of glycolysis, 2 ATP molecules are formed, which is significantly less energy production during oxidative, proceeds much faster than oxidative phosphorylation [34]. In conditions when the cell needs energy supply in the shortest possible time, glycolysis is the preferred option for obtaining it. The infected cell is forced to additionally work for the needs of the virus, which is why rapid reproduction of ATP is necessary. Using HCV as an example, it had been shown that viral proteins were able to inhibit the action of various components of the respiratory chain and increase the expression of glycolytic enzymes, such as glucokinase, hexokinase, and pyruvate kinase [34]. Glycolysis is not always the only dominant way of obtaining energy in cells infected with the virus. Thus, it was found that in HIV-infected lymphocytes, energy metabolism occurs in addition to glycolysis by glutaminolysis, as well as by oxidation of fatty acids [51].
Model of viral effect on mitochondria by the example of Sars-CoV-2
Coronavirus infection caused by SARS-CoV-2 is characterized by high morbidity and significant mortality. The pathogenesis of SARS-CoV-2 may be associated with the development of a strong inflammatory reaction, which can lead to serious damage to the lungs, as well as to their chronic pathology [44]. SARS-CoV-2 enters alveolar epithelial cells through the ACE-2 receptor, which plays a dual role in the development of COVID-19: On the one hand, it is a receptor that allows SARS-CoV-2 to enter the cell, and on the other hand, ACE-2 prevents severe course of COVID-19 [52], in particular, ACE-2 stimulates the production of type IIFN, an antiviral agent [53]. The direct function of ACE-2 is associated with the suppression of the renin-angiotensin system action by converting angiotensin II to angiotensin (1-7) [54]. It is noted that during COVID-19, SARS-CoV-2 causes suppression of ACE-2 expression, which leads to the accumulation of angiotensin II, which initiates vasoconstriction, thrombus formation, reduces the rate of alveolar fluid clearance, and also increases the transcription of IL6 (a pro-inflammatory cytokine), which leads to increased inflammation in the lungs [54]. Alveolar macrophages and pneumocytes secrete pro-inflammatory cytokines that increase the expression of Cell Adhesion Molecules (CAMs), which increase the permeability of the lung endothelium, leading to viral dissemination and infiltration of neutrophils and inflammatory monocytes. Cytokines stimulate the production of new leukocytes, which migrate to the lungs and further increase inflammation, which can lead to a cytokine storm [55].
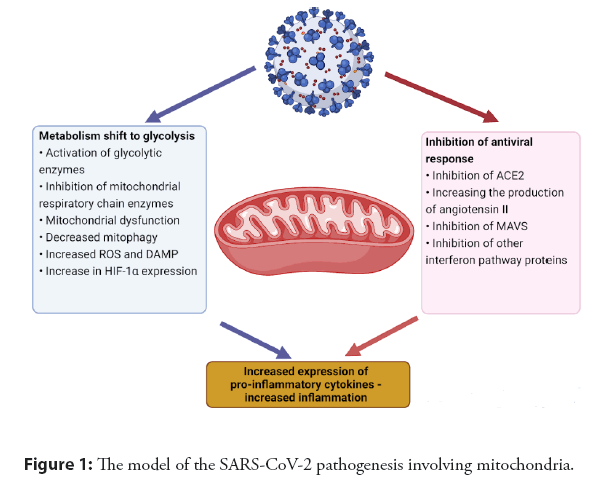
The impact of SARS-CoV-2 on mitochondria consists of two processes: Metabolic reprogramming of the infected cell and inhibition of the antiviral immune response. The model of the SARS-CoV-2 pathogenesis involving mitochondria is shown in Figure 1.
Figure 1: The model of the SARS-CoV-2 pathogenesis involving mitochondria.
Like many viruses, SARS-CoV-2 shifts energy metabolism from oxidative phosphorylation to glycolysis, probably by affecting the expression or activity of glycolytic enzymes and enzymes of the mitochondrial respiratory chain [56]. In addition, macrophages, getting into the focus of inflammation in the lungs, where the level of oxygen is much lower than in the surrounding tissues, are forced to obtain energy by using less oxygen. SARS-CoV-2 proteins can interact with subunits of the respiratory chain complexes, inhibiting their action, which leads to mitochondrial dysfunction, which causes electron leakage from the electron transport chain and subsequent accumulation of excess Reactive Oxygen Species (ROS) [57]. ROS increase the expression of Hypoxia-Inducible Factor-la (HIF-la), which in turn induces transcription of the pro-inflammatory cytokine IL-ip and further enhances glycolysis [57]. The resulting ROS can additionally pose a direct threat to mitochondria: By destroying macromolecules, ROS contribute to the formation of defective mitochondria, which release molecular structures-DAMP (Damage Association Molecular Patterns) into the cytoplasm, which helps maintain the inflammatory response. Possible inhibition of mitophagy under the influence of SARS-CoV-2 also contributes to the development of the inflammatory response [58]. SARS-CoV-2 is also able to affect the angiotensin-aldosterone system through mitochondria since the ACE2 receptor is also located on the surface of mitochondria. By inhibiting the action of ACE2, SARS-CoV-2 activates the production of angiotensin II, which leads to the activation of the NLRP3 inflammasome and pyroptosis, followed by an increase in inflammation [58].
The observed increase in the inflammatory immune response in severe forms of COVID-19 is combined with the weak T-cell response, which is one of the main driving forces of antiviral immunity [58]. In infected cells, SARS-CoV-2 suppresses the triggering of the interferon response, which is initiated through the mitochondrial signaling protein MAVS [58]. In addition to suppressing the spread of the virus in the population of nearby cells and launching an adaptive immune response, IFNI also has an anti-inflammatory effect by blocking the production of IL-1 [59]. It was found that some SARS-CoV-2 proteins were able to interact with MAVS, blocking the induction of type I interferon [57]. And for the SARS-CoV-2 ORF9b protein, which is the MAVS receptor and is also involved in the activation of interferon, had been shown the possibility of binding to the mitochondrial protein TOM70 [60]. Thus, the mitochondria are an important target of SARS-CoV-2, by acting on which it changes the cell metabolism, and also inhibits the antiviral interferon response, and induces an inflammatory response.
Discussion
The main groups of drugs from COVID-19 are aimed either at reducing the inflammatory response or having a direct antiviral effect. The therapeutic strategy aimed at developing drugs that are directed at protecting mitochondrial functions from the effects of SARS-CoV-2 is a promising direction because it assumes that the created drugs will have a double effect: To enhance the antiviral immune response and inhibit the development of inflammation. An additional advantage is a possibility of using these potential drugs in the early stages of viral infection, which can help prevent the development of moderate and severe forms of COVID-19.
This also does not cancel the use of such drugs in patients with severe inflammation that has already developed, which can be reduced by their administration. One potential therapeutic strategy is to enhance the activity of the MAVS signaling protein. So, it was shown that O-GlcNAcylation MAVS led to enhanced innate antiviral response against RNA viruses [61]. D-glucosamine here has been considered a potential drug substance [61]. Another MAVS-related approach relies on restoring MAVS levels in infected Sars-CoV-2 cells by administration of mesenchymal stem cells expressing elevated levels of the given protein [62]. Another possible strategy is the inhibition of the glycolytic pathway, which, as noted earlier, increases coronavirus infection. Studies [63,64] have shown that administration of 2-deoxy-d-glucose, which is a proven inhibitor of glycolysis, inhibited the spread of Sars-CoV-2 in cell culture. It also makes sense to consider other signaling targets of the interferon pathway in addition to MAVS to select potential therapeutic compounds that activate their action or block their binding to SARS-CoV-2 proteins. Since, in addition to glycolysis, infected cells can also use glutaminolysis [65], enzymes and substrates involved in this process can also be considered potential targets in COVID-19 therapy.
Conclusion
The mitochondrion is an important cellular target for viruses, targeting which they can regulate mitosis and apoptosis, affect the metabolic pathways of the cell and trigger an immune response. SARS-CoV-2, acting on mitochondria, shifts energy metabolism from oxidative phosphorylation to glycolysis and also inhibits the production of interferon, which leads to the development of a strong inflammatory reaction characteristic of COVID-19. As potential therapeutic strategies aimed at preventing negative effects after interaction of SARS-CoV-2 with mitochondria, approaches have been proposed that increase the activity or expression of MAVS-mitochondrial antiviral protein, as well as alter pathological metabolism by inhibiting glycolysis by introducing inhibitory substrates.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Russian Science Foundation (Grant # 22-65-00005). The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Authors’ Contributions
Writingoriginal draft preparation, AVB; writing review and editing, AVG, AGK, NGN, ADZ, ANO, AVP. All authors read and approved the final version of the manuscript.
References
- EBSCO. Ten most impactful infectious disease outbreaks of 2020. (2020).
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)
- Alizon S, Sofonea MT. SARS-CoV-2 virulence evolution: Avirulence theory, immunity and trade-offs J Evol Biol. 34(12): 1867-1877 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Cevik M. Kuppalli K, Kindrachuk J, et al. Virology, transmission, and pathogenesis of SARS-CoV-2. British Med J. (2020)
[CrossRef] [Google Scholar] [PubMed]
- Li C, He Q, Qian H, et al. Overview of the pathogenesis of COVID-19 (Review). Exp Ther Med. 22(3): 1-10 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Mitochondria-the cell: A molecular approach. (2021).
- Sobenin IA, Sazonova MA, Postnov AY, et al. Mitochondrial mutations are associated with atherosclerotic lesions in the human aorta. Clin Dev Immunol. 832464 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Sobenin IA, Sazonova MA, Postnov AY, et al. Association of mitochondrial genetic variation with carotid atherosclerosis. PLoS One. 8(7): e68070 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Sobenin IA, Mitrofanov KY, Zhelankin AV, et al. Quantitative assessment of heteroplasmy of mitochondrial genome: Perspectives in diagnostics and methodological pitfalls. Biomed Res Int. 292017 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Sobenin IA, Sazonova MA, Postnov AY, et al. Changes of mitochondria in atherosclerosis: possible determinant in the pathogenesis of the disease. Atherosclerosis. 227(2): 283-288 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Reshi L, Wang HV, Hong JR. Modulation of mitochondria during viral infections. Mitochondrial Dis. IntechOpen. (2018).
- Tilokani L. Nagashima S, Paupe V, et al. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 62(3): 341 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Puchenkova OA, Nadezhdin SV, Soldatov VO, et al. Study of antiatherosclerotic and endothelioprotective activity of peptide agonists of EPOR/CD131 heteroreceptor. Pharm Pharmacol. 8(2): 100-111 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Yu R. Lendahl U, Nistér M, et al. Regulation of mammalian mitochondrial dynamics: Opportunities and challenges. Front Endocrinol (Lausanne). 11(11): 374 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Schwarz TL. Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol. 5(6): (2013).
[CrossRef] [Google Scholar] [PubMed]
- Chen G, Kroemer G, Kepp O. Mitophagy: An emerging role in aging and age-associated diseases. Front Cell Dev Biol. 8: 200 (2020).
- Zakaryan FI, Stamminger T. Nuclear remodelling during viral infections. Cell Microbiol. 13(6): 806-813 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Roe T, Reynolds TC, Yu G, et al. Integration of murine leukemia virus DNA depends on mitosis. European Mol Biol Org. 12(5): 2099 (1993).
[CrossRef] [Google Scholar] [PubMed]
- Shaw P, Brown J. Focus issue on nuclear architecture and dynamics: Nucleoli: Composition, function, and dynamics. Plant Physiol. 158(1): 44 (2012).
- Izumi RE, Valdez B, Banerjee R, et al. Nucleolin stimulates viral internal ribosome entry site-mediated translation. Virus Res. 76(1): 17-29 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Gustin KE, Samow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J Virol. 76(17): 8787 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Glingston RS, Deb R, Kumar S, et al. Organelle dynamics and viral infections: at cross roads. Microbes Infect. 21(1): 20 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Verchot J. Wrapping membranes around plant virus infection. Curr Opin Virol. 1(5): 388-395 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Wolff G, Melia CE, Snijder EJ, et al. Double-membrane vesicles as platforms for viral replication. Trends Microbiol. 28(12): 1022 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Ravindran MS, Bagchi P, Cunningham CN, et al. Opportunistic intruders: How viruses orchestrate ER functions to infect cells. Nat Rev Microbiol. 14(7): 407 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Grangeon R, Agbeci M, Chen J, et al. Impact on the endoplasmic reticulum and Golgi apparatus of turnip mosaic virus infection. J Virol. 86(17): 9255 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Quiner CA, Jackson WT. Fragmentation of the Golgi apparatus provides replication membranes for human rhinovirus 1A. Virology. 407(2): 185 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Rohde J, Emschermann F, Knittler MR, et al. Orf virus interferes with MHC class I surface expression by targeting vesicular transport and Golgi. BMC Vet Res. 8: 114 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Allison AC, Sandelin K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 117(6): 879-887 (1963).
[CrossRef] [Google Scholar] [PubMed]
- Ju X, Yan Y, Liu Q, et al. Neuraminidase of Influenza A Virus Binds Lysosome-Associated Membrane Proteins Directly and Induces Lysosome Rupture. J Virol. 89(20): 10347 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Aits S, Jäättelä M. Lysosomal cell death at a glance. J Cell Sci. 126(9): 1905-1912 (2013).
[Cross Ref] [Google scholar] [Pub Med]
- You J, Hou S, Malik-Soni N, et al. Flavivirus infection impairs peroxisome biogenesis and early antiviral signaling. J Virol. 89(24): 12349-1236 (2015).
[Cross Ref] [Google scholar] [Pub Med]
- Lazarow PB. Viruses exploiting peroxisomes. Curr Opin Microbiol. 14(4): 458-469 (2011).
[Cross Ref] [Google scholar] [Pub Med]
- Li X, Wu K, Zeng S, et al. Viral Infection Modulates Mitochondrial Function. Int J Mol Sci. 22(8): 4260 (2021).
- Kim SJ, Syed GH, Khan M, et al. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc Natl Acad Sci. 111(17): 6413-6418 (2014).
[Cross Ref] [Google scholar] [Pub Med]
- Gou H, Zhao M, Xu H, et al. CSFV induced mitochondrial fission and mitophagy to inhibit apoptosis. Oncotarget. 8(24): 39382 (2017).
[Cross Ref] [Google scholar] [Pub Med]
- Kim SJ, Khan M, Quan J, et al. Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. PLoS pathog. 9(12): e1003722 (2013).
[Cross Ref] [Google scholar] [Pub Med]
- Barbier V, Lang D, Valois S, et al. Dengue virus induces mitochondrial elongation through impairment of Drp1-triggered mitochondrial fission. Virology.500: 149-160 (2017).
[Cross Ref] [Google scholar] [Pub Med]
- Shi CS, Qi HY, Boularan C, et al. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol. 193(6): 3080-3089 (2014).
[Cross Ref] [Google scholar] [Pub Med]
- Nomura-Takigawa Y, Nagano-Fujii M, Deng L, et al. Non-structural protein 4A of Hepatitis C virus accumulates on mitochondria and renders the cells prone to undergoing mitochondria-mediated apoptosis. J Gen Virol. 87(7): 1935-1945 (2006).
[Cross Ref] [Google scholar] [Pub Med]
- Zhao P, Han T, Guo JJ, et al. HCV NS4B induces apoptosis through the mitochondrial death pathway. Virus Res. 169(1): 1-7 (2012).
[Cross Ref] [Google scholar] [Pub Med]
- Takada S, Shirakata Y, Kaneniwa N, et al. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 18(50): 6965-6973 (1999).
[Cross Ref] [Google scholar] [Pub Med]
- Nasirudeen AM, Wang L, Liu DX. Induction of p53-dependent and mitochondria-mediated cell death pathway by dengue virus infection of human and animal cells. Microbes Infect. 10(10-11): 1124-32 (2008).
[Cross Ref] [Google scholar] [Pub Med]
- Elesela S, Lukacs NW. Role of mitochondria in viral infections. Life. 11(3): 232 (2021).
[Cross Ref] [Google scholar] [Pub Med]
- Li XD, Sun L, Seth RB, et al. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci. 102(49): 17717-177122 (2005).
[Cross Ref] [Google scholar] [Pub Med]
- Wei C, Ni C, Song T, et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol. 185(2): 1158-1168 (2010).
[Cross Ref] [Google scholar] [Pub Med]
- Valle-Casuso JC, Angin M, Volant S, Passaes C, et al. Cellular metabolism is a major determinant of HIV-1 reservoir seeding in CD4+ T cells and offers an opportunity to tackle infection. Cell Metab. 29(3): 611-626 (2019).
[Cross Ref] [Google scholar] [Pub Med]
- He Z, Zhu X, Wen W, et al. Dengue virus subverts host innate immunity by targeting adaptor protein MAVS. J Virol. 90(16): 7219-7230 (2016).
[Cross Ref] [Google scholar] [Pub Med]
- Riedl W, Acharya D, Lee JH, et al. Zika virus NS3 mimics a cellular 14-3-3-binding motif to antagonize RIG-I-and MDA5-mediated innate immunity. Cell Host Microbe. 26(4): 493-503 (2019).
[Cross Ref] [Google scholar] [Pub Med]
- Yarbrough ML, Zhang K, Sakthivel R, et al. Primate-specific miR-576-3p sets host defense signalling threshold. Nat Commun. 5(1): 1-10 (2014).
[Cross Ref] [Google scholar] [Pub Med]
- Sun Y, Zheng H, Yu S, et al. Newcastle disease virus V protein degrades mitochondrial antiviral signaling protein to inhibit host type I interferon production via E3 ubiquitin ligase RNF5. J Viro. 93(18): e00322 (2019).
[Cross Ref] [Google scholar] [Pub Med]
- Yalcin HC, Sukumaran V, Al-Ruweidi MK, et al. Do changes in ace-2 expression affect SARS-CoV-2 virulence and related complications: A closer look into membrane-bound and soluble forms. Int J Mol Sci. 22(13): 6703 (2021).
[Cross Ref] [Google scholar] [Pub Med]
- Ziegler CG, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 181(5): 1016-1035 (2020).
[Cross Ref] [Google scholar] [Pub Med]
- Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open. 4(02): e138-e144 (2020).
[Cross Ref] [Google scholar] [Pub Med]
- Polidoro RB, Hagan RS, de Santis Santiago R, et al. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front Immunol. 11: 162 (2020).
[Cross Ref] [Google scholar] [Pub Med]
- Codo AC, Davanzo GG, de Brito Monteiro L, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 32(3): 437-446 (2020).
[Cross Ref] [Google scholar] [Pub Med]
- Mehrzadi S, Karimi MY, Fatemi A, et al. SARS-CoV-2 and other coronaviruses negatively influence mitochondrial quality control: Beneficial effects of melatonin. Pharmacol Ther. 224: 107825 (2021).
[Cross Ref] [Google scholar] [Pub Med]
- Nunn AV, Guy GW, Brysch W, et al. SARS-CoV-2 and mitochondrial health: implications of lifestyle and ageing. Immun Ageing. 17(1): 1-21 (2020).
[Cross Ref] [Google scholar] [Pub Med]
- Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 369(6504): 718-724 (2020).
[Cross Ref] [Google scholar] [Pub Med]
- Jiang HW, Zhang HN, Meng QF, et al. SARS-CoV-2 ORF9b suppresses type I interferon responses by targeting TOM70. Cell Mol Immunol. 17(9): 998-1000 (2020).
[Cross Ref] [Google scholar] [Pub Med]
- Song N, Qi Q, Cao R, et al. MAVS O-GlcNAcylation is essential for host antiviral immunity against lethal RNA viruses. Cell Rep. 28(9): 2386-2396 (2019).
- Babajani A, Hosseini-Monfared P, Abbaspour S, et al. Targeted mitochondrial therapy with over-expressed MAVS protein from mesenchymal stem cells: a new therapeutic approach for COVID-19. Front Cell and Dev Biol. 9: 695362 (2021).
[Cross Ref] [Google scholar] [Pub Med]
- Bhatt AN, Kumar A, Rai Y, et al. Glycolytic inhibitor 2-Deoxy-D-glucose attenuates SARS-CoV-2 multiplication in host cells and weakens the infective potential of progeny virions. Life Sci. 295: 120411 (2022).
[Cross Ref] [Google scholar] [Pub Med]
- Balkrishna A, Thakur P, Singh S, et al. Glucose antimetabolite 2-Deoxy-D-Glucose and its derivative as promising candidates for tackling COVID-19: Insights derived from in silico docking and molecular simulations. (2020).
- Chambers JW, Maguire TG, Alwine JC et al. Glutamine metabolism is essential for human cytomegalovirus infection. J Virol. 84(4): 1867-1873 (2010).
[Cross Ref] [Google scholar] [Pub Med]