Review Article - Interventional Cardiology (2012) Volume 4, Issue 4
Managing no-reflow during percutaneous coronary intervention
- Corresponding Author:
- Michael Derntl
Department of Cardiology
Hospital Rudolfstiftung
Juchgasse 25, 1030 Vienna, Austria
Tel: +43 171 165 2207
Fax: +43 171 165 2209
E-mail: michael.derntl@wienkav.at;
franz.weidinger@wienkav.at
Abstract
Keywords
acute myocardial infarction, embolization, microcirculation, percutaneous coronary intervention, thrombectomy, vasoconstriction
The term ‘no-reflow’ describes a condition of myocardial tissue hypoperfusion despite patent epicardial coronary arteries. Initially, it was used to describe the inability to reperfuse a previously ischemic region [1]. Microvascular obstruction is considered to be the underlying cause of noreflow and may be provoked by various mechanisms. For diagnosis of no-reflow, other possibilities such as dissection, local thrombus formation, stenosis or epicardial spasm have to be excluded. In ≥30% of patients, after thrombolysis or mechanical intervention for acute myocardial infarction (AMI), no-reflow has been reported [2–5]. No-reflow has been observed in 0.6–2% of cases during percutaneous coronary intervention (PCI) and has been identified especially during PCI of thrombus-containing lesions, saphenous vein grafts (SVG) or when performing atherectomy, despite optimal anticoagulation with heparin [6–10]. There is evidence that no-reflow has a strong negative impact on clinical outcome after PCI [3,11–16]. Patients with no-reflow during PCI have a higher incidence of early postinfarction complications, adverse left ventricular remodeling, repeat hospitalizations for heart failure and higher mortality [2,3]. Despite continuous improvements of PCI procedures, no-reflow remains a constant threat. Prevention, diagnosis and treatment of no-reflow are essential to improve outcome after PCI. The purpose of this review is to give an overview about pathophysiology, predictors and management options for no-reflow.
Classification
According to the duration of the preceding myocardial ischemia, no-reflow may be classified as interventional or reperfusion no-reflow.
▪ Interventional no-reflow.
Interventional no-reflow occurs following noninfarct PCI and involves heart muscle that was not exposed to prolonged ischemia prior to the procedure. It is unpredictable, sudden in onset and presents clinically as acute ischemia with chest pain and electrocardiography changes. It is known that patients with interventional noreflow that complicates PCI have higher rates of myocardial infarction and mortality [17].
▪ Reperfusion no-reflow
Reperfusion no-reflow follows PCI for reperfusion of an infarct-related coronary artery in the setting of AMI. Reperfusion no-reflow is preceded by ischemic cell injury. It is limited to the irreversibly damaged necrotic zone and may be exacerbated during reperfusion. Clinically, it may present with continued chest pain and failure of ST-resolution. Reperfusion no-reflow is an independent predictor of adverse clinical outcome after AMI and is associated with increased mortality [18].
Diagnosis of no-reflow
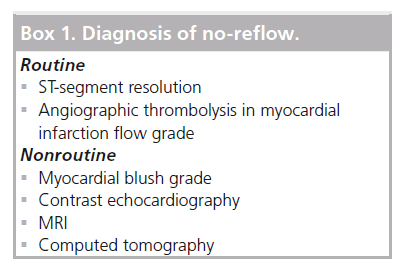
This is a brief overview of various modalities for the diagnosis of no-reflow. Different sensitivities of diagnostic tests explain the wide variety of reported incidence of the no-reflow phenomenon. Routine and nonroutine diagnostic options for no-reflow are listed in Box 1.
▪ Electrocardiography
Due to the fact that electrocardiography ST-segment resolution is a marker of myocardial tissue reperfusion, persistence of ST-segment elevation in AMI may be an indicator of microvascular obstruction and no-reflow.
▪ Angiographic assessment
According to the Thrombolysis In Myocardial Infarction (TIMI) flow grades, coronary angiography allows a semiquantitative grading of epicardial coronary flow [19]. It is known that reduced coronary flow after primary angioplasty (TIMI flow grade: 0–2) is associated with worse outcome, even when no significant epicardial obstruction remains [18]. However, angiographic epicardial flow is a poor substitute for tissue perfusion and microvascular obstruction often occurs unrecognized. More sensitive markers of tissue perfusion have been identified and provide prognostic information beyond that of TIMI flow grade. With the use of computerized myocardial blush grade analysis a qualitative assessment of myocardial contrast density exists. Catheter-based methods, such as with a Doppler guidewire or pressure/temperature guidewire for the assessment of the index of microcirculatory resistance, may also be used [20].
▪ Echocardiography
Noninvasive assessment of myocardial perfusion with myocardial contrast echocardiography may demonstrate microvascular no-reflow even in patients with angiographic TIMI flow grade 3 after PCI [21].
▪ MRI/computed tomography
Tissue hypoenhancement in contrast-enhanced MRI or multidetector computed tomography indicates impaired myocardial perfusion and correlates with evidence of microvascular obstruction [22,23].
▪ Biomarkers
A rise in serum cardiac biomarkers after PCI reflects myocardial necrosis secondary to tissue hypoperfusion and ischemia. More than 70% of patients may exhibit elevated troponin values after an otherwise successful elective PCI [24].
Pathophysiology of no-reflow
No-reflow was described for the first time by Kloner et al. [25]. In a canine model, no-reflow occurred after prolonged coronary occlusion followed by reperfusion. Contrary to epicardial spasm, no-reflow during coronary intervention generally responds poorly to intracoronaryadministered nitroglycerin. This phenomenon corresponds with the observation that the microcirculation responds poorly to nitroglycerin [26–29]. During ischemia, small vessel vasoconstriction may occur due to impaired local synthesis of endothelium-derived relaxing factor. Calcium antagonists act directly on the vascular smooth muscle rather than endothelium-derived relaxing factor, and constitute a more effective treatment for no-reflow during coronary intervention. Taken together, this observation suggests that no-reflow may be due to distal microvascular spasm caused by the release of potent vasoconstrictors. The severity of microvascular obstruction that develops after both elective and infarct-related PCI may be influenced by pre-existing microvascular obstruction and/or endothelial dysfunction. This may explain the association of diabetes mellitus and hyperlipidemia with the no-reflow phenomenon.
Four pathogenetic mechanisms may contribute to the development of no-reflow.
▪ Distal embolization
Previous experimental studies have shown that myocardial blood flow decreases irreversibly when more than 50% of coronary capillaries are obstructed [30]. During PCI, emboli of different sizes can originate from atherosclerotic plaques or coronary thrombus [31]. Okamura et al. used a Doppler guidewire during coronary intervention in patients with AMI, to count the number of embolic particles [32]. The average number of emboli during primary PCI was 25. Emboli can obstruct prearterioles and may induce noreflow. Distal embolization plays an important role in situations in which the amount of debris is largest, particularly during SVG PCI [33].
▪ Ischemia-related injury
Endothelial dysfunction may occur after prolonged ischemia. Morphological changes include endothelial protrusions and membranebound bodies, which may fill the capillaries up to luminal obliteration. Moreover, endothelial gaps with extravascular erythrocytes are common after ischemia [34]. These morphological findings are accompanied by a reduction of regional myocardial blood flow within the previously ischemic region and may result in no-reflow [35]. Furthermore, interstitial edema due to myocardial cell swelling might cause microvascular compression [36].
▪ Reperfusion-related injury
Reperfusion of ischemic tissues is often associated with microvascular dysfunction, which is characterized as impaired endotheliumdependent dilation in arterioles and the trafficking of leukocytes and plasma protein extravasation in postcapillary venules. Activated endothelial cells in all segments of the microcirculation produce more oxygen radicals but less nitric oxide in the initial period following reperfusion [37]. The resulting imbalance leads to the production and release of inflammatory mediators and enhances the biosynthesis of adhesion molecules that mediate leukocyte endothelial cell adhesion [37]. Activated platelets and neutrophils can aggregate and obstruct capillaries [38,39]. Vasoconstrictive mediators released by activated neutrophils, platelets and damaged endothelium contribute to sustained vasoconstriction of the coronary microcirculation [40]. Several inflammatory mediators are involved in a complex interaction between platelets, neutrophils and endothelial cells. TNF-a, IL-1b, selectin and endothelin-1 (ET-1) seem to play an important role in reperfusion-related injury and consecutive no-reflow [41–43]. Natriuretic peptides might suppress ET-1 expression, limiting infarct size when administered before and during coronary occlusion [44]. Reperfusion might also cause irreversible injury to myocytes [45]. Ischemia may result in a cellular calcium overload that triggers uncontrolled hypercontraction and stimulates opening of the mitochondrial permeability transition pore (m-PTP), which further enhances calcium overload. Cyclosporine, which blocks the m-PTP, has been shown to reduce infarct size by 20% when administered intravenously in patients undergoing primary PCI [46]. Furthermore, ischemic preconditioning might also reduce infarct size by blockade of m-PTP [45,47].
▪ Individual susceptibility
Predisposition to no-ref low can be genetic and/or acquired. Conditions such as diabetes or hypercholesterolemia have been associated with impaired microvascular reperfusion after primary PCI and enlarged reperfusion injury [48,49].
Predictors of no-reflow
▪ Predictors of distal embolization
Thrombus burden at a lesion site in the coronary artery is a major risk factor for distal embolization. Angiographic morphologic features of infarct-related arteries as a predictor for noreflow have been proposed by Yip et al. [50]. The score to assess thrombus burden included the following characteristics: 1: an angiographic thrombus with the greatest linear dimension being more than three times the reference lumen diameter; 2: cutoff pattern (lesion morphology with an abrupt cutoff without taper before the occlusion); 3: presence of accumulated thrombus (>5 mm of linear dimension) proximal to the occlusion; 4: presence of floating thrombus proximal to the occlusion; 5: persistent contrast medium distal to the obstruction; and 6: reference lumen diameter of the infarct-related artery being >4 mm. Yip et al. reported that all of these characteristics were independent predictors of no-reflow in 800 patients undergoing primary PCI. Distal embolization of thrombotic debris typically occurs after stent placement in large coronary vessels. In small vessels, the stent might fix the thrombus to the vessel wall, especially if the thrombus is not fresh anymore. Similar findings have been described by Limbruno et al. [51]. In a series of patients with ST elevation myocardial infarction (STEMI) undergoing primary PCI with distal filter protection, they found that Yip’s score was an independent predictor of total debris volume captured in the filter’s basket. Another risk factor for no-reflow is SVG PCI. The incidence of major adverse cardiac events doubles with SVG PCI compared with native-artery PCI [52].
▪ Predictors of ischemia-related injury
Time to treatment in PCI plays an important role for the occurrence of the no-reflow phenomenon. The longer the time to reperfusion, the higher is the prevalence of no-reflow [53]. A relationship between myocardial thickness and occurrence of no-reflow was proposed by Turschner et al. [54]. They showed that prolonged ischemia followed by reperfusion is associated with increased thickness of the myocardium due to tissue edema, which eventually leads to no-reflow for mechanical reasons. The extent of the ischemic region is another determinant of no-reflow, as demonstrated by Iwakura et al. A higher prevalence of no-reflow when the left anterior descending is the infarctrelated artery as compared with other epicardial coronary arteries proposes that a larger extent of the ischemic area is a predictor of no-reflow [55].
▪ Predictors of reperfusion-related injury
Several studies have demonstrated that platelets may play a role in no-reflow. Campo et al. demonstrated a relationship between platelet reactivity on admission and prevalence of no-reflow [56]. Huczek et al. showed that mean platelet volume on admission predicts impaired reperfusion and long-term mortality in AMI treated with primary percutaneous coronary intervention [57]. Niccoli et al. suggested that plasma levels of thromboxane-A2 (TxA2) predict noreflow [58]. Other data indicate that depletion of antioxidants might be associated with the no-reflow phenomenon in AMI [59]. The strong vasoconstrictor ET-1 seems to play a key role in no-reflow. ET-1 levels on admission are an independent predictor of no-reflow [60]. Another easily available predictor is the neutrophil count, which has been related to microvascular injury after primary PCI [61].
▪ Predictors of individual susceptibility
Acquired and genetic factors might play a role in the development of the no-reflow phenomenon. Studies have suggested that conditions such as diabetes and hypercholesterolemia might predispose to no-reflow [48,49]. Iwakura et al. revealed a relationship between hyperglycemia and the no-reflow phenomenon in patients with AMI [62]. Furthermore, data revealed an association between C-reactive protein and myocardial perfusion in patients with ST-elevation AMI [63]. Preinfarction angina, another aquired factor, might have a protective effect due to induction of ischemic preconditioning [64]. Vignali et al. suggested that the 1976T>C polymorphism of the adenosine 2A receptor gene is associated with a higher prevalence of no-reflow [65]. Other data revealed that patients with no-reflow show a more compact fibrin network [66]. This might be a genetically mediated resistance to lysis. Among the individual predictors of noref low, coronary anatomy, as quantified by the SYNTAX score, has also recently been demonstrated to be a reliable predictor [67].
Prevention & treatment of no-reflow
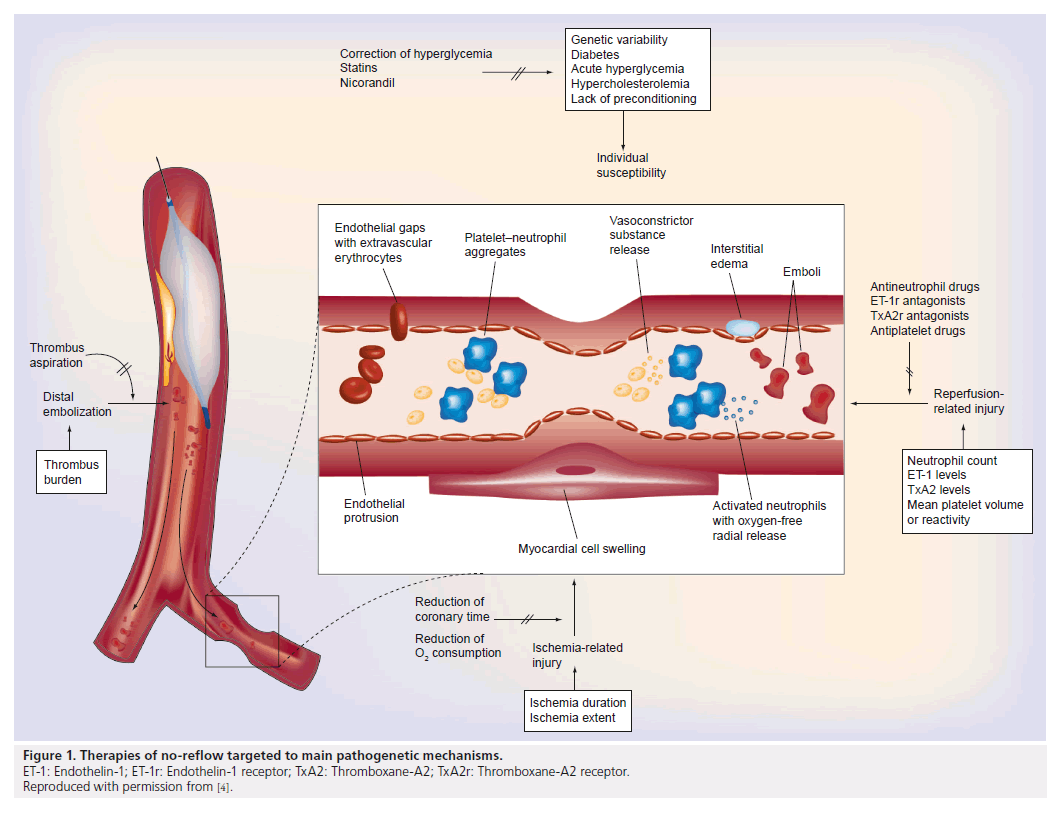
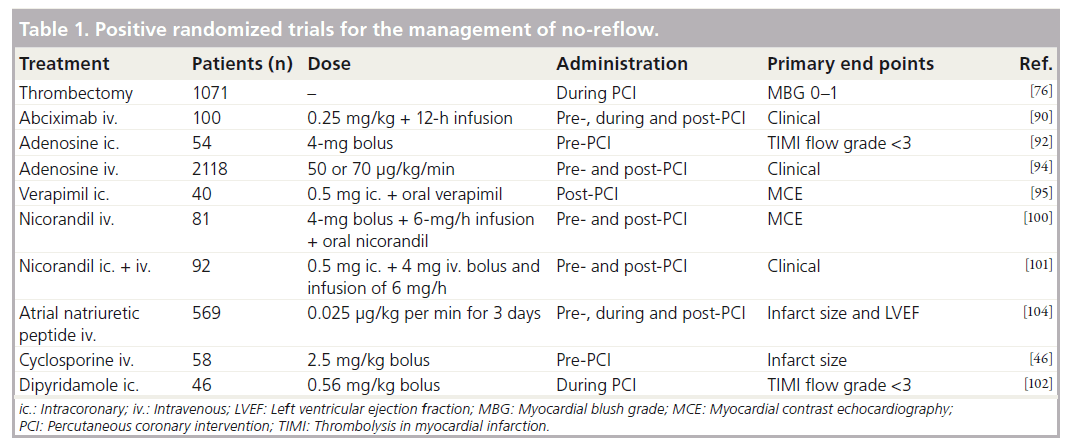
Prevention and treatment options of noreflow can be divided in pharmacological and mechanical approaches. A number of therapeutic strategies have been tested for prevention and treatment of no-reflow with inconsistent results, but the reason may be a random use for all patients without considering the underlying pathogenetic components. Furthermore, often experimental data cannot be translated into clinical practice [68]. As a function of the pathophysiology of no-reflow, management options should be used individually (Figure 1). Table 1 lists selected positive randomized trials for the management of no-reflow.
Figure 1. Therapies of no-reflow targeted to main pathogenetic mechanisms.
ET-1: Endothelin-1; ET-1r: Endothelin-1 receptor; TxA2: Thromboxane-A2; TxA2r: Thromboxane-A2 receptor.
Reproduced with permission from [4].
▪ Prevention of distal embolization
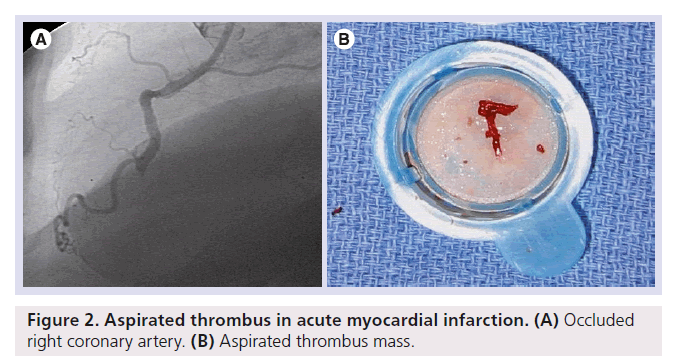
PCI in thrombus-containing lesions carries a high risk of no-reflow. With the technique of direct stent implantation, balloon-induced thrombus fragmentation is avoided and the atherothrombus is fixed under the stent struts. Loubeyre et al. demonstrated improved reperfusion in patients randomized to direct stenting as compared with standard primary PCI [69]. However, only patients with good distal visualization of the infarct-related artery after guidewire passage are suitable for direct stenting. The use of thrombectomy and distal protection devices is a more sophisticated technical approach for the prevention of distal embolization, although early studies using this technique showed no significant benefit compared with standard primary PCI [70,71]. However, the negative results of these trials should be interpreted within the limitations of their design, which was characterized by the enrollment of patients at low risk for no-reflow and by the use of first-generation, complex devices. A further study that used another still complex thrombectomy device in high-risk patients did show improvement of myocardial reperfusion [72]. The first randomized trial assessing the role of thrombectomy, performed with a simple manual aspiration catheter, was the REMEDIA trial [73]. This study proved that manual thrombectomy was safe and resulted in better myocardial perfusion as compared with standard primary PCI. Especially in the subset of patients with a high thrombus burden, a benefit was evident. A further substudy of the REMEDIA trial showed that thrombus aspiration significantly reduced no-reflow [74]. Svilaas et al. confirmed the improvement in reperfusion associated with manual thrombus aspiration as compared with standard primary PCI [75]. The TAPAS study was the first to show that improvement of myocardial perfusion by manual thrombus aspiration results in a lower mortality at 12-month follow-up [76]. In a recent study, in patients with large anterior STEMI undergoing primary PCI, infarct size at 30 days was not significantly reduced by manual aspiration thrombectomy [77]. However, in this study, thrombus mass after wire passage was not taken into account. Taken together, manual thrombus aspiration should be used in the setting of primary PCI in patients with a high thrombus burden [78,79]. Figure 2 shows an aspirated thrombus mass in AMI. Furthermore, embolic protection devices should be used during SVG PCI when technically feasible [80,81]. However, distal embolic protection devices do not improve survival or re-infarction rates in patients undergoing native-artery PCI [82,83]. The reason for this might be that distal protection devices must cross the thrombotic lesion and so the risk of releasing thrombi during this passage is much higher in native vessels compared with the bigger SVG. In a recent retrospective study, the infusion of intracoronary drugs using a novel perfusion catheter seemed to be safe and could help to improve myocardial perfusion in a selected group of patients presenting with ACS who developed no-reflow during PCI [84]. Another issue is the role of antiplatelet therapy pretreatment in AMI. Mangiacapra et al. compared clopidogrel 600 and 300 mg loading doses in patients with ST-segment elevation myocardial infarction. Patients receiving the 600-mg loading dose of clopidogrel showed a significantly lower incidence of post- PCI myocardial blush grade 0 or 1 and the no-reflow phenomenon was significantly less common [85].
▪ Prevention of ischemia-related injury
Strategies of reducing total ischemic time might reduce the prevalence of the no-reflow phenomenon. Pharmacologic drugs reducing myocardial oxygen consumption and, consequently, the severity of ischemia might improve outcome [86]. Beneficial effects of carvedilol, fosinopril and valsartan on coronary no-reflow have been demonstrated [87,88].
▪ Prevention & treatment of reperfusion-related injury
Several studies have demonstrated that, in particular, drugs with the effect of counteracting endothelial dysfunction or platelet and neutrophil activation may be a prevention and/or treatment option for reperfusion no-reflow.
Antiplatelets
▪ Glycoprotein IIb/IIIa antagonists
Petronio et al. demonstrated that abciximab improves myocardial perfusion when started during primary PCI and infused for 12 h thereafter [89]. In a small randomized study of abciximab versus tirof iban in patients undergoing primary PCI, Danzi et al. demonstrated similar rates of final TIMI flow grade 3 (86 vs 88%), adverse cardiac remodeling and clinical events at 1 month in both arms [90]. Stone et al. showed recently that in patients with large anterior STEMI presenting early after symptom onset and undergoing primary PCI with bivalirudin anticoagulation, infarct size at 30 days was significantly reduced by bolus intracoronary abciximab delivered to the infarct lesion site [77].
By contrast, glycoprotein IIb/IIIa receptor antagonists have failed to mitigate the impact of distal embolization in SVG intervention [91].
Vasodilators
▪ Adenosine
Adenosine is mainly produced by the degradation of adenosine triphosphate, which antagonizes platelets and neutrophils, reduces calcium overload and oxygen free radicals, and induces vasodilation. Marzilli et al. showed that intracoronary administration of 4 mg of adenosine as an adjunct to primary PCI in AMI resulted in a lower rate of no-reflow as compared with the control arm [92]. Intravenous adenosine has been tested in two large randomized trials [93,94]. Both studies showed a significant reduction of infarct size, but in-hospital and 6-month clinical outcomes were similar to those observed in the placebo group.
▪ Calcium-channel blocker
Taniyama et al. conducted a small randomized study and showed in patients with first STEMI that intracoronary verapamil compared with placebo was associated with better microvascular function [95]. Accordingly, intracoronary verapamil has been successfully used to reverse no-reflow after primary PCI [96]. For the prevention of no-reflow in vein grafts, nicardipin may be used [97].
▪ Nitroprusside
Nitroprusside is a nitric oxide donor that does not depend on intracellular metabolism to derive nitric oxide. Two small registries showed an improvement in final TIMI flow grade after administration of intracoronary nitroprusside, given in an attempt to reverse no-reflow [98,99].
▪ Nicorandil
Nicorandil is a hybrid drug consisting of an ATP-sensitive potassium ion channel opener and nicotinamide nitrate. Intravenous infusion of nicorandil for 24 h after primary PCI resulted in better angiographic, functional and clinical outcomes compared with placebo in two randomized studies [100,101].
▪ Dipyridamole
Dipyridamole is a thromboxane synthase inhibitor and stops the effects of TxA2 (platelet aggregation and vasoconstriction). In a small randomized trial it was superior to verapimil for the treatment of no-reflow during primary angioplasty [102].
Hormones
▪ Epinephrine
Skelding et al. analyzed 29 consecutive patients in whom intracoronary epinephrine was administered for refractory no-reflow [103]. Intracoronary epinephrine resulted in a significant improvement in coronary flow. These findings indicate that intracoronary epinephrine may exert salutary effects in patients suffering refractory no-reflow following elective or acute coronary interventions.
▪ Atrial natriuretic peptide
Kitakaze et al. randomized 277 patients to receive intravenous atrial natriuretic peptide and 292 patients to receive placebo [104]. They showed that atrial natriuretic peptide treatment was associated with a significant reduction in infarct size, an increase in left ventricular ejection fraction and an improved myocardial perfusion.
Mitochondrial permeability transition pore inhibitors
▪ Cyclosporine
Cyclosporine inhibits the opening of mitochondrial permeability transition pores and attenuates myocardial injury that occurs at the time of reperfusion. In a small randomized trial, administration of cyclosporine at the time of reperfusion was associated with a smaller infarct size than that seen with placebo [46].
Novel therapies
▪ Exenatide
Exenatide is a glucagon-like peptide-1 analog. In patients with STEMI who are undergoing primary PCI, intravenous administration of exenatide at the time of reperfusion increased myocardial salvage [105].
▪ Ischemic pre- & post-conditioning
One or several short cycles of ischemia before (preconditioning) or after (post-conditioning) a sustained coronary occlusion with subsequent reperfusion reduces the ultimate infarct size [45]. Ischemic post-conditioning, a mechanical maneuver at the onset of reperfusion, reduces infarct size after ischemia and was first described in 2003 by Zhao et al. [106]. Staat et al. showed that post-conditioning by coronary angioplasty protects the human heart during AMI [107]. Similar results were achieved by remote ischemic conditioning prior to PCI [108]. Current research is being addressed to understand the molecular mechanism of this protection.
▪ Prevention of individual susceptibility
Unfortunately, genetically determined susceptibility to microcirculatory injury cannot be influenced yet. However, predisposition, as with diabetes and hypercholesterolemia, can be treated. Treatment of hyperglycemia might be an important target in the prevention of no-reflow. The DIGAMI study demonstrated a reduction of infarct size following a periprocedural reduction of blood glucose [109]. However, the DIGAMI 2 follow-up study demonstrated no effect on mortality [110]. In addition, the treatment of hypercholesterolemia by the use of statins might reduce reperfusion injury. Iwakura et al. demonstrated that chronic statin therapy in patients with or without hypercholesterolemia is associated with a lower prevalence of no-reflow [111].
Conclusion
The no-reflow phenomenon occurs both in elective and primary PCI and is associated with adverse outcomes. Microvascular obstruction is believed to be the underlying cause of no-reflow, which is induced by various pathophysiologic mechanisms. Coronary no-reflow results in prolonged myocardial ischemia. Owing to its negative prognostic relationship, this complication requires prompt diagnosis and treatment. Patients undergoing PCI for AMI and intervention of SVG are high-risk subsets. Several trials have demonstrated some benefit of intracoronary calcium-channel blockers, adenosine, nitroprusside, nicorandil and systemic abciximab in the treatment of noreflow. Fewer data support the use of epinephrine, atrial natriuretic peptide, cyclosporine and dipyridamole. In the setting of high thrombus burden, thrombectomy and/or distal protection devices should be used for the prevention of no-reflow.
Future perspective
Future directions in no-reflow research include elucidation and targeted activation of intracellular cardioprotective signaling pathways. For example, ET-1 antagonists have shown beneficial effects in animal models [112]. M-PTP blockers or selective TxA2 antagonists might be other promising approaches for the treatment of no-reflow [46,58].
The development of better mechanical devices to prevent or reduce distal embolization is another area of potential advance in this field.
Executive summary
Myocardial tissue hypoperfusion despite patent epicardial coronary arteries
▪ No-reflow can be caused by microvascular obstruction.
▪ There are four pathogenetic mechanisms: distal embolization, ischemia-related injury, reperfusion-related injury and individual susceptibility.
▪ No-reflow has a negative impact on clinical outcome due to myonecrosis of the heart muscle.
Classification according to the duration of the preceding myocardial ischemia
▪ Interventional no-reflow: occurs following noninfarct percutaneous coronary intervention (PCI).
▪ Reperfusion no-reflow: follows PCI for reperfusion of an infarct-related coronary artery in the setting of acute myocardial infarction.
Different sensitivity of diagnostic tests explains the wide variety of reported incidence
▪ Routine diagnostic tests: angiographic thrombolysis in myocardial infarction flow grade; electrocardiography ST-segment resolution (in ST elevation myocardial infarction).
▪ Nonroutine diagnostic tests: myocardial blush grade; contrast echocardiography; computed tomography; MRI.
Prevention & treatment of no-reflow can be divided into pharmacological and mechanical approaches
▪ Administration of an intracoronary vasodilator (adenosine, calcium-channel blocker or nitroprusside) is reasonable to treat PCI-related no-reflow that occurs during primary or elective PCI.
▪ In the setting of acute myocardial infarction, evidence for a beneficial effect on no-reflow exists for abciximab, adenosine, nicorandil and nitroprusside.
▪ Epinephrine, atrial natriuretic peptide, cyclosporine and dipyridamole might have beneficial effects.
▪ Thrombectomy and/or embolic protection devices should be used for the prevention of no-reflow in the setting of high thrombus burden and during saphenous vein graft intervention.
Future perspective
▪ The elucidation and targeted activation of intracellular cardioprotective signaling pathways is a possible research direction.
▪ Development of more sophisticated mechanical devices will be seen in the future.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- Krug A, Du Mesnil de Rochemont, Korb G. Blood supply of the myocardium after temporary coronary occlusion. Circ. Res. 19(1), 57–62 (1966).
- Ito H, Tomooka T, Sakai N et al. Lack of myocardial perfusion immediately after successful thrombolysis: a predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation 85(5), 1699–1705 (1992).
- Ito H, Maruyama A, Iwakura K et al. Clinical implications of the ‘no reflow’ phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 93(2), 23–28 (1996).
- Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 54(4), 281–292 (2009).
- Eeckhout E, Kern MJ. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur. Heart J. 22(9), 729–739 (2001).
- Piana RN, Paik GY, Moscucci M et al. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation 89, 2514–2518 (1994).
- Abbo KM, Dooris M, Glazier S. Features and outcome of no-reflow after percutaneous coronary intervention. Am. J. Cardiol. 75, 778–782 (1995).
- Baim DS, Carozza JP, Kuntz RE et al. Managaing the embolization problem during saphenous vein graft intervention. Cathet. Cardiovasc. Intervent. 47(2), 155–156 (1999).
- Topol EJ, Yadov YS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation 101(5), 570–580 (2000).
- Piana R, Paik GY, Moscucci M et al. Incidence and treatment of ‘no reflow’ after percutaneous coronary intervention. Circulation 89, 2514 (1994).
- Brosh D, Assali AR, Mager A et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am. J. Cardiol. 99(4), 442–445 (2007).
- Gibson CM, Cannon CP, Murphy SA et al. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 105(2), 1909–1913 (2002).
- McLaughlin MG, Stone GW, Aymong E et al. Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J. Am. Coll. Cardiol. 44(6), 1215–1223 (2004).
- Bolognese L, Carrabba N, Parodi G et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 109(9), 1121–1126 (2004).
- Galiuto L, Garramone B, Scarà A et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J. Am. Coll. Cardiol. 51(5), 552–559 (2008).
- Wu KC, Zerhouni EA, Judd RM et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97, 765–772 (1998).
- Abbo KM, Dooris M, Glazier S et al. Features and outcome of no-reflow after percutaneous coronary intervention. Am. J. Cardiol. 75, 778–782 (1995).
- Morishima I, Sone T, Okumura K et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J. Am. Coll. Cardiol. 36(4), 1202–1209 (2000).
- The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N. Engl. J. Med. 312(14), 932–936 (1985).
- Pijls NH, De Bruyne B. Coronary Pressure (2nd Edition). Kluwer Academic Publishers, MA, USA (2000).
- Ito H, Okamura A, Iwakura K et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation 93(11), 1993–1999 (1996).
- Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation 94(12), 3318–3326 (1996).
- Jacquier A, Boussel L, Amabile N et al. Multidetector computed tomography in reperfused acute myocardial infarction. Assessment of infarct size and no-reflow in comparison with cardiac magnetic resonance imaging. Invest. Radiol. 43(11), 773–781 (2008).
- Bonz AW, Lengenfelder B, Strotmann J et al. Effect of additional temporary glycoprotein IIb/IIIa receptor inhibition on troponin release in elective percutaneous coronary interventions after pretreatment with aspirin and clopidogrel (TOPSTAR trial). J. Am. Coll. Cardiol. 40(4), 662–668 (2002).
- Kloner RA, Ganote CE, Jenning RB. The ‘no-reflow’ phenomenon after temporary coronary occlusion in dogs. J. Clin. Invest. 54, 1496–1508 (1974).
- Winbury JJ, Howe BB, Hefner MA. Effect of nitrates and other coronary dilators on large and small coronary vessels: an hypothesis for the mechanism of action of nitrates. J. Pharmacol. Exp. Ther. 168(1), 70–95 (1969).
- Sellke FW, Myers PR, Bates JN et al. Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am. J. Physiol. 258(2 Pt 2), H515–H520 (1990).
- Sellke FW, Tomanek RJ, Harrison DG. l-cysteine selectively potentiates nitroglycerininduced dilation of small coronary microvessels. J. Pharmacol. Exp. Ther. 258(1), 365–369 (1991).
- Kurz MA, Lamping KG, Bates JN et al. Mechanisms responsible for the heterogeneous coronary microvascular response to nitroglycerin. Circ. Res. 68(3), 847–855 (1991).
- Hori M, Inoue M, Kitakaze M et al. Role of adenosine in hyperemic response of coronary blood flow in microembolization. Am. J. Physiol. 250(3 Pt 2), H509–H518 (1986).
- Skyschally A, Leineweber K, Gres P et al. Coronary microembolization. Basic Res. Cardiol. 101, 373–382 (2006).
- Okamura A, Ito H, Iwakura K et al. Detection of embolic particles with the Doppler guide wire during coronary intervention in patients with acute myocardial infarction: efficacy of distal protection device. J. Am. Coll. Cardiol. 45(2), 212–215 (2005).
- Mauri L, Rogers C, Baim DS. Devices for distal protection during percutaneous coronary revascularization. Circulation 113, 2651–2656 (2006).
- Reffelmann T, Kloner RA. The no-reflow phenomenon: a basic mechanism of myocardial ischemia and reperfusion. Basic Res. Cardiol. 101, 359–372 (2006).
- Ambrosio G, Weisman HF, Mannisi JA et al. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 80(6), 1846–1861 (1989).
- Tranum-Jensen J, Janse MJ, Fiolet WT et al. Tissue osmolality, cell swelling, and reperfusion in acute regional myocardial ischemia in the isolated porcine heart. Circ. Res. 49(2), 364–381 (1981).
- Carden DL, Granger DN. Pathophysiology of ischemia-reperfusion injury. J. Pathol. 190, 255–266 (2000).
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N. Engl. J. Med. 57, 1121–1135 (2007).
- Engler RL, Schmid-Schönbein GW, Pavelec RS. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am. J. Pathol. 111, 98–111 (1983).
- Ito BR, Schmid-Schönbein G, Engler RL. Effects of leukocyte activation on myocardial vascular resistance. Blood Cells 16, 145–163 (1990).
- Lefer AM, Tsao PS, Aoki N et al. Mediation of cardioprotection by transforming growth factor-b. Science 249(4964), 61–64 (1990).
- Furuichi K, Wada T, Iwata Y et al. Interleukin-1-dependent sequential chemokine expression and inflammatory cell infiltration in ischemia-reperfusion injury. Crit. Care Med. 34(9), 2447–2455 (2006).
- Chamoun F, Burne M, O’Donnell M, Rabb H. Pathophysiologic role of selectins and their ligands in ischemia reperfusion injury. Front. Biosci. 5, E103–E109 (2000).
- Baxter GF. Natriuretic peptides and myocardial ischaemia. Basic Res. Cardiol. 99, 90–93 (2004).
- Skyschally A, Schulz R, Heusch G. Pathophysiology of myocardial infarction: protection by ischemic pre- and postconditioning. Herz 33, 88–100 (2008).
- Piot C, Croisille P, Staat P et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 359, 473–481 (2008).
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117, 3152–3156 (2008).
- Collet JP, Montalescot G. The acute reperfusion management of STEMI in patients with impaired glucose tolerance and Type 2 diabetes. Diabetes Vasc. Dis. Res. 2(3), 136–143 (2005).
- Golino P, Maroko PR, Carew TE. The effect of acute hypercholesterolemia on myocardial infarct size and the no-reflow phenomenon during coronary occlusion-reperfusion. Circulation 75, 292–298 (1987).
- Yip HK, Chen MC, Chang HW et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest 122(4), 1322–1332 (2002).
- Limbruno U, De Carlo M, Pistolesi S et al. Distal embolization during primary angioplasty: histopathologic features and predictability. Am. Heart J. 150(1), 102–108 (2005).
- Hoffmann R, Hamm C, Nienaber CA et al. Implantation of sirolimus-eluting stents in saphenous vein grafts is associated with high clinical follow-up event rates compared with treatment of native vessels. Coron. Artery Dis. 18(7), 559–564 (2007).
- Nallamothu BK, Bradley EH, Krumholz HM. Time to treatment in primary percutaneous coronary intervention. N. Engl. J. Med. 357, 1631–1638 (2007).
- Turschner O, D’hooge J, Dommke C et al. The sequential changes in myocardial thickness and thickening which occur during acute transmural infarction, infarct reperfusion and the resultant expression of reperfusion injury. Eur. Heart J. 25(9), 794–803 (2004).
- Iwakura K, Ito H, Kawano S et al. Predictive factors for development of the no-reflow phenomenon in patients with reperfused anterior wall acute myocardial infarction. J. Am. Coll. Cardiol. 38(2), 472–477 (2001).
- Campo G, Valgimigli M, Gemmati D et al. Value of platelet reactivity in predicting response to treatment and clinical outcome in patients undergoing primary coronary intervention: insights into the STRATEGY study. J. Am. Coll. Cardiol. 48(11), 2178–2185 (2006).
- Huczek Z, Kochman J, Filipiak KJ et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Coll. Cardiol. 46(2), 284–290 (2005).
- Niccoli G, Giubilato S, Russo E et al. Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention. Eur. Heart J. 29(15), 1843–1850 (2008).
- Matsumoto H, Inoue N, Takaoka H et al. Depletion of antioxidants is associated with no-reflow phenomenon in acute myocardial infarction. Clin. Cardiol. 27(8), 466–470 (2004).
- Niccoli G, Lanza GA, Shaw S et al. Endothelin-1 and acute myocardial infarction: a no-reflow mediator after successful percutaneous myocardial revascularization. Eur. Heart J. 27(15), 1793–1798 (2006).
- Takahashi T, Hiasa Y, Ohara Y et al. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. Am. J. Cardiol. 100(1), 35–40 (2007).
- Iwakura K, Ito H, Ikushima M et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 41, 1–7 (2003).
- Hoffmann R, Suliman H, Haager P et al. Association of C-reactive protein and myocardial perfusion in patients with ST-elevation acute myocardial infarction. Atherosclerosis 186, 177–183 (2006).
- Karila-Cohen D, Czitrom D, Brochet E et al. Decreased no-reflow in patients with anterior myocardial infarction and pre-infarction angina. Eur. Heart J. 20(23), 1724–1730 (1999).
- Vignali L, Talanas G, Saia F et al. Genetic association between the 1976T>C polymorphism in the adenosine A2 receptor and angiographic no-reflow phenomenon (abstr). Il giornale italiano di Cardiologia Invasiva 3(Suppl. 1), 109 (2007).
- Zalewski J, Undas A, Godlewski J et al. No-reflow phenomenon after acute myocardial infarction is associated with reduced clot permeability and susceptibility to lysis. Arterioscler. Thromb. Vasc. Biol. 27(10), 2258–2265 (2007).
- Magro M, Nauta ST, Simsek C et al. Usefulness of the SYNTAX score to predict “no reflow” in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am. J. Cardiol. 109(5), 601–606 (2012).
- Dirksen MT, Laarman GJ, Simoons ML, Duncker DJ. Reperfusion injury in humans: a review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc. Res. 74(3), 343–355 (2007).
- Loubeyre C, Morice MC, Lefèvre T et al. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J. Am. Coll. Cardiol. 39, 15–21 (2002).
- Ali A, Cox D, Dib N et al. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J. Am. Coll. Cardiol. 48(2), 244–252 (2006).
- Dangas G, Stone GW, Weinberg MD et al. Contemporary outcomes of rescue percutaneous coronary intervention for acute myocardial infarction: comparison with primary angioplasty and the role of distal protection devices (EMERALD trial). Am. Heart J. 155(6), 1090–1096 (2008).
- Young JJ, Cox DA, Stuckey T et al. Prospective, multicenter study of thrombectomy in patients with acute myocardial infarction: the X-Tract AMI registry. J. Interv. Cardiol. 20(1), 44–50 (2007).
- Burzotta F, Trani C, Romagnoli E et al. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombusaspiration in primary and rescue angioplasty (REMEDIA) trial. J. Am. Coll. Cardiol. 46(2), 371–376 (2005).
- Galiuto L, Garramone B, Burzotta F et al. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention: a myocardial contrast echocardiography substudy of the REMEDIA trial. J. Am. Coll. Cardiol. 48(7), 1355–1360 (2006).
- Svilaas T, Vlaar PJ, van der Horst IC et al. Thrombus aspiration during primary percutaneous coronary intervention. N. Engl. J. Med. 358, 557–567 (2008).
- Vlaar PJ, Svilaas T, van der Horst IC et al. Cardiac death and reinfarction after 1 year in the thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet 371(9628), 1915–1920 (2008).
- Stone GW, Maehara A, Witzenbichler B et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA 307(17), 1817–1826 (2012).
- Burzotta F, Crea F. Thrombus-aspiration: a victory in the war against no reflow. Lancet 371(9628), 1889–1890 (2008).
- Vlaar PJ, Svilaas T, van der Horst IC et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 371(9628), 1915–1920 (2008).
- Coolong A, Baim DS, Kuntz RE et al. Saphenous vein graft stenting and major adverse cardiac events: a predictive model derived from a pooled analysis of 3958 patients. Circulation 117(6), 790–797 (2008).
- Stone GW, Rogers C, Hermiller J et al. Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto-coronary bypass grafts. Circulation 108(5), 548–553 (2003).
- Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur. Heart J. 29(24), 2989–3001(2008).
- Stone GW, Webb J, Cox DA et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 293(9), 1063–1072 (2005).
- Maluenda G, Ben-Dor I, Delhaye C et al. Clinical experience with a novel intracoronary perfusion catheter to treat noreflow phenomenon in acute coronary syndromes. J. Interv. Cardiol. 23(2), 109–113 (2010).
- Mangiacapra F, Muller O, Ntalianis A et al. Comparison of 600 versus 300-mg clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary coronary angioplasty. Am. J. Cardiol. 106(9), 1208–1211 (2010).
- Opie LH. Reperfusion injury and its pharmacologic modification. Circulation 80, 1049–1062 (1989).
- Zhao J, Yang Y, You S et al. Carvedilol preserves endothelial junctions and reduces myocardial no-reflow after acute myocardial infarction and reperfusion. Int. J. Cardiol. 115(3), 334–341 (2007).
- Zhao JL, Yang YJ, You SJ et al. Pretreatment with fosinopril or valsartan reduces myocardial no-reflow after acute myocardial infarction and reperfusion. Coron. Artery Dis. 17, 463–469 (2006).
- Petronio AS, De Carlo M, Ciabatti N et al. Left ventricular remodeling after primary coronary angioplasty in patients treated with abciximab or intracoronary adenosine. Am. Heart J. 150, 1015 (2005).
- Danzi GB, Sesana M, Capuano C et al. Comparison in patients having primary coronary angioplasty of abciximab versus tirofiban on recovery of left ventricular function. Am. J. Cardiol. 94(1), 35–39 (2004).
- Roffi M, Mukherjee D, Chew DP et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation 106(24), 3063–3067 (2002).
- Marzilli M, Orsini E, Marraccini P et al. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation 101(18), 2154–2159 (2000).
- Mahaffey KW, Puma JA, Barbagelata NA et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J. Am. Coll. Cardiol. 34, 1711–1720 (1999).
- Ross AM, Gibbons RJ, Stone GW et al. A randomized, double-blinded, placebocontrolled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 45(11), 1775–1780 (2005).
- Taniyama Y, Ito H, Iwakura K et al. Beneficial effect of intracoronary verapamil on microvascular and myocardial salvage in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 30(5), 1193–1199 (1997).
- Werner GS, Lang K, Kuehnert H et al. Intracoronary verapamil for reversal of no-reflow during coronary angioplasty for acute myocardial infarction. Catheter Cardiovasc. Interv. 57, 444–451 (2002).
- Habibzadeh MR, Thai H, Movahed MR. Prophylactic intragraft injection of nicardipine prior to saphenous vein graft percutaneous intervention for the prevention of no-reflow: a review and comparison to protection devices. J. Invasive Cardiol. 23(5), 202–206 (2011).
- Pasceri V, Pristipino C, Pelliccia F et al. Effects of the nitric oxide donor nitroprusside on no-reflow phenomenon during coronary interventions for acute myocardial infarction. Am. J. Cardiol. 95, 1358–1361 (2005).
- Airoldi F, Briguori C, Cianflone D et al. Frequency of slow coronary flow following successful stent implantation and effect of nitroprusside. Am. J. Cardiol. 99(7), 916–920 (2007).
- Ito H, Taniyama Y, Iwakura K et al. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J. Am. Coll. Cardiol. 33(3), 654–660 (1999).
- Ono H, Osanai T, Ishizaka H et al. Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation. Am. Heart J. 148(4), E15 (2004).
- Tanzilli G, Greco C, Pascery V et al. Dipyridamole versus verapamil for treatment of no-reflow during primary angioplasty. Catheter Cardiovasc. Interv. 76(6), 787–793 (2010).
- Skelding KA, Goldstein JA, Mehta L et al. Resolution of refractory no-reflow with intracoronary epinephrine. Catheter Cardiovasc. Interv. 57(3), 305–309 (2002).
- Kitakaze M, Asakura M, Kim J et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet 370(9597), 1483–1493 (2007).
- Lønborg J, Vejlstrup N, Kelbæk H et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Heart J. 33(12), 1491–1499 (2012).
- Zhao ZQ, Corvera JS, Halkos ME et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 285(2), H579–H588 (2003).
- Staat P, Rioufol G, Piot C et al. Postconditioning the human heart. Circulation 112(14), 2143–2148 (2005).
- Bøtker HE, Kharbanda R, Schmidt MR et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375(9716), 727–734 (2010).
- Malmberg K, Rydén L, Efendic S et al. Randomized trial of insulinglucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J. Am. Coll. Cardiol. 26(1), 56–65 (1995).
- Malmberg K, Rydén L, Wedel H et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur. Heart J. 26(7), 650–661 (2005).
- Iwakura K, Ito H, Kawano S et al. Chronic pre-treatment of statins is associated with the reduction of the no-reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur. Heart J. 27(5), 534–539 (2006).
- Galiuto L, DeMaria AN, del Balzo U et al. Ischemia-reperfusion injury at the microvascular level: treatment by endothelin A-selective antagonist and evaluation by myocardial contrast echocardiography. Circulation 102(25), 3111–3116 (2000).
▪ Study revealed the strong negative impact of no-reflow on clinical outcome.
▪ Described the no-reflow phenomenon for the first time.
▪ First randomized trial assessing the role of thrombectomy performed with a simple manual aspiration.
▪ Study showed that improvement of myocardial perfusion by manual thrombus aspiration results in a lower mortality at 12-month follow-up.
▪ Showed that abciximab improves myocardial perfusion when started during primary percutaneous coronary intervention and infused for 12 h thereafter.
▪ Study showed significant reduction of infarct size after administering intravenous adenosine in acute myocardial infarction.
▪ Showed that intracoronary verapamil was associated with better microvascular function compared with placebo.