Research Article - Imaging in Medicine (2019) Volume 11, Issue 3
Magnetic Resonance Imaging Of Patients With Complications From Polyacrylamide Hydrophilic Gel Injection For Facial Plasty
Wang Xiaoyan1, Zhang Yan1*, Cheng Jing-Liang1, Hu Ying, Wang An-Fei1, Meng Fang-Fang2 and Liu Xi3
1Department of Magnetic Resonance, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
2Imaging Department, Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
3Department of Clinical Medicine, Xinxiang Medical University, Xinxiang, China
Corresponding Author:
Zhang Yan
Department of Magnetic Resonance, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
E-mail: wangxiaoyan0372@sina.cn
Abstract
Objective: To investigate the magnetic resonance imaging (MRI) signal features and MRI appearance of common complications resulting from polyacrylamide hydrogel (PAHG) injection for facial plasty?
Methods: MRI and clinical data of 42 patients treated by PAHG injection for facial plasty were retrospectively analyzed and the MRI features were compared with clinical data. All images were acquired using a SIEMENS Skyra 3.0T MR imaging unit. MR sequences, including FSE-T1WI, FSE-FS-T2WI, STIR and DWI, were acquired with an 8-channel brain coil. MRI slices through the maxillofacial region in the transverse, coronal and sagittal planes were analyzed.
Results: PAHG in the facial region appears as a variety of shapes owing to the anatomical space where it is present. It appears as a low signal intensity on T1-weighted images, high signal intensity on T2-weighted images and fat saturation T2-weighted images (the best contrast is obtained on fat saturation T2-weighted images), and high signal intensity on DWI. Of a total of 42cases, induration was observed in 23 cases, hydrogel migration in 13 cases, secondary deformity in three cases, and infection in three case.
Conclusions: MRI can provide diagnostic information for common postoperative complications of PAHG facial injection. It is an ideal examination method to diagnose postoperative complications of PAHG and to perform postoperative follow-up.
Keywords
magnetic resonance imaging ■polyacrylamide hydrophilic ■facial plasty ■complications
Introduction
Polyacrylamide (PAM) is a water-soluble polymer, which is synthesized from acrylamide monomer. Since 1987, hydrophilic polyacrylamide gel has been used by plastic surgeons in the former Soviet Union for cosmetic surgery of the face, breast, limbs and male and female organs [1]. As a medical soft tissue filling material, it was introduced into China by Ukraine in 1997, and has been mainly used in breast augmentation since then. Because of the small amount of trauma associated with its use [2], it was easily accepted by patients. Thereafter, the scope of application gradually expanded, the number of patients increased, and complications gradually became evident [3-5]. With improvements in living standards, more and more beauty-loving people have undergone cosmetic surgery. Since 1997, polyacrylamide hydrogel (PAHG) has been used in China. Because of its ease of application, PAHG has been widely used in patients with local soft tissue defects such as of the head, face, breast, buttocks and lower limbs. However, with the passage of time, various complications gradually appeared [6]. To date, there has been no evaluation of the long-term safety of polyacrylamide injected into the human body either at home or abroad. Because of its long-term presence within the human body for several years or even decades, it not only causes physical pain to users, but also causes huge psychological trauma because of the particular sites where it is used. Some serious complications have especially aroused great concern in society in general. It is necessary to select suitable examination methods to evaluate the status of a hydrogel in vivo, to find out and concurrently show signs as soon as possible, and to take effective treatment measures in time [7].
Magnetic resonance imaging (MRI) has the advantages that it does not involve radiation, and provides high resolution of soft tissue and multi-directional imaging [8]. It can clearly show the shape and distribution of PAHG, thus providing an objective basis for clinical diagnosis and treatment. In this paper, MRI data from 42 patients after hydrogel injection were analyzed retrospectively, and the imaging characteristics of MRI were discussed in order to accurately judge the hydrogel status in the maxillofacial region before treatment.
Methods
█ General information
Forty two female patients with maxillofacial discomfort were treated by facial injection of PAHG at our hospital. They ranged in age from 20 to 58 years, with a mean of 37 years. The injection sites were located in the temporal region, cheek, eyelid, eyebrow, forehead, nose and chin. The time since the injection ranged from 6-10 years, and the specific injection volume was not recorded in the original data.
█ Inspection equipment and scanning method
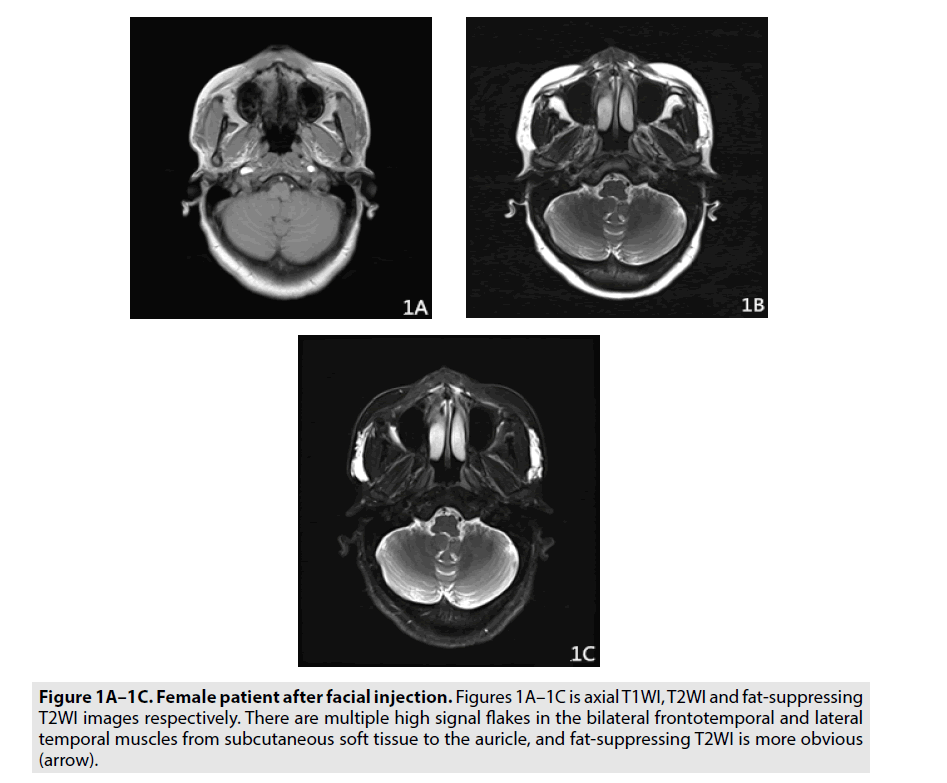
A Siemens Prisma 3.0T MR scanner was used to scan all patients. The scanning method was as follows: Patients were placed in the supine position and the head and neck were scanned with phased array coils. Transverse fast spin echo sequence T1-weighted imaging (FSE TIWI), fat saturation (FS) fast spin echo T2- weighted imaging (FSE T2WI), coronal FSE T2WI scanning, sagittal FSE T2WI scanning and diffusion-weighted imaging were routinely performed. The sequence parameters were axial T1WI (TR/TE: 220 ms/2.6 ms, matrix 520 × 520, 4 mm thickness, 4 mm thickness, 4 mm thickness, 1 mm interlayer spacing), axial T2WI (TR/TE: 6450 ms/111 ms, 6450 ms/111 ms, matrix 290 × 520, 4mm thickness, 1 mm interlayer spacing), axial fat-T2WI TR TR/TE: 6550 ms/113 ms, 6550 ms/113 ms, matrix 330 × 520, 520 × 520, 4 mm interlayer thickness, 4 mm, 1 mm interlayer spacing, 1 mm, coronal FSE T2WI (TR/TE: TR/TE: 7730 ms/TE/ TE/12330 ms, 320 mm, 320 mm, 320 1 mm) (FIGURES 1A-1C).
Figure 1A-1C. Female patient after facial injection. Figures 1A–1C is axial T1WI, T2WI and fat-suppressing T2WI images respectively. There are multiple high signal flakes in the bilateral frontotemporal and lateral temporal muscles from subcutaneous soft tissue to the auricle, and fat-suppressing T2WI is more obvious (arrow).
Results
█ Signal manifestation of PAHG
PAHG injections have different shapes because of their different anatomical clearances and injection doses. They can show abnormal signal shadows such as flat, spindle, lump, strip and cone shapes. Most of them have clear boundaries, while a few show diffuse abnormal signal shadows with unclear boundaries. The hydrogel MRI signal is similar to that of water, the T1WI signal is slightly low, and the muscle signal is similar or slightly lower; the T2WI signal is high, and there is a thin low signal envelope around which a thin fibrous tissue membrane is formed after hydrogel injection.
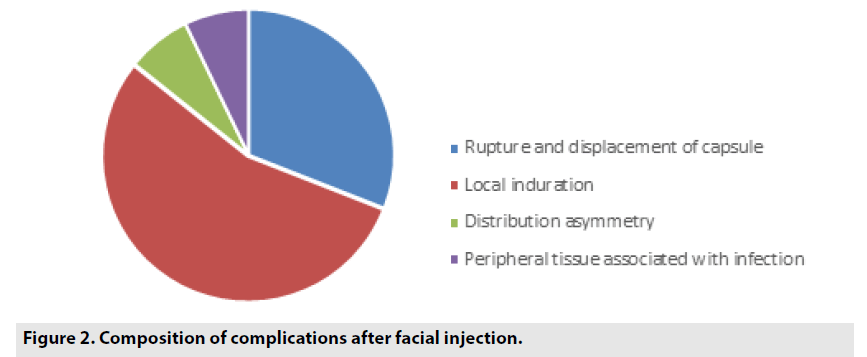
Common complications after PAHG facial injection in 42 cases are recorded in TABLE 1 and FIGURE 2).
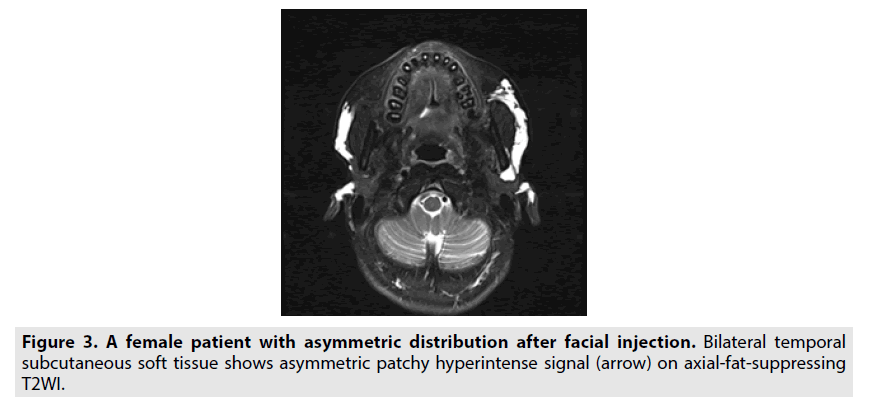
Asymmetric hydrogel distribution: An asymmetric distribution could be caused by a discrepancy in the levels of bilateral injection. Most of the cases were asymmetric due to hydrogel displacement, accounting for approximately 80% (FIGURE 3).
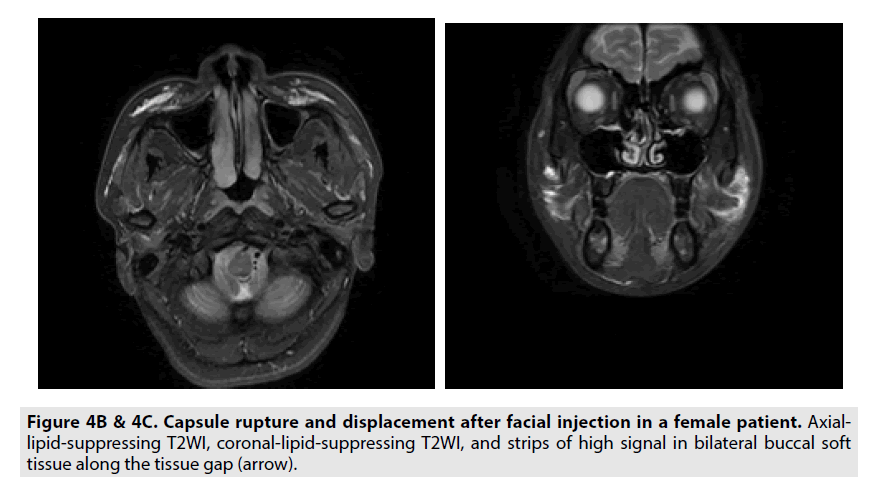
Rupture of hydrogel coating: In all cases, the capsule was ruptured to varying degrees. The aggregated capsule ruptured, revealing that the contour of the injection mass was relatively complete and the internal signal was not uniform. On FSE FS T2WI and STIR, multiple linear and low-signal segregations could be seen in the high signal mass. Free capsule rupture with hydrogel diffusion is characterized by irregular morphology, uneven edges with pseudopodal protrusions; FSE FS T2WI and STIR are characterized by high signal hydrogel diffusion to the surrounding area, forming multiple irregular mass images.
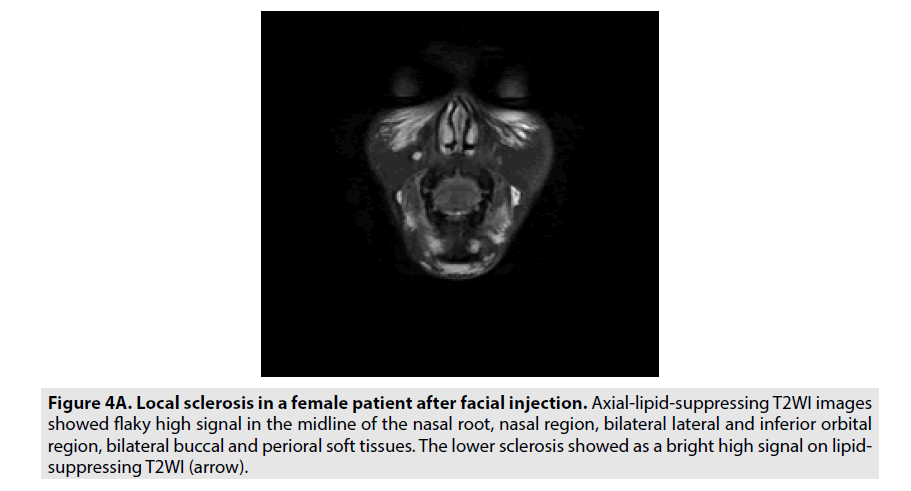
Local hardening of hydrogels: Round or quasi-circular hydrogel masses were scattered subcutaneously, glandularly, interstitially or intramuscularly. FSE FS T2WI sequence and STIR showed bright areas of high signal (FIGURE 4A).
Figure 4a. Local sclerosis in a female patient after facial injection. Axial-lipid-suppressing T2WI images showed flaky high signal in the midline of the nasal root, nasal region, bilateral lateral and inferior orbital region, bilateral buccal and perioral soft tissues. The lower sclerosis showed as a bright high signal on lipidsuppressing T2WI (arrow).
Hydrogel shift: Hydrogels migrated along the potential maxillofacial space, and sometimes migrated to the deep facial space or even to the glands (FIGURES 4B and 4C).
Complicated infection of peripheral tissues:
Irregular patchy T2WI sequences and STIR were mixed with bright areas of high signal around the abnormal hydrogel signal (FIGURES 5A and 5B).
Discussion
PAH is a colorless transparent gel polymer composed of 2.5–5.0% polyacrylamide and water. Although acrylamide monomer can be absorbed by the skin, respiratory tract and digestive tract, it is distributed in the kidney, liver, brain, spinal cord and sciatic nerve. It has neurotoxic effects in various animals, but its polymer has been proven to be a nondegradable, safe, non-toxic and biocompatible material [9]. Because of its advantages, such as reduced pain, reduced trauma, rapid effects and good hand feeling, it was quickly accepted by society. Since its introduction in 1997 in China, hydrogel has been widely used in breast augmentation, temporoplasty and buttock augmentation as a filling material for human soft tissue implantation. However, because it can decompose into highly-toxic monomeric molecules in the human body, hydrogel has been listed as a suspected carcinogen and has now been banned. At the same time, complications such as hydrogel diffusion, displacement, pain and infection have been found to occur after injection [10]. Consequently, regular follow-up observation of the performance of hydrogel in vivo on MRI and early detection of complications have gradually attracted the attention of the recipients.
█ PAHG MR signal characteristics
PAHG is a water-soluble material. A certain proportion of water is added before injecting into the human body, and the water content after swelling could reach over 97% [11]. As a result, its MRI signal is similar to that of water. PAHG exhibits hypointensity on T1WI and hyperintensity on T1WI in facial skin and adipose tissue in each space, making it easy to recognize. PAHG exhibits hyperintensity on T2WI, while adipose tissue produces a medium hyperintensity on T2WI, slightly lower than the PAHG signal, thus the two are not easy to distinguish. However, the PAHG signal intensity is low after the fat signal is suppressed, making it easier to distinguish. Thus, the contrast between PAHG and peripheral facial tissue on T2WI is the highest, giving the clearest results. In coronal T2WI images, sometimes the lipid suppression is incomplete and the lipid signal is still high, so that it is easily confused with the high signal of hydrogel. STIR sequences should be scanned in order to avoid misjudgment. At the same time, the application of the STIR sequence is better to investigate the presence of a small amount of hydrogel in the subcutaneous adipose layer. Jin Guang-wei et al., [12] carried out an MRI study after hydrogel augmentation mammoplasty, and considered FSE FS T2WI as the best sequence to observe hydrogel distribution. According to their observation of MR images, they believe that after PAHG facial injection, FS T2WI images are superior to T1WI images, and a T2WI image is superior to a T1WI image. Clinicians should therefore combine the comprehensive analysis of FS T2WI images and T2WI images. Meanwhile STIR sequences should be added to the examination of partial anatomic structures with inadequate fat inhibition and a small amount of hydrogels in the subcutaneous fat layer.
█ Complications after PAH injection
Hydrogel diffusion: It has been reported that a hydrogel capsule can rupture any time between 1 week and 3 years after injection [13], which is the most common complication. According to the types of hydrogel capsule rupture, it can be divided into aggregation type and free type [14]. (1) The aggregation type of capsule rupture refers to a situation where the injection mass contour is intact, which is massive or nodular. The clinical manifestations are sclerosis, hard feeling and no obvious tenderness. On MRI, the internal signal is not uniform, and multiple linear low-signal segregation scan be seen in FSE FS T2WI and STIR sequences. (2) Free capsule rupture refers to the distribution of injected material in the interstitial space in the form of dispersed masses, some of which even reach the muscles, glands or subcutaneous fat. The clinical manifestations are multiple diffuse subcutaneous or intramuscular sclerosis, accompanied by pain. The morphology of the injected material is irregular on MRI, and the high signal injections on FSE FS T2WI and STIR are patchy and nodular. Most of the capsule ruptures in this group were free ruptures.
Local hardening of hydrogels: There are many potential interstitial layers in facial anatomy. The liquid colloidal hydrogel can diffuse and translocate after injection due to pressure, muscle contraction, body position and other factors, and the morphology is unstable after injection.. Improper post-operative management can aggravate the spread of foreign bodies. Accidental inflammation of subcutaneous tissue caused by incorrect injection can cause sclerosis [15]. The formation mechanism of lumps or sclerosis has not yet been clarified. Some researchers believe that fillers stimulate fibroblast proliferation, capsule formation, and capsule contracture and will make fillers form spherical lumps or nodules, while in patients with different physiques, the severity of occurrence is also different [16]. Others believe that the uneven filling caused by inappropriate injection level, location or irregular injection method may be one of the main reasons for the formation of sclerotic lumps.
Hydrogel free shift: Lin et al., [17] found that displacement accounted for 7.6% of complications after hydrogel augmentation mammoplasty. Hydrogel migration was also the most common complication after facial injection. Guo [18] believed that the reasons for early hydrogel displacement were improper operation, incorrect post-operative management and improper protection of the recipients. The main reasons for late displacement might be related to the physical characteristics of the hydrogel itself (i.e., a certain degree of fluidity). Facial skin is thin and soft, subcutaneous tissue is loose, and there are many potential spaces within each anatomical structure. Honeycomb tissue fills each space, and blood vessels and nerves pass through. Some spaces contain salivary glands and lymph nodes. Honeycomb tissue is accompanied by blood vessels and nerve bundles, leading from one space to another, so that adjacent spaces communicate with each other. Therefore, PAHG injected into the face is prone to displacement under the action of gravity, masticatory muscles and facial expression muscles [19]. In addition, because hydrogel is directly injected into the body without any guidance, incorrect injection levels, tissue elasticity, gravity, posture, muscle contraction, and post-operative massage can cause hydrogel to diffuse and migrate along the superficial facial musculoaponeurotic system to the surrounding or even deep facial areas, resulting in unstable morphology after injection. Most of the free hydrogels are distributed in subcutaneous tissue, fat and muscle in the form of masses of different sizes. Some of them even reach the parapharyngeal space, mastoid process and parotid gland beyond the superficial space.
Hydrogel distribution asymmetry: Due to local tissue elasticity, density, pressure, external force and gravity and other factors, injection materials will diffuse and migrate into the surrounding tissue gap, leading to the redistribution of hydrogels.
Peripheral tissues associated with infection: Early infection may result from inadequate aseptic operation and bacterial contamination of fillings. Late infection may be related to decreased immunity [20]. Consequently, subcutaneous injection into the face should be avoided as far as possible, and intracutaneous injection of PAHG should be strictly prohibited. If the level of facial injection is too shallow, it will lead to poor hand feeling and differences in local skin color in the filled area, and even skin necrosis. Because the face is the predisposing site for acne and folliculitis, the injection level is too shallow, and it is easy to cause secondary infection [21]. If infection invades the brain, it can lead to bacterial meningitis or brain abscess. If pathogenic microorganisms and their metabolites enter the blood in large quantities, infectious shock will easily occur and even endanger life [22].
PAHG has caused irreversible damage to some patients. Once complications occur during hydrogel facial injection, foreign bodies cannot be removed. Even if complications occur only with small injection doses, cosmetic surgery can transform into deformity surgery because the face is exposed. If complications occur, it is not appropriate to squeeze to cause filling diffusion, which makes the treatment more difficult. In the past, multi-level and multi-site injection was used in some operations, but because PAHG is closely combined with surrounding tissues, it is difficult to remove it by aspiration alone, so at present a small incision under direct vision is mostly used. Surgery cannot completely remove the filler, so in some patients the risks of infection, sclerosis and displacement will remain after removal [23]. Surgical removal of PAHG should be the first choice after complications associated with PAHG injection cosmetology [24].
Conclusion
In summary, MRI has the advantages of nonradiation, high resolution of soft tissue and three-dimensional imaging. It can clearly display the shape and distribution of PAHG. MRI can clearly show the range and distribution of PAHG displacement, suggesting whether there is free PAHG in subcutaneous, muscular space or gland, and can determine the range of PAHG displacement. Consequently it is an ideal imaging method for evaluating the effect of PAHG after facial injection and ascertaining whether there are complications such as displacement and sclerosis. Therefore, after hydrogel injection, routine MRI examination should be performed and regular follow-up review should be carried out, so that complications such as hydrogel diffusion and displacement can be detected early, and timely treatment can be carried out.
Acknowledgement
The authors of the present study would like to thank the patients and their families for support and collaboration, and we thank International Science Editing (http://www. internationalscienceediting.com) for editing this manuscript.
References
- Ting WU, Xiao-min LUO, Wang BO et al. The systemic review of follow-up study of hydrophilic polyacrylamide gel injection for soft tissue filling. Chin. J. Pharmacovigil. 3: 93-96, (2006).
- Jian-min MO, Meng-Jun C, Bin Z et al. Application of medical polyacrylamide hydrogel in augmentation of buttock and lower extremit. Chin. J. Aest. Plast. Surg. 13: 100-102, (2002).
- Hua-Xin HU, Xue-Jun L, Hui Z et al. Clinical results of breast augmentaton with polyacrylamide hydrogel injection.Chin. J. Plast. Surg. 18: 18-83, (2002).
- Wen-li C, Liu Y. A case of fungal infection after augmentation mammoplasty with polyacrylamide hydrogel. Chin. J. Aesth. Med. 11: 116, (2002).
- Hui-Sheng L, Kai-Hua L, The complication of polyacrylamide gel injection for soft tissue filling and its treatment. Chin. J. Aesth Med. 11: 117-120, (2002).
- Tao L, Yu-Zhi X, Zheng D et al. MRI analysis of polyacrylamide hydrogel injection for facial plasty. Chin. J. Aesth. Med. 17: 963-965, (2008).
- Cai-Yun W, Zhi-Ting W, Xiao-Jun Z et al. Magnetic resonance imaging of patients with polyacrylamide hydrophilic gel injection for facial plasty. Chin. J. Med. Aesth. Cosmet. 18: 423-425, (2012).
- Li-Ying X, Xiang-Cai K, Yi-ming Z et al. Applications of magnetic resonance imaging in diagnosis of the complications after breast augmentation with polyacrylamide hydrogel injection. Chin. J. Plast. Surg. 20: 197-199, (2003).
- Cheng N, Xu S, Deng H et al. Migrationg of impants; a problem with ingectable polyacrylamide gel in aesthetic plastic surgery. Aesth. Plast. Surg. 30: 215-225, (2006).
- Li-An Z, Jie L, Ying W, et al. Complications and treatment of facial plastic surgery with medical polyacrylamide hydrogel. Chin. J. Med. Aesth. Cosmet. 9: 366-367, (2003).
- Li-An Z, Qin L, Ying W et al. Failure and treatment of facial plastic surgery with polyacrylamide hydrogel in 2 cases. J. Plast. Reconstr. Surg. l: 49-51, (2004).
- Guang-Wei J, Cong-Feng W, Xia L et al. Significance of MRI before surgery to remove polyacrylamide hydrogel used for augmentation mamma-plasty. Chin. J. Med. Aesth. Cosmet. 4: 233-235, (2008).
- Jin-Cai G, Yu-Rong W. MR imaging of rupture of breast implant and diffusion of injected hydrophilic polyacrylamide gel. Radiol. Pract. 19: 338-340, (2004).
- Ling F, Peng-Cheng L, Rong H et al. MR images of rupture and leakage of breast implants. Chin. J. Radiol. 36: 938-941, (2002).
- Kang SS, Zhang ZW, Chou HY et al. Complication of polyacrylamide hydrogel injection for facial plasty. Chin. J. Plast. Surg. 19: 325-327, (2003).
- Yu L, Wang J, Zhang B et al. Treatment of breast injection with polyacrylamide hydrogel with infiltrated fascia capsule removal: report on 104 cases. Aesth. Plast. Surg. 36: 1120-1127, (2012).
- Jun L, Yun-Liang Q, Qun Y et al. Clinical analysis of complications of polyacrylamide hydrogel injection for augmentation mammoplasty in 118 cases. Chin. J. Plast. Surg. 23: 101-102, (2007).
- Wei G. Polyacrylamide hydrogel injection for augmentation mammoplasty: Analysis of 16 cases of hydrogel distant migration. Chin. J. Misdiag. 8: 7761-7762, (2008).
- Chen Z, Jiang Y, Chang S et al. Treatment of buccal displacement after temporal augmentation with polyacrylamide hydrogel and analysis of its causes. Chin. J. Aesth. Plast. Surg. 14: 277-278, (2003).
- Feng XL, Yi CX, Peng C et al. Analysis and treatment complications induced by polyacrylamide hydrogel injection. Chin. J. Plast. Surg. 19: 331-333, (2003).
- Dong-Sheng X, Qi-Ming Z, Sheng YC et al. The application of polyacrylamide hydrogel (PAHG) in face-plasty. Chin. J. Aesth. Med. 10: 69-70, (2001).
- Xiao-Li W. Clinical study of self-made negative pressure drainage versus adjustable negative pressure drainage in the treatment of infection after temporal filling with PAHG. Chin. J. Aesth. Plast. Surg. 28: 670-672, (2017).
- Yin-Ke T. Complications of polyacrylamide hydrogel injection and its treatment. J. Clin. Surgery. 625: 453-455, (2017).
- He J, Liu X, Jianbo Y et al. Complications and treatment of breast augmentation with polyacrylamide hydrogel injection. Chin. J. Med. Aesth. Beauty. 20: 13-17, (2014).