Review Article - Imaging in Medicine (2011) Volume 3, Issue 1
Evolution of CT colonography
Stefanie Weinstein†1, Rizwan Aslam1 & Judy Yee11Veterans Affairs Medical Center, 4150 Clement Street (114), San Francisco, CA 94121, USA
- Corresponding Author:
- Stefanie Weinstein
Veterans Affairs Medical Center
4150 Clement Street (114)
San Francisco, CA 94121, USA
Tel: +1 415 750 2120
Fax: +1 415 750 6944
E-mail: Stefanie.Weinstein@va.gov
Abstract
Many public health organizations, including the American Cancer Society, recommend colorectal carcinoma screening for all average risk adults beginning at the age of 50 years. Colorectal cancers are known to develop from precursor adenomatous polyps that progress from small to large size and from dysplasia to cancer over the course of many years. Despite the potential for prevention, patient compliance is markedly suboptimal. Additional screening options could lead to improved detection rates for early discovery of polyps and cancers and thus lead to fewer cancer deaths. Many early studies have confirmed the ability of CT colonography to accurately detect polyps and colorectal cancers. This article will provide an overview of the current ‘state of the art’ of CT colonography, focusing on relevant recent research in the areas of colonic cleansing and distention, data acquisition, interpretation methods, validation and extracolonic findings. New guidelines, including the indications for CT colonography, are also discussed.
Keywords
2D/3D interpretation ▪ colonic cleansing ▪ colonic distention ▪ colorectal cancer screening ▪ computer-aided detection ▪ CT colonography ▪ extracolonic findings ▪ flat lesions
Colonic cleansing
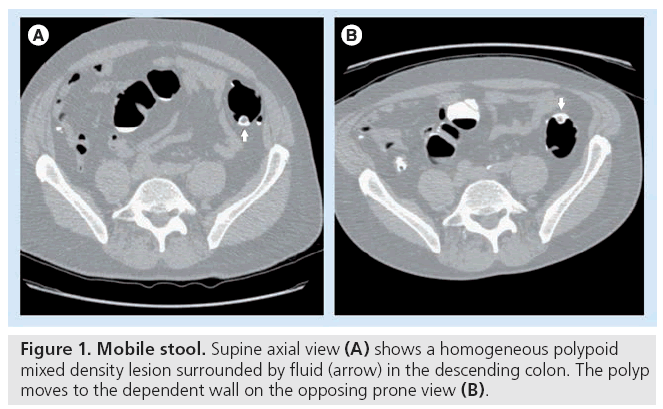
Adequate bowel preparation for CT colonography (CTC) is imperative. A well-cleansed colon is necessary to facilitate polyp detection and limit both false-positives and false-negatives by allowing differentiation of polyps from folds and residual stool that affect both 2D and 3D analyses [1]. Residual solid stool can mimic a true polyp (false-positive) or obscure polyps (false negative) and lengthen interpretation times (Figure 1) [2]. Oral intake should be restricted to clear liquids 24 h before the examination. The addition of a cathartic or a laxative promotes evacuation of colonic contents.
Laxatives: wet & dry
A myriad of colonic cleansing preparations are available that vary in cleaning ability, safety and patient tolerance [3]. The major preparations can be classified as either ‘dry’ or ‘wet’. The dry preparations include the saline cathartics and the wet preparations consist of various lavage solutions of polyethylene glycol (PEG) [4]. The saline cathartics, which include sodium phosphate (NaP) or magnesium citrate, are osmotic inorganic cathartic salts that are not absorbed, and remain in the lumen of the bowel, where they cause an increase in intraluminal fluid and stimulate peristalsis and subsequent evacuation. NaP is typically administered as a single dose of 45 ml. Some have advocated the use of a double dose (90 ml) of NaP, but a single dose has been shown to be just as effective [5]. Complications with NaP have been widely reported, such as electrolyte disturbances including hyperphosphatemia, hypocalcemia, hypernatremia, hypokalemia and acute phosphate nephropathy. Therefore, this cathartic preparation should be avoided in the setting of electrolyte abnormalities, renal failure, congestive heart failure, ascites and in combination with ACE inhibitors [6,7].
Magnesium citrate is less likely to result in pronounced electrolyte imbalances, although fluid intake should be maintained to prevent dehydration [8,9]. A recent study by Borden et al. comparing colonic cleaning and fluid retention for CTC with double-dose magnesium citrate and single dose NaP in 118 and 115 patients, respectively, showed excellent cleansing with 88.6 and 88.1% of respective groups having no significant residual stool. These results suggest that retained stool and fluid is similar with both types of saline cathartics. Magnesium citrate is preferred in patients at risk for potential electrolyte disturbances due to its inherent decreased risk of this complication compared with NaP [10].
Lavage preparations of PEG are generally considered to be wet preparations. Although PEG does not cause large fluid shifts, the preparation leaves considerable residual fluid in the colon (Figure 2). Residual fluid is less of a problem at optical colonoscopy because it can be suctioned at the time of the procedure. However, retained fluid pools can obscure significant portions of the bowel wall in CTC [11]. Typically, patients are required to ingest 4 l of PEG within 2–3 h on the day before the study. While PEG has been reported to be safer and therefore may be preferred in the elderly, it has the lowest adherence due to poor tolerance [12]. Patients have difficulty with compliance due to abdominal discomfort, bloating and nausea [13]. Adverse effects, including electrolyte disturbances, vomiting, allergic reactions and aspiration, are still reported but occur to a lesser degree [9]. Studies comparing the efficacy of oral NaP and PEG before undergoing colonoscopy have found no significant difference in the quality of bowel cleansing between these two agents [14–16]. A meta-analysis study by Belsey et al., identified 58 publications reporting adverse events in 131 patients following oral administration of either PEG (22 patients) or NaP (109 patients) [16].
Figure 2: Submerged peduculated polyp. Supine axial image (arrow in A) shows a peduculated polyp in the sigmoid colon. The polyp is submerged in fluid on the prone image (B). On abdominal windows, the polyp is soft tissue density (arrow in C) and is faintly visible submerged in the fluid (arrow in D).
Fecal & fluid tagging
Fecal tagging is achieved by ingestion of highdensity contrast agent(s). Residual solid or liquid colonic contents become homogeneously high in attenuation and, thus, can be differentiated from polyps of soft tissue density. Barium sulfate and/ or iodinated contrast is administered with meals 24–48 h prior to imaging. Different densities of barium-based tagging agents have been proposed and have been shown to be effective [17,18]. Fundamentally, barium labels the solid stool remaining in the colon and iodinated contrast material tags residual fluid pools. Iodine-based agents (either ionic or nonionic regimens), because they are hypertonic, also cause fluid shifts and, therefore, have an additional cathartic effect. A 2009 retrospective study using nonlaxative or minimum-laxative protocols found the homogeneity of tagged fecal matter more effective with better iodine-based regimens than with barium [19]. A large multicenter trial included fecal and fluid tagging (16 g of barium sulfate given 24 h before and 60 ml iodinated contrast given the evening before the CT scan) and patients underwent a full bowel-cleansing regimen with either PEG, magensium citrate or phospho-soda and bisacodyl tablets [20].
Minimal preparation & noncathartic CTC
There has been much recent investigation into ‘minimal preparation’ or ‘prepless’ CTC as a result of limited patient tolerance and poor patient compliance with full bowel cleansing regimes. Early studies examining polyp detection in the setting of tagging without cathartic preparation or with reduced cathartic preparation have reported favorable results [21,22]. In a 2008 study by Jensch et al., 40 patients were randomized to different cathartic levels (varying doses of biscadcoyl with and without magnesium citrate). There were no differences in image quality and patient acceptance rates were lower with the more aggressive regimens [23]. A recent study in 2010 by Keeling et al., evaluated limited-preparation CTC in 67 elderly patients (age range: 75–89 years) with limited functional status. Limited low-dose CTC yielded good to excellent image quality, which excluded relevant large lesions (masses and polyps > 1 cm), including any colonic cancers with the potential to obstruct or metastasize. While small cancers and polyps may have been missed in this study, these findings were considered to be less relevant in this patient population given the limited life expectancy [24]. Another study by Jensch et al. illustrated limitations of current noncathartic preparations. In this study, CTC with fecal tagging only was performed in patients at increased risk of colorectal cancer. There were more false-positives identified on noncathartic CTC compared with blinded colonoscopy (42 false-positives in 168 patients using a threshold of 6 mm). Per-patient sensitivities were not significantly different for CT colonoscopy and colonoscopy. Sensitivities and specificities of CTC for patients with lesions 6 mm or larger, and 10 mm or larger, were 76 and 82%, and 79 and 97%, respectively [22].
Another recent study, performed in 50 patients undergoing CTC with a taggingonly bowel preparation, found that a low fiber diet the day prior to the examination may lead to better tagging and decreased solid stool, although this does not appear to have an effect on polyp detection [25].
‘Nonconventional’ preparation CTC studies have typically involved mainly small cohorts and high-risk patient populations, and preparation protocols have varied widely. There are currently no standardized protocols for minimal bowel preparation or fecal tagging regimens that are universally accepted [26]. A recent study comparing 1-day versus 2-day iodine-based bowel preparation protocols found improved tolerance and comparable performance for the 1-day preparation [27]. In the future, minimum laxative CTC may be offered to select patient cohorts such as those with limited mobility or limited tolerance with the understanding that there may an increased number of false-positives.
Same day fecal tagging after incomplete colonoscopy has also been evaluated and compared with the standard colon cleansing prior to colonoscopy. Additional fecal tagging can reduce residual f luid and may improve distention. Although there may be increased fecal residue, the tagged nature helps differentiate stool residue from polyps [28]. There is no consensus or recommendation at this point on the optimum same day fecal tagging regime.
Electronic cleansing
New electronic subtraction algorithms are currently being explored. The concept of ‘electronic cleansing’ involves manipulating images so that high-density tagged material is removed or subtracted without compromising polyp detection. In practice, electronic subtraction is challenging due to heterogeneous fecal tagging and partial volume artifacts [29]. Electronic cleansing is limited by ‘oversubtraction’, which can occur where polyps are subtracted with the stool. There are mixed results in the literature as to whether electronic stool subtraction improves or decreases the sensitivity of CTC [30]. A recent study by Serlie et al., found that electronic cleansing led to shortened interpretation times, and found lesions uncovered by electronic cleansing to have comparable conspicuity with lesions surrounded by air, and allowed for easier identification and improved reader confidence [31]. These algorithms will continue to be implemented as lower volume cleansing techniques continue to advance, and there is further development of improved subtraction software. Structureanalysis cleansing is a new electronic cleansing technique that avoids cleansing artifacts. This technique uses local morphologic information to identify submerged polyps while removing tagged material with the air-tagging boundaries that otherwise can be a source of partial volume effects and cleansing artifacts [32].
Colonic cleansing is essential for a diagnostic CTC examination. Fecal and fluid tagging and electronic cleansing are used to distinguish retained solid or fluid material from colorectal polyps. Minimal preparation or ‘prepless’ CTC has the potential to improve patient compliance with CTC.
Colonic distention
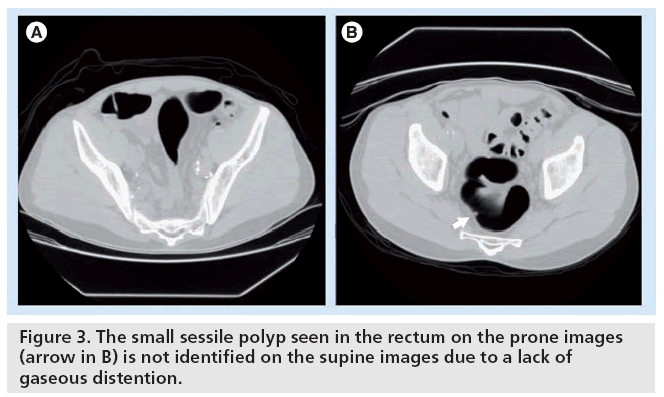
Poorly distended or collapsed segments may obscure polyps or mimic annular obstructing carcinomas. Decreased sensitivity and specificity have been reported with decompressed segments (Figure 3) [33,34]. A scout image should be performed prior to CT with the patient in the supine position to evaluate for adequate distention. Additional gas can be administered in supine and prone positions as needed. Scan acquisition is performed in the supine and prone positions to improve chances of distending the transverse and sigmoid colon and to redistribute remaining material/fluid and air. At our institution, we frequently perform an additional scan in the decubitus position to optimize distention of the sigmoid colon. Improved per-patient and per-polyp sensitivities have been reported with prone and supine imaging [33–35]. CO2 is preferred to air because it is absorbed through the colonic wall and exhaled via the lungs, and therefore has been better tolerated owing to decreased postprocedural gas and pain. Several studies have confirmed reduced abdominal pain in patients with the use of CO2 rather than air [36]. Current CTC technique favors use of an automated insufflation device for CO2 administration that results in improved colonic distention compared with manual inflation. The maximum pressure setting is set at a safe level (up to 25 mmHg) to minimize the risk of colonic perforation, and some have a mechanism for deflation if there is too much pressure [37]. The perforation risk with electronic CO2 insufflation is negligible in the screening population, compared with optical colonoscopy. The few reported perforations during CTC have involved manual insufflation [38].
Spasmolytic agents
The use of spasmolytic agents, such as glucagon or Buscopan®, has been controversial, although current CTC technique does not include spasmolytics for all patients. Based on data from barium enema studies, antispasm drugs have been reported to decrease patient cramping after the procedure [39]. Other studies, however, have found that glucagon does not significantly improve sensitivity for colorectal polyp detection and its use would contribute to increased cost with potential for harmful side effects [40]. Thus, there are insufficient data to recommend the routine use of spasmolytics, but they may be used in the setting of colonic spasm or abdominal discomfort that might restrict bowel insufflation [41]. Buscopan is not available in the USA, but has been reported to be effective in Europe.
Scan protocol
With the advent of spiral or helical CT in the early 1990s, CT scanning has become rapid enough to allow for thinner slices, shorter scan times and reduced radiation dose. Meta-analysis studies have demonstrated that CTC data acquired at 1-mm-thin sections have improved sensitivity or specificity for polyp detection in both phantom and human datasets. For example, Lui and colleagues evaluated various slice thicknesses at CTC, including a 1.25-mm section every 1 mm (thin) and 5-mm section every 2 mm (thick). They demonstrated improved specificity and decreased false-positive findings with thin sections, but there was no significant difference in polyp detection sensitivity between thin and thick sections [42–44]. At present, typical protocols use a collimation of 0.625–3.0 mm, ideally with scan times of less than 10 s, allowing for single breath hold [9]. A recent consensus by the 2009 American College of Radiology (ACR) practice guidelines recommended that CTC be performed using a multidetector row CT scanner with greater than or equal to four detector rows, slice thickness equal to or less than 3 mm, and reconstruction interval of less than or equal to 2 mm [41]. The same dataset may be used to evaluate for extracolonic pathology at 2.5- or 5-mm slice thickness [9].
Radiation dose
The effective radiation dose must be taken into consideration when determining a scan protocol. Radiation dose should be kept to a minimum, while maintaining diagnostic image quality. As CTC becomes widely available for colon cancer screening of the general population, there must be consideration of radiation exposure. Although thinner sections improve polyp detection, as slice thickness is reduced, the radiation dose is increased to maintain constant noise [45]. As noise increases, it becomes more difficult to differentiate stool from polyps. Attention to current (mAs), voltage (kVP) and automated tube current modulation is needed to keep radiation exposure as low as reasonably achievable. (ALARA principle) [12]. Due to the high tissue contrast between gas and colonic wall, reduction in mAs can be obtained without limiting polyp detection, at least for polyps larger than 10 mm in diameter. Studies have shown acceptable sensitivity for detection of polyps with low effective radiation doses. In a study by Macari et al., CTC was performed on 105 patients with a reduced radiation dose protocol consisting of 50 mAs, 120 kVp and 1.25-mm slice thickness with 1-mm reconstruction intervals. Sensitivity of 70% for 6–9 mm polyps and 93% for polyps over 10 mm were reported [46]. Van Gelder and colleagues looked at ultra-low-dose scans ranging from 0.05 to 12 mSv and found overall sensitivity for polyps 5 mm or larger to decrease at lower doses but remained at 74% for 0.2 mSv [47]. A recent study by Fisichella et al. examined 50 patients at high risk for colorectal cancer who underwent CT at standard dose, 40–160 mA, and low dose, 10–50 mA, followed by same day optical colonoscopy and looked at artifacts and the presence of polyps. It was found that reduction of the effective dose to 1 mSv significantly affected image quality on 3D images but the detection of lesions larger than 6 mm was not significantly compromised [48]. Use of automatic exposure control (which modulates tube current according to the z axis of the patient and the rotational angle of the CT gantry) can individualize dose; decreasing dose for small patients and increasing dose for larger patients. The CT protocol may also vary depending on the clinical indication. For example, while low-dose CTC is recommended for screening purposes, a diagnostic examination in a symptomatic patient may require the use of routine dose and intravenous contrast. Higher radiation dose exposure may be required with obese patients or if additional scanning is required in a decubitus position. Liedenbaum and colleagues evaluated 34 research institutions performing CTC and found median effective dose per institution for screening CTC of 5.7 mSv compared with 9.1 mSv for daily routine CT examinations. They found no difference in effective dose with different numbers of detector rows but noted the trend towards increased use of dose modulation [49]. With current dose ranges for screening CTC ranging closer to 5 mSv, Brenner and Georgsson concluded that the benefit/risk ratio was high for CTC. They estimated the potential absolute lifetime risk of radiation-induced cancer risk for a single CTC examination at 50 years to be approximately 0.14% (previous estimates) or approximately 1 in 700. This potentially could be further reduced by a factor of 5–10 with optimized low-dose protocols depending upon the assumptions underlying the estimates of the radiation risk models [47,50–52].
Most estimates of radiation-induced cancer risk come from the study of Japanese atomic bomb survivors, in the ‘Life Span Study’, which evaluated long-term follow-up of the effects of radiation exposure. Patients were exposed to a wide range of radiation doses in the range of 40 mSv [53]. Analysis of cancer risk is based on the use of a linear no-threshold assumption with low doses only estimated by extrapolating models based on the results from the exposures of these patients. The Biological Effect of Ionizing Radiation VII report extrapolating radiation risk from data from atomic bomb survivors, states that at doses less than 1000 mSv, ‘statistical limitations make it difficult to evaluate cancer risk in humans’ [54]. An accurate assessment of cancer risk is difficult given that the radiation dose received at CTC is considerably smaller than has been implicated in radiation exposure studies and is not a whole-body exposure as assumed in the atomic bomb survivor cohort.
Since CTC involves the use of ionizing radiation, the effective radiation dose must be monitored and kept to a minimum, while still maintaining diagnostic image quality. An accurate cancer risk is difficult to calculate based on estimates from prior radiation-induced cancer risk studies.
Data interpretation
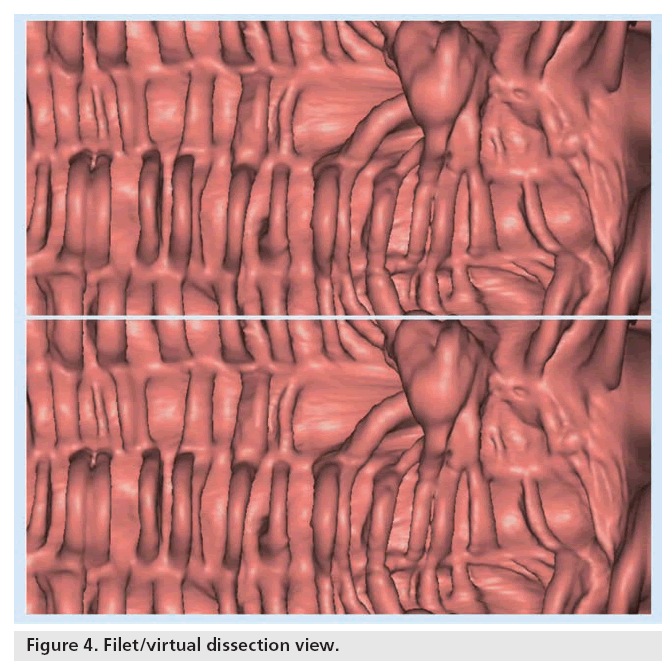
The initial scan data from a CTC examination consists of a large number of images that need to be interpreted efficiently. Both 2D and 3D techniques are used concomitantly with the choice of primary interpretation based on personal preference and individual accuracy. Typically, one display technique is used as the primary interpretation method and the other used for problem solving, although both are considered complementary reading tools. Polyps appear as ovoid or rounded, homogeneous soft tissue densities that are typically fixed in location. By contrast, residual stool is heterogeneous in density and mobile. Interpretation of CTC studies with tagging requires a modification of traditional reading methods. Wide bone windows are needed to help find polyps hidden in the denser fluid and stool [55]. New techniques being explored include the ‘filet view’ or ‘virtual dissection’ view where the colon is cut open along a centerline along the long axis to lay flat and ‘sliced’ open for display (Figure 4) [56]. Additional training is needed as skill sets are different from conventional CTC interpretation. New 3D software is available that offers a translucency view or color map overlay based on lesion density to distinguish tagged stool from soft tissue density of polyps [57]. Other software can track portions of colonic wall by painting examined surfaces a different color so that the reader can review any missed areas. There are multiple commercial workstations with US FDAapproved software targeted at CTC analysis.
Some are thin-client web-based solutions, while others are standalone workstations or integrated into picture archiving and communication system (PACS), which vary in ease of use and capabilities. All should allow for multiplanar reformations and 3D endoluminal viewing with the option of new features, including panoramic views, virtual dissection, translucency rendering and wide-angle views, which may improve polyp detection and improve reader accuracy and efficiency. Novel displays and techniques include an unfolded cube display and a display that uses the location of the tenia coli to coregister the supine and prone scans [12,58,59].
Training
When learning how to interpret a CTC study, a radiologist should ideally perform the primary interpretation at a workstation, on endoscopically confirmed cases, and receive feedback on performance. Large multicenter trials have varied widely in reader skills. The American College of Radiology Practice Guidelines recommends that readers review at least 45–50 cases [60,61]. Readers in the National CT Colonography Trial were required to have read 500 CTC studies and undergo a qualifying examination of 20 cases. Mentorship or assistance in cases is encouraged as less experienced readers may miss lesions. A second reader, or use of a computer-aided design (CAD) system, may help readers reconsider a possible lesion and therefore increase accuracy and sensitivity [62]. Training should not only include interpretation skills, but also provide additional teaching on the indications, contraindications, methods of patient preparation and scan protocol. There should also be participation in a quality assurance program [63]. Radiologists can attend various training courses through professional societies, university programs and the ACR training facility. More trained readers will be needed to accommodate the potential demand when CTC becomes widely implemented as a screening test.
CT colonography readers should be welltrained in both 2D and 3D interpretation. Accuracy and efficiency increase with training and experience.
Computer-aided detection
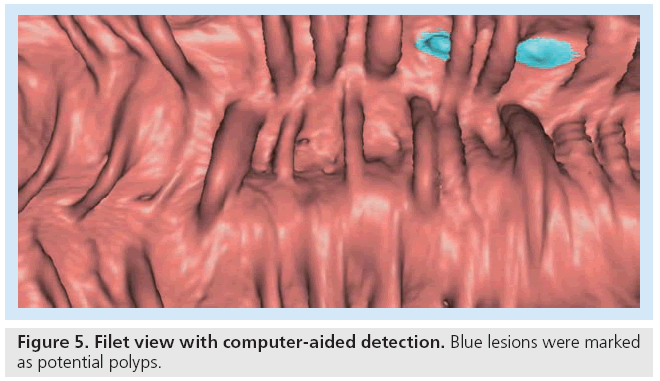
Computer-aided detection, introduced in the late 1990s, has the potential to play an important role in improving the diagnostic ability of CTC and decreasing reader variability [64]. Radiologists can use CAD as either a first, concurrent or second reader. Using CAD as a first reader has not been widely accepted. In the concurrent reader paradigm, CAD markers are visible during the radiologist interpretation. While this may reduce interpretation time and increase sensitivity, a drawback is that the CAD markers may become a distraction. In the second reader option, the radiologist reviews his or her findings separately from the CAD and then revises the final interpretation. The radiologist has the final decision to accept or reject any lesion. Sensitivity is likely to be increased, but interpretation times may be longer with this format and could reduce overall specificity [65,66]. Experts recommend the use of CAD as a ‘second reader’ after initial interpretation without CAD (Figure 5) [62]. An appropriate CAD system should have a high sensitivity for detection of clinically significant polyps (above a size threshold) and low false-positive detection rate and should only add minimal extra reading time. Common falsepositives include the ileocecal valve, thickened folds, residual fecal matter and the rectal tube that can mostly be avoided by correlation with the 2D images [64]. False negatives are due to flat or small polyps, poor distention, adherent contrast material and residual fecal matter, which can be reduced by various techniques, including fecal and fluid tagging, software correction and fluid subtraction [64]. Several large studies are under way to prove the benefit of CAD and support its reimbursement. In standalone trials, Summers et al. found sensitivities for CAD of 89.3% for identifying adenomatous polyps at least 1 cm in size. For 8- and 10-mm adenoma size thresholds, sensitivities of CAD were not significantly different from optical colonoscopy [67]. Van Ravenstein et al., looked at CAD sensitivities for polyps 6 mm or larger, with results ranging from 85 to 100% and found their CAD system generalizable to data from different medical centers and different patient populations [68]. Other groups have looked at sensitivity of different CAD systems in colonic phantoms and found similar outcomes [69]. Integration of CAD to CTC software and hardware platforms will increase confidence and perhaps accuracy of interpretation, particularly for new readers. Larger trials evaluating CAD in the clinical settings are required to prove that CAD improves sensitivity without time penalty. In addition, the issues of appropriate training, cost efficiency and standardization need to be addressed. iCAD, Inc. has recently received FDA approval for its VeraLook CAD.
Computer-aided detection may assist in CTC sensitivity and reduce reader interpretation time.
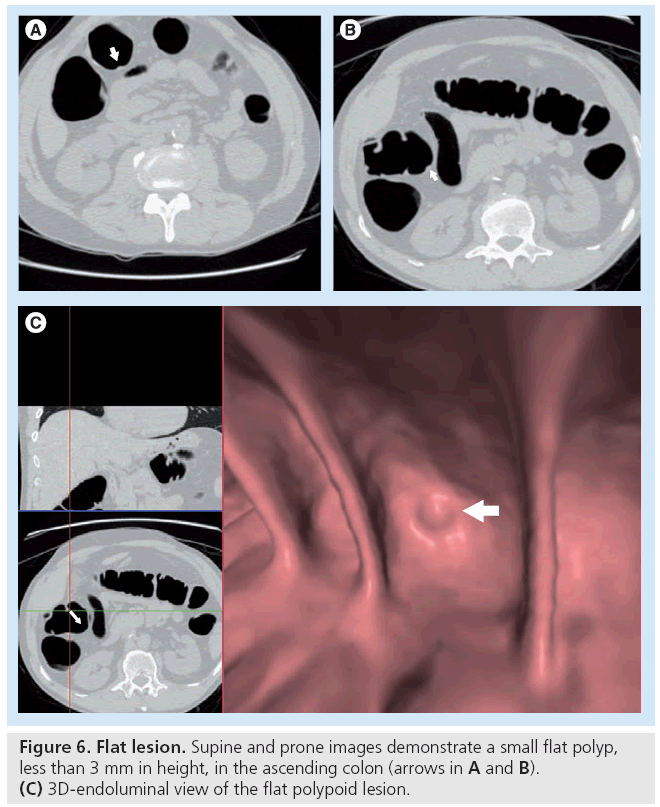
Flat lesions
The significance of flat or nonpolypoid lesions is debatable. Polypoid lesions include sessile polyps, which have a broad base, and pedunculated polyps that have a head and stalk. These account for most advanced adenomas and cancers [70]. The true prevalence and potential clinical significance of ‘flat’ or ‘nonpolypoid’ colorectal lesions in the US screening population is controversial [71]. The lack of standardized definitions, variations in study population and differences in approaches to detection contribute to the disagreement. The term ‘flat’ lesion is misleading as these lesions are usually superficially elevated with a small minority containing a central depression, whereas completely flat lesions are exceedingly rare. Flat lesions are considered less conspicuous than polypoid lesions of a similar size on both CTC and optical colonoscopy, but may be detectable with appropriate techniques and awareness (Figure 6). Phantom and clinical studies, however, have shown reasonable sensitivity at CTC with the use of oral contrast and combined 3D and 2D reading methods. One study found sensitivities of 80% for flat lesions 6 mm or greater in an asymptomatic screening population using fecal and fluid tagging and primary 3D review. By contrast, a smaller study, without fecal tagging and primary 2D review, detected less than 50% of flat lesions but only ten patients were evaluated [72]. Potential clinical relevance varies in the literature, with some reporting less aggressive and often non-neoplastic histology in flat versus polypoid lesions [73]. In a recent study from Pickhardt et al., a prospective screening study evaluating 5107 asymptomatic patients found flat lesions, using 3 mm as the maximum threshold, to have less aggressive histology features [74]. Flat lesions appeared to be more likely to have hyperplastic histology [74–77]. Unlike the situation in Asia, there is no substantial evidence that small, flat lesions are a common problem in the West [71]. With technical advances, the ability to detect flat lesions has improved as well. Therefore, while flat lesions remain challenging, they should not represent a major drawback to widespread screening [72–78]. A nonpolypoid subtype, the carpet lesion, is a laterally or superficially spreading tumor. Carpet lesions can be subtle, and although they have a lower rate of malignancy, they may have villous features and dysplasia [12]. More work will be needed in the areas of these lesions where size alone may not be the main determinant of risk of neoplasia.
The clinical significance and prevalence of flat lesion is controversial. CTC has shown reasonable sensitivities in detecting flat lesions with optimized scanning techniques.
Extracolonic findings
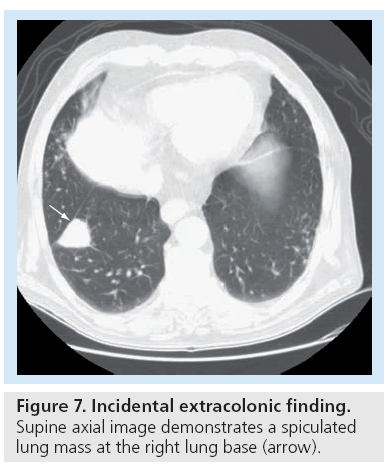
CT colonography can detect extracolonic lesions that range from benign to more significant findings, including cancers outside of the colon at an early or asymptomatic stage. Most incidental findings are benign and determined to be clinically inconsequential, with further work-up leading to additional healthcare cost, patient anxiety, and potentially increased patient morbidity and exposure to unnecessary radiation. Some findings, however, can lead to an earlier diagnosis of clinically significant disease that could result in decreased morbidity and mortality as well as long-term cost savings. The detection of extracolonic abnormalities may become limited with lower dose CT technique due to increased image noise. New iterative reconstruction or denoising CT image reconstruction filters promise to improve the image quality of low-dose scans [78]. The incidence of ‘significant’ extracolonic findings has been shown to be higher with the use of IV contrast [79]. Studies performed in asymptomatic screening patients have found extracolonic findings in 41–69% of patients, although the majority of findings are insignificant. It is estimated that 9–14% of patients, however, will have significant extracolonic findings, of whom, 1–2% will need intervention. Xiong and colleagues performed a meta-analysis of primary studies of extracolonic findings with 3488 patients and found extracolonic lesions in 2015, 188 undergoing further diagnostic work-up and 0.8% requiring immediate treatment. Extracolonic malignancies were identified in 2.7% with almost half identified at the early stage [80]. Several studies have evaluated the cost of work-up of incidental findings and largely showed that there was an additional cost ranging from US$12 to $36 per CTC study [78]. Although most incidental findings are benign, 1–3% are more significant, requiring surgery with an expectant cost increase. Most significant extracolonic findings are usually malignancies or large aortic aneurysms (Figure 7) [81]. In a recent study by Pickhardt et al., unsuspected cancer was detected in 0.56% of an asymptomatic population of greater than 10,000 patients undergoing screening CTC at two centers. Invasive colorectal cancer was found in 22 patients (0.21%) and extracolonic cancer in 36 patients (0.35%). The detection rate of unsuspected cancer is 1 per 200 asymptomatic adults, including one invasive colorectal carcinoma per 500 cases and one extracolonic cancer per 300 cases. Thus, detection of asymptomatic, early-stage and localized extracolonic cancer represents an additional benefit of screening CTC [82].
Figure 7: Incidental extracolonic finding. Supine axial image demonstrates a spiculated lung mass at the right lung base (arrow).
The majority of incidental extracolonic findings identified on CTC are benign, although occasional clinically important lesions may be detected at earlier and curable stages.
Validation studies
Single-center studies, multicenter trials and meta-analyses have demonstrated excellent performance of CTC using colonoscopy as the reference standard. Early trials that were mostly single-center studies showed mixed results using a wide range of cohorts, including symptomatic, surveillance and screening populations [83–86]. Per-polyp sensitivity for medium-sized polyps (6–9 mm) and large polyps (greater than 10 mm) ranged from 47 to 82% and 75 to 93%, respectively. Per-patient sensitivity ranged from 76 to 93% and 84 to 100%. Cotton and colleagues looked at 600 patients with symptoms of possible colorectal cancer or a history of polyps and found low sensitivity [87]. A study by Rockey et al., also showed disappointing results [88]. However, these studies did not use high spatial resolution multidetector CT techniques, primary 3D interpretation or tagging [55]. Doshi and colleagues reviewed the false-negative interpretations from the Cotton et al. study and found significantly improved per-polyp and per-patient sensitivities, suggesting the initial results may have been due to insufficient training and expertise.
Subsequent studies have demonstrated improved sensitivities for polyp detection with more consistent excellent results. These studies stress the importance of rigorous techniques and extensive training [89]. Pickhardt et al. evaluated asymptomatic adults and showed that CTC could achieve comparable performance with optical colonoscopy [90]. CTC had similar sensitivity to colonoscopy of 94% for polyps 10 mm and larger, compared with sensitivity of 88.7% for polyps 6 mm and larger. The American College of Radiology Imaging Network (ACRIN) National CTC Trial, the largest multicenter trial evaluating CTC performance, involved 2531 asymptomatic subjects from 15 sites, and demonstrated per-patient sensitivity and specificity of 90 and 86%, respectively, for adenomatous colorectal lesions over 1 cm. All readers had previously interpreted 500 CTC cases as experience or attended a 1.5-day course and taken a certification examination. Unlike most prior studies, fluid and fecal tagging, colonic distention with CO2, multidetector CT with 2D and 3D detection on dedicated software systems were included [9,20]. Since this landmark study, several other large screening trials in the USA and Europe have reported similar results, including a study from the Mayo Clinic [91] and a large Italian Multicenter Polyp Accordance CTC Study (IMPACT). The Italian study demonstrated per patient sensitivity of 90.7% for adenomas larger than 10 mm, and 84.2% for adenomas larger than 6 mm. The prevalence of advanced neoplasia was higher than in other studies (19% of all lesions 6 mm or larger), although this was not unexpected as they evaluated a population of patients at above average risk of developing colorectal cancer [63,92]. In addition, Graser et al., in a Munich study, reported sensitivities of 92% for polyps greater than or equal to 10 mm and 97% for advanced neoplasia [93]. Several meta-analyses have also demonstrated CTC’s ability to detect clinically significant polyps. Halligan and colleagues performed a meta-analysis that included 24 studies with a total of 2610 patients. Sensitivity and specificity for 10 mm or larger lesions were 93 and 97%, respectively, which decreased to 86 and 96% for 6–9 mm polyps [94]. Mullhal and colleagues evaluated 33 studies, which included data on 6393 patients. Pooled sensitivity for lesions measuring 10 mm or larger, 6–9 and 5 mm or smaller were 85, 70 and 48%, respectively, and specificities were 97, 93 and 92%, respectively [95]. A large clinical outcomes study in 2007 compared CTC and optical colonography (OC) screening programs and showed the advantages of using CTC. Over 6000 asymptomatic patients underwent either primary screening CTC or primary screening OC at the University of Wisconsin, WI, USA, comparing the relative yield of each screening approach for the detection of advanced neoplasia. The number of cases of advanced neoplasia identified was similar in both screening arms (123 with CTC vs 121 with colonoscopy). However, CTC resulted in fewer total polypectomies overall. An additional seven patients in the OC group had perforations, whereas there were none in the CTC group. Results support the use of CTC as a primary colorectal screening tool and to select those who would benefit from therapeutic colonoscopy [96].
Indications
Colon cancer screening beginning at 50 years of age in asymptomatic average-risk patients has resulted in a decrease in morbidity and mortality associated with colorectal cancer [97]. Despite the potential for prevention, only 42% compliance is acheived compared with 67–85% for breast cancer for screening for women over 40 years of age [201]. In 2008, new joint guidelines were issued by the American Cancer Society, the US Multi- Society Task Force and the American College of Radiology for Colorectal Cancer Screening. These guidelines included CTC as a valid screening option for detection of adenomatous polyps and colorectal cancer in the average risk population [98]. A recent multicenter study by Regge et al. included 937 patients with risk factors for colorectal cancer who underwent CTC and found a 85% sensitivity and 88%specificity for detecting advanced neoplasia 6 mm or larger. CT could therefore be used as a tool for surveillance of patients at increased risk for colorectal cancer, although patients would still have to undergo optical colonoscopy for biopsy or polypectomy [92]. CTC is also indicated for surveillance of new colorectal polyps following polypectomy or after prior CTC when a polyp smaller than 1 cm has been identified and is being followed radiographically. Other important indications for CTC are in patients who have had incomplete OC or patients at increased risk for complications during colonoscopy, including those on anticoagulation therapy, those with pulmonary disease and those who are a sedation risk [12,99].
Target polyp size
The size threshold used for referral of patients for polypectomy determines overall CTC efficacy and affects the economics and cost–effectiveness of CTC. As a primary screening tool, a major issue for CTC will be the polyp size threshold chosen to trigger colonoscopy. A small polyp threshold size of 6 mm will trigger a larger number of colonoscopies compared with a larger polyp size of 10 mm or greater. According to the ACRIN trial, 12% of patients would have been referred for colonoscopy if threshold was 6 mm, and 4% of patients with lesions 10 mm or larger [20]. The screening target for the prevention of colorectal cancer is the advanced adenoma, defined as an adenoma with a substantial villous component (>25%), size greater than 10 mm, or with high-grade dysplasia or early invasive cancer [100]. While the accuracy of CTC for large polyps is high, the performance for smaller 6–9 mm polyps is more variable in the range of 50 to 95%. Management of intermediate polyps (between 6–9 mm) is therefore controversial as to whether the benefits of polypectomy outweigh the risk and cost associated with colonoscopy. A small percentage of 6–9 mm adenomas, however, will show advanced histology with a range of 2.7 to 5.3% [101,102]. Cost–effectiveness studies may vary depending on the prevalence of one or two advanced adenomas in this size range; therefore, 3-year CTC surveillance or polypectomy is recommended (C2 using the C-RADS classification). Based on existing data for the risk of future advanced neoplasia in multiple adenomas on colonoscopy, patients with three or more small polyps should be referred for polypectomy [103].
While prevalence of diminutive polyps (5 mm or smaller) is higher than for 6–9 mm polyps, the prevalence of advanced neoplasia is quite lower within a screening population [104]. Data from longitudinal endoscopic and barium studies reveals slow growth of these colorectal polyps.
Few data exist for the performance of CTC in detecting lesions 5 mm or less, as most large CTC trials do not report diminutive lesions. Even at OC, the gastrointestinal experts feel that the cost and risk of polypectomy and pathologic assessment outweigh the low yield of detecting an advanced adenoma. Rather, these lesions are more likely to be hyperplastic polyps, tubular adenomas or pseudolesions and should not be referred for colonoscopy or polypectomy. Presumably, an interval of 5 years’ surveillance would allow a polyp to grow to a more relevant size if it truly has aggressive histology. Furthermore, even if diminutive polyps are more aggressively managed, the attempted matching with OC can be difficult [102]. CTC can be used to follow these small unresected colorectal lesions given its excellent polyp measurement capabilities and good accuracy and reproducibility for size measurement [105]. A study with no polyps of 6 mm or larger is therefore considered a negative study according to the CT Colonography Reporting and Data System (CRADS) [106]. Future studies that define the natural history of 1–9 mm polyps on CTC should provide more data to support noninvasive follow-up of small and diminutive polyps [107]. Leaving small polyps in place, and instead following with serial examinations, is a departure from current screening paradigms that have stressed a ‘no polyp left behind’ policy. Cumulative costs, procedural risks and radiation exposure issues have to be considered.
Polyps less than 6 mm in size are more likely to be of benign histology, and therefore aggressive management of these lesions would not be cost effective.
Safety/complications
The US Preventive Services Task Force and Medicare have raised concerns about the potential harm of CTC, including perforation rates, detection and work-up of extracolonic findings and risk of radiation-related cancer [51,108,202]. Reported complications are usually related to the risk associated with bowel preparation, similar to that of optical colonoscopy. Colonic perforation during CTC typically occurs in patients with known colonic pathology. Obstructive lesions, hernias, active inflammatory bowel disease, recent biopsy or recent polypectomy have all been found to be associated with perforation [52]. There have been reports, however, of colonic perforation in patients without known colonic disease [109,110]. A large multicenter study of over 21,000 examinations, including screening and diagnostic examinations, found the risk of perforation was exceedingly low with symptomatic perforation rate of 0.005% and a significant complication rate of 0.18% [111]. Two patients developed renal failure related to the bowel preparation and one had chest pain. Most ‘perforations’ typically occur as asymptomatic extraluminal gas that would not have been detected on colonoscopy [52]. Since CTC detects tiny amounts of free air that would otherwise not be detected by an alternative examination, the symptomatic perforation rate should be the relevant rate evaluated, which has ranged from 0.005 to 0.03%. By contrast, colonic perforation rates range from 0.06 to 0.19% and up to 0.2% for therapeutic colonoscopy, higher than for CTC [112]. Some have suggested a wait time of 5 days to 2 weeks between incomplete colonoscopy and CTC after deep biopsy or polypectomy owing to increased risk of perforation due to a small defect in the colon mucosa from polypectomy [113].
Conclusion
CT colonography has the potential to improve colon cancer screening compliance and, thus, it is poised to have a significant impact on the prevention of colorectal cancer. CTC is comparable with OC when state-of-the-art techniques are applied. Fluid and fecal tagging has allowed for easier discrimination of stool from polyps and the use of CO2 allows for reliable distention. The previous mindset of universal polypectomy for any polyp is changing due to the low yield of identifying malignancy or premalignancy in diminutive polyps and costs and complications of unnecessary referral to colonoscopy and polypectomy.
The prerequisites for a CTC examination include a multidetector scanner, postprocessing hardware and software, training for both radiologists and staff, and establishment of an ongoing screening and follow-up program. There needs to be a consensus on reporting of extracolonic findings, with attention to cost containment. Current CTC technique uses low radiation dose protocols to ensure minimal or no risk to patients. Favorable coverage and reimbursement decisions will allow more rapid dissemination of CTC as a primary screening tool.
Future perspective
Full integration of CAD into CTC software and hardware platforms is already underway. iCAD, Inc. has recently received FDA approval for its VeraLook computer-aided detection. Nonlaxative PET and CTC fusion may be potential areas of development to aid in polyp and cancer detection. In addition, there are likely to be further developments in modalities that do not require radiation, such as MR colonography, particularly in patients who require more frequent colon evaluation.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- Yee J: CT colonography: examination prerequisites. Abdominal Imaging 27(3), 244–252 (2002).
- Fletcher JG, Johnson CD, Welch TJ et al.: Optimization of CT colonography technique: prospective trial in 180 patients. Radiology 216(3), 704–711 (2000).
- Delagge M, Kaplan R: Efficacy of bowel preparation with the use of a prepackaged, low fiber diet with a low sodium, magnesium citrate cathartic vs. a clear liquid diet with a standard sodium phosphate cathartic. Aliment Pharmacol. Ther. 21, 1491 (2005).
- Gelfand DW, Chen MYM, Ott DJ: Preparing the colon for the barium enema examination. Radiology 178, 609–613 (1991).
- Kim DH, Pickhardt PJ, Hinshaw JL et al.: Prospective blinded trial comparing 45ml and 90ml doses of oral sodium phosphate for bowel preparation before computed tomographic colonography. J. Comput. Assist. Tomogr. 31, 53 (2007).
- Ehrenpresis ED, Nogueras JJ, Botoman VA et al.: Serum electrolyte abnormalities secondary to Fleet’s phospho-soda colonoscopy prep. Surg. Endosc. 10, 1022–1024 (1996).
- Vukasin P, Weston LA, Beart RW: Oral Fleet phospho-soda laxative induced hyperphosphatemia and hypocalcemic tetany in an adult. Dis. Colon Rectum 40, 497–499 (1997).
- Wiberg JJ, Turner GC, Nutall FQ: Effect of phosphate or magnesium cathartics on serum calcium. Arch. Intern. Med. 138, 1114–1116 (1978).
- Yee J: CT colonography: techniques and applications. Radiol. Clin. North Am. 47, 133 (2009).
- Borden ZS, Pickhardt PJ, Kim DH et al.: Bowel preparation for CT colonography: blinded comparison of magnesium citrate and sodium phosphate for catharsis. Radiology 254, 138–144 (2010).
- Macari M, Lavelle M, Pedrosa I et al.: Effect of different bowel preparations on residual fluid at CT colonography. Radiology 218, 274 (2001).
- Poullos Peter D, Beaulieu CF: Current techniques in the performance, interpretation and reporting of CT colonography. Gastrointest. Endoscopy Clin. N. Am. 20, 169–192 (2010).
- Frommer D: Cleaning ability and tolerance of three bowel preparations for colonoscopy. Dis. Colon Rectum 40(1), 100–104 (1997).
- Golub RW, Kerner BA, Wise WE et al.: Colonoscopic bowel preparations – which one? A blinded, prospective, randomized trial. Dis. Colon Rectum 38, 594–599 (1995).
- Marshall JB, Pineda JJ, Barthel JS et al.: Prospective, randomized trial comparing a new sodium phosphate solution with polyethylene glycol-electrolyte lavage for colonoscopy preparation. Gastrointest. Endosc. 39, 631–634 (1993).
- Belsey J, Epstein O, Heresbach D: Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment. Pharmacol. Ther. 29, 15–28 (2008).
- Taylor SA, Slater A, Burling DN et al.: CT colonography: optimization, diagnostic performance and patient acceptability of reduced-laxative regimens using barium based faecal tagging. Eur. Radiol. 18, 32 (2008).
- Lefere PA, Gryspeerdt SS, Dewyspelaere J et al.: Dietary fecal tagging as a cleansing method before CT colonography: initial results polyp detection and patient acceptance. Radiology 224, 393 (2002).
- Nagata K, Singh AH, Sangwaiya MJ et al.: Comparative evaluation of the fecal-tagging quality in CT colonography: barium vs. iodinated oral contrast agent. Acad. Radiol. 16, 1393–1399 (2009).
- Johnson CD, Chen M-H, Toledano AY et al.: Accuracy of CT colonography for detection of large adenomas and cancers. N. Engl. J. Med. 359, 1207–1217 (2008).
- Lefere P, Gryspeerdt S, Marrannes J et al.: CT colonography after fecal tagging with a reduced cathartic cleansing and a reduced volume of barium. AJR Am. J. Roentgenol. 184, 1836–1842 (2005).
- Jensch S, deVries AH, Peinga J et al.: CT colonography with limited bowel preparation: performance characteristics in an increasedrisk population. Radiology 247, 12–32 (2008).
- Jensch S, de Vries AH, Pot D et al.: Image quality and patient acceptance of four regimens with different amounts of mild laxatives for CT colonography. Am. J. Roentgenol. 191, 158 (2008).
- Keeling AN, Slattery MM, Leong S et al.: Limited-preparation CT colonography in frail elderly patients: a feasibility study. AJR Am. J. Roentgenol. 194, 1279–1287 (2010).
- Liedenbaum MH, Denters MJ, de Vries AH et al.: Low-fiber diet in limited bowel preparation for CT colonography: influence on image quality and patient acceptance. AJR Am. J. Roentgenol. 195, W31–W37 (2010).
- Johnson CD, Chen M-H, Toledano AY et al.: Accuracy of CT colonography for detection of large adenomas and cancers. N. Engl. J. Med. 359, 1207–1217 (2008).
- Liedenbaum MH, de Vries AH, Gouw CIBF et al.: CT Colonography with minimal bowel preparation: evaluation of tagging quality, patient acceptance and diagnostic accuracy in two iodine-base preparation schemes. Eur. Radiol. 20, 367–376 (2010).
- Gryspeerdt S, Lefere P, Herman M et al.: CT colonography with fecal tagging after incomplete colonoscopy. Eur. Radiol. 15, 1192 (2005).
- Lefere P, Gryspeerdt S, Marrannes J et al.: CT colonography after fecal tagging with a reduced cathartic cleansing and a reduced volume of barium. AJR Am. J. Roentgenol. 184, 1836–1842 (2005).
- Zalis ME, Perumpillichira JJ, Magee C et al.: Tagging-based, electronically cleansed CT colonography: evaluation of patient comfort and image readability. Radiology 239, 149 (2006).
- Serlie IW, de Vries AH, van Vliet LJ et al.: Lesion conspicuity and efficiency of CT colonography with electronic cleansing based on a three-material transition model. AJR Am. J. Roentgenol. 191, 1493 (2008).
- Cai W, Yoshida H, Zalis ME et al.: Informatics in radiology: electronic cleansing for noncathartic CT colonography: a structure-analysis scheme. Radiographics 30(3), 585–602 (2010).
- Yee J, Kumar NN, Hung RK et al.: Comparison of supine and prone scanning separately and in combination at CT colonography. Radiology 226, 653 (2003).
- Fletcher JG, Johnson CD, Welch TJ et al.: Optimization of CT colonography technique: prospective trial in 180 patients. Radiology 216, 704 (2000).
- Chen SC, Lu DS, Hecht JR, Kadell BM: CT colonography: value of scanning in both the supine and prone positions. AJR Am. J. Roentgenol. 172(3), 595–599 (1999).
- Shinners TJ, Pickhardt PJ, Thaylor AJ, Jones AJ, Olsen CH: Patient-controlled room air insufflaton versus automated carbon dioxide delivery for CT colonography. AJR Am. J. Roentgenol. 186(6), 1491–1496 (2006).
- Burling D, Taylor SA, Halligan S et al.: Automated insufflation of carbon dioxide for MDCT colonography: distention and patient experience compared with manual insufflation. AJR Am. J. Roentgenol. 186, 96–103 (2006).
- Pickhardt PJ: Incidence of colonic perforation at CT colonograph: review of existing data and implications for screening of asymptomatic adults. Radiology 239, 313 (2006).
- Bova JG, Jurdi RA, Bennett WF: Antispasmodic drugs to reduce discomfort and colonic spasm during barium enemas: comparison of oral hyoscyamine, i.v. glucagon and no drug. AJR Am. J. Roentgenol. 161(5), 965–968 (1993).
- Yee J, Hung RK, Akerkar GA, Wall SD: The usefulness of glucagon hydrochloride for colonic distention in CT colonography. AJR Am. J. Roentgenol. 173(1), 169–172 (1999).
- American College of Radiology: ACR Practice Guideline for the Performance of Computed Tomography (CT) Colonography in Adults. American College of Radiology, Philadelphia, PA, USA (2009).
- Mulhall BP, Veerappan GR, Jackson JL: Meta-analysis: computer tomographic colonography. Ann. Intern. Med. 142, 635–650 (2005).
- Lui YW, Macari M, Israel G et al.: CT colonography data interpretation: effect of different section thicknesses – preliminary observations. Radiology 229, 791–797 (2003).
- Chung DJ, Huh KC, Choi WJ Kim JK: CT colonography using 16-MDCT in the evaluation of colorectal cancer. AJR Am. J. Roentgenol. 184(1), 98–103 (2005).
- McCollough CH: Optimization of multidetector array CT acquisition parameters for CT colonography. Abdom. Imaging 27, 253 (2002).
- Macari M, Bini EJ, Xue X et al.: Colorectal neoplasms: prospective comparison of thin section low dose multi-detector row CT colonography and conventional colonoscopy for detection. Radiology 224, 383–392 (2002).
- van Gelder RE, Venema HW, Florie J et al.: CT colonography: feasibility of substantial dose reduction – comparison of medium to very low doses in identical patients. Radiology 232(2), 611–620 (2004).
- Fisichella, VA, Bath M, Johnsson AA et al.: Evaluation of image quality and lesion perception by human readers on 3D CT colonography: comparison of standard and low radiation dose. Eur. Radiol. 20(3), 630–639 (2010).
- Liedenbaum MH, Venema HW, Stocker J: Radiation dose in CT colonography – trends in time and differences between daily practice and screening protocols. Eur. Radiol. 17, 2616–2621 (2007).
- Brenner DJ, Georgsson MA: Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenerology 129, 328–337 (2005).
- Hall EJ, Brenner DJ: Cancer risks from diagnostic radiology. Br. J. Radiol. 81, 362 (2008).
- Gonzalez BA, Kim KP, Yee J: CT colonography: perforation rates and potential radiation risks. Gastrointest. Endosc. Clin. N. Am. 20, 279–291 (2010).
- Preston DL, Ron E, Tokuoka S et al.: Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 168(1), 1–64 (2007).
- National Research Council, Committee to Assess the Health Risks from Exposure to Low Levels of Ionizing Radiation: Health Risks From Exposure To Low Levels Of Ionizing Radiation. Monson R (Ed.). National Academies Press, Washington DC, USA (2006).
- Rizwan A, Yee J: Computed tomographic colonography. Expert Rev. Gastroenterol. Hepatol. 2(3), 371–384 (2008).
- Johnson KT, Johnson CD, Fletcher JG et al.: CT colonography using 360 degree virtual dissection: a feasibility study. AJR Am. J. Roentgenol. 186, 90 (2006).
- Pickhardt PJ: Translucency rendering in 3D endoluminal CT colonography: a useful tool for increasing polyp specificity and decreasing interpretation time. AJR Am. J. Roentgenol. 183, 429 (2004).
- Vos FM, van Gelder RE, Serlie IW et al.: Three-dimensional display modes for CT colonography: conventional 3D virtual colonoscopy versus unfolded cube projection. Radiology 228(3), 878–885 (2003).
- Huang A, Roy DA, Summers RM et al.: Teniae coli-based circumferential localization system for CT colonography: feasibility study. Radiology 243(2), 551–560 (2007).
- Dachman A, Bekeny K, Zintsmaster M et al.: Formative evaluation of standardized training for CT colonographic image interpretation by novice readers. Radiology 249, 167–177 (2008).
- American College of Radiology: ACR Practice Guidelines for the Performance of Computed Tomography (CT) Colonography in Adults. American College of Radiology, Philadelphia, PA, USA (2005).
- Slater A, Taylor SA, Tam E et al.: Reader error during CT colonography: causes and implications for training. Eur. Radiol. 16, 2275–2283 (2006).
- McFarland EG, Fletcher JG, Pickhardt P et al.: ACR Colon Cancer Committee White Paper: status of CT colonography 2009. J. Am. Coll. Radiol. 6, 756–772 (2009).
- Summers, Ronald M: Improving the accuracy of CTC interpretation: computer-aided detection. Gastrointest. Endoscopy Clin. N. Am. 20, 245–257 (2010).
- Petrick N, Haider M, Summers RM et al.: CT colonography and computer-aided detection as a second reader: observer performance study. Radiology 246(1), 148–156 (2008).
- Mang T, Peloschek P, Plank C et al.: Effect of computer-aided detection as a second reader in multidetector-row CT colonography. Eur. Radiol. 17(10), 2598–2607 (2007).
- Summers RM, Yao J, Pickhardt PJ et al.: Computed tomographic virtual colonoscopy computer-aided polyp detection in a screening population. Gastroenterology 129(6), 1832–1844 (2005).
- van Ravenstein VF, van Wijk C, Truyen R et al.: Computer aided detection of polyps in CT colonography using logistic regression. IEEE Trans. Med. Imaging 29(1), 120–131 (2009).
- Lee MW, Kim SH, Park Shim et al.: An anthropomorphic phantom study of computer-aided detection performance for polyp detection on CT colonography: a comparison of commercially and academically available systems. AJR Am. J. Roentgenol. 193(2), 445–454 (2009).
- Kin DH, Pickhardt PJ, Taylor AJ et al.: CT colonography versus colonoscopy for the detection of advanced neoplasia. N. Engl. J. Med. 357(14), 1403–1412 (2007).
- Park SH, Lee SS, Choi EK et al.: Flat colorectal neoplasms: definition, importance, and visualization on CT colonography. AJR Am. J. Roentgenol. 188(4), 953–959 (2007).
- Park SH, Hyun KH, Kim YA et al.: Flat polyps of the colon: detection with 16- MDCT colonography – preliminary results. AJR Am. J. Roentgenol. 186, 1611–1617 (2006).
- O’Brien MJ, Winawer SJ, Zauber AG et al.: Flat adenomas in the national polyp study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin. Gastroenterol. Hepatol. 2(10), 905–911 (2004).
- Pickhardt PJ, Kim DH, Robbins JB: Flat (nonpolypoid) colorectal lesion identified at CT colonography in a U.S. screening Population. Acad. Radiol. 17, 784–790 (2010).
- Pickhardt PJ, Choi JR, Hwang I et al.: Nonadenomatous polyps at CT colonography: prevelance, size distribution, and detection rates. Radiology 232(3), 784–790 (2004).
- Fidler JL, Johnson CD, MacCarty RL et al.: Detection of flat lesions in the colon with CT colonography. Abdom. Imaging 27(3), 292–300 (2002).
- MacCarty RL, Johnson CD, Fletcher JG et al.: Occult colorectal polyps on CT colonography: implications for surveillance. AJR Am. J. Roentgenol. 186(5), 1380–1383 (2006).
- Yee J, Sadda S, Aslam R, Yeh B: Extracolonic findings at CT colonography. Gastrointest. Endoscopy Clin. N. Am. 20, 305–322 (2010).
- Spreng A, Netzer P, Mattich J, Dinkel HP, Vock P, Hoppe H: Importance of extracolonic findings at IV contrast medium-enhanced CT colonography versus those at non-enhanced CT colonography. Eur. Radiol. 15, 2088–2095 (2005).
- Xiong T, Richardson M, Woodroffe R et al.: Incidental lesions found on CT colonography: their nature and frequency. Br. J. Radiol. 78, 22–29 (2005).
- Yee J, Kumar NN, Godara S et al.: Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology 236(2), 519–526 (2005).
- Pickardt PJ, Kim DH, Meiners RJ et al.: Colorectal and extracolonic cancers detected at screening CT colonography in 10286 asymptomatic adults. Radiology 255, 83–88 (2010).
- Fenlon HM, Nunes DP, Schroy PC et al.: A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N. Engl. J. Med. 341, 1496–1503 (1999).
- Van Dam J, Cotton P, Johnson CD et al.: AGA future trends report: CT colonography. Gastroenterology 127, 970–984 (2004).
- Muhall BP, Veerappan GR Jackson JL: Meta-analysis: computed tomography colonography. Ann. Intern. Med. 142 (8), 635–650 (2005).
- Yee J, Akerkar GA, Hung RK, Steinauer-Gebauer AM, Wall SD, McQuaid KR: Colorectal neoplasia: performance character after colonography detection in 300 patients. Radiology 219, 685–692 (2001).
- Cotton PB, Durkalski VL, Pineau BC et al.: Computed tomographic colonography (virtual colonoscopy), a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA 291(14), 1713–1719 (2004).
- Rockey DC, Paulson R, Niedzwiecki D et al.: Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 365(9456), 305–311 (2005).
- Doshi T, Rusinak D, Halvorsen R et al.: CT colonography: false-negative interpretations. Radiology 244, 165–173 (2007).
- Pickhardt PJ, Choi JR, Hwang I et al.: Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N. Engl. J. Med. 349(23), 2191–2200 (2003).
- Johnson CD, Fletcher JG, MacCarty RL et al.: Effect of slice thickness and primary 2D versus 3D virtual dissection on colorectal lesion detection at CT colonography in 452 asymptomatic adults. AJR Am. J. Roentgenol. 189, 672–680 (2007).
- Regge D, Laudi C, Galatola G et al.: Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA 301, 2453–2461 (2009).
- Graser A, Stieber P, Nagel D et al.: Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 58, 241–248 (2009).
- Halligan S, Altman DG, Tyalor SA et al.: CT colonography in the detection of colorectal polyps and cancer: systematic review, meta analysis, and proposed minimum data set for study level reporting. Radiology 237(3), 893–904 (2005).
- Mulhall BP, Veerappan GR, Jackson JL: Meta-analysis: computed tomographic colonoscopy. Ann. Intern. Med. 893–904 (2005).
- Kim DH, Pickardt PJ, Taylor AJ et al.: CT colonography versus colonoscopy for the detection of advanced neoplasia. N. Engl. J. Med. 357, 1403–1412 (2007).
- Ransohoff DF, Sandler RS: Clinical practice: screening for colorectal cancer. N. Engl J. Med. 346, 40–44 (2002).
- Levin B, Lieberman DA, McFarland B et al.: Screening and surveillance for the detection of colorectal cancer and adenomatous polyps, 2008; a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 58, 130–160 (2008).
- Macari M, Berman P, Decker M, Milano A, Megibow AJ: Usefulness of CT colonography in patients with incomplete colonoscopy. AJR Am. J. Roentgenol. 173, 561–564 (1999).
- Kim DH, Pickhardt PJ, Taylor AJ: Characteristics of advanced adenomas detected at CT colonographic screening: implications for appropriate polyp size thresholds for polypectomy versus surveillance. AJR Am. J. Roentgenol. 188, 940–944 (2007).
- Winawer SJ, Zauber AG: The advanced adenoma as the primary target of screening. Gastrointest. Endosc. Clin. N. Am. 12(1), 1–9 (2002).
- Pickhardt PJ, Kim DH: Performance of CT colonography for detecting small, diminutive, and flat polyps. Gastrointest. Endoscopy Clin. N. Am. 20, 209–226 (2010).
- Winawer SJ, Zauber AG, Fletcher RH et al.: Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 130(6), 1872–1885 (2006).
- Lieberman D, Moravec M, Holub J et al.: Polyp size and advanced histology is patients undergoing colonoscopy screnning: implications for CT colonography. Gastroenterology 135(4), 1100–1105 (2008).
- Park SH, Choi EK, Lee SS et al.: Polyp measurement reliability, accuracy and discrepancy: optical colonoscopy versus CT colonography with pig colonic specimens. Radiology 244(1), 157–164 (2007).
- Zalis ME, Barish MA, Choi JR et al.: CT colonography reporting and data system: a consensus proposal. Radiology 236(1), 3–9 (2005).
- Lieberman D: Debate: small (6–9 mm) and diminutive (1–5 mm) polyps noted on CTC: how should they be managed. Gastrointest. Endoscopy Clin. N. Am. 20, 239–243 (2010).
- US Preventive Services Task Force: Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 149(9), 627–637 (2008).
- Young BM, Fletcher JG, Earnest F et al.: Colonic perforation at CT colonography in a patient without known colonic disease. AJR Am. J. Roentgenol. 186, 119–121 (2006).
- Bassett JT, Liotta RA, Barlow D et al.: Colonic perforation during screening CT colonography using automated CO2 insufflation in an asymptomatic adult. Abdom. Imaging 33, 598–600 (2008).
- Pickhardt PJ: Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology 239, 313–316 (2006).
- Sosna J, Blachar A, Amitai M et al.: Colonic perforation at CT colonography: Assessment of risk in a multicenter large cohort. Radiology 239, 457–463 (2006).
- Stevenson G: Normal anatomy and techniques of examination of the colon:barium, CT and MRI. In: Margulis and Burheme’s Alimentary Tract Radiology. Freeny PC, Stevenson GW (Eds). Mosby, St Louis, MO, USA, 692–724 (1994).
- Centers for Disease Control and Prevention, National Center for Health Statistics. Summary health statistics for US adults: National Health Interview Survey (2005) www.cdc.gov/nchs/data/series/sr_10/ sr10_232.pdf
- Decision memo for screening computed tomography (CTC) for colorectal cancer. Centers for Medicare and Medicaid Services (2009) www.cms.gov/mcd/viewdecisionmemo. asp?from2=viewdecisionmemo. asp&id=220& (Accessed January 2010)
• The American College of Radiology Imaging Network (ACRIN) study was one of the largest multicenter studies to compare the effectiveness of state-of-the-art CT colonography (virtual colonoscopy) to conventional colonoscopy.
• Guidelines issued by the American College of Radiology (ACR) to optimize and standardize CT colonography performance.
• Meta-analysis looking at data from many studies comparing CTC with colonoscopy.
• Review of the current status and rationale of the updated ACR practice guidelines for CT colonography.
• Early paper demonstrating utility of CTC for detecting clinically important extracolonic findings.
• Compared the performance of CTC and colonoscopy for detecting advanced neoplasia in similar population groups. The number of polypectomies and complications were considerably smaller in the CTC group.
Websites