Review Article - Imaging in Medicine (2010) Volume 2, Issue 6
Diffusion tensor imaging of white matter pathology in the mouse brain
Jiangyang Zhang*
Department of Radiology, Johns Hopkins University School of Medicine, 217 Traylor Bldg, 720 Rutland Ave., Baltimore, MD, 21205, USA
- *Corresponding Author:
- Jiangyang Zhang
Department of Radiology, Johns Hopkins University
School of Medicine, 217 Traylor Bldg
720 Rutland Ave. Baltimore, MD, 21205, USA
Tel.: +1 410 502 9856
E-mail:jzhang3@jhmi.edu
Abstract
Diffusion tensor imaging has been increasingly used for studying white matter pathology in rodent models of neurological diseases. Here, applications of diffusion tensor imaging in detecting major and subtle white matter pathology in the mouse CNS are reviewed, followed by several technical details that may be helpful in designing studies that involve diffusion tensor imaging of rodent brain and spinal cord.
Keywords
brain; diffusion tensor imaging; mouse; MRI; pathology; spinal cord ; white matter
Over the past two decades, with the development of modern gene technology, it has become increasingly popular to use rodent models for studying neurological diseases and testing novel treatments. Rodent models enable us to examine detailed neuropathology and study the genetic control of disease processes. While histology has been the standard tool for neuropathology, its invasiveness hinders longitudinal studies that are critical for defining spatial and temporal characteristics of disease processes. It is also difficult to use histology to perform comprehensive analysis of neuropathology. For example, quantification of axonal density and lesion volume using histology can be labor intensive and time consuming. Quantitative noninvasive imaging markers that can measure the extent and progression of neuropathology are desirable. Since white matter (WM) pathology is the hallmark of several neurological diseases, (e.g., multiple sclerosis), and is directly linked to functional deficits, imaging WM pathology in rodent models is an important area of research with the potential to be translated to the clinics.
MRI is an ideal imaging modality for examining WM pathology noninvasively. It can be applied longitudinally and provides an array of tissue contrasts that are sensitive to several aspects of WM pathology. T1/T2 MRI and magnetization transfer imaging have been used to visualize inflammation and disrupted myelin in the brain and spinal cord. Diffusion tensor imaging (DTI), a mature MRI technique, has been demonstrated to be sensitive to injured WM [1–3]. DTI measures the extent of water molecule diffusion along multiple axes and fits the measurements to a tensor model [4]. A diffusion tensor is a 3 × 3 symmetric matrix, from which three eigenvalues (l1, l2, and l3, in descending order) and their corresponding eigenvectors (v1, v2, and v3) can be computed. The eigenvalues are the apparent diffusion coefficients along the three orthogonal axes defined by the eigenvectors. From the three eigenvalues, mean diffusivity and various measures of diffusion anisotropy can be calculated at each pixel [5]. The eigenvector associated with the largest eigenvalue is called the primary eigenvector, which indicates the primary axis of water diffusion. Differences in diffusivity, diffusion anisotropy, and the primary axis of water diffusion between different tissue structures form the basic DTI contrasts. These contrasts are unique because they reflect the organization and conditions of WM microstructures, and the structural information encoded by them is complementary to conventional magnetic resonance (MR) modalities. For example, diffusion anisotropy has been shown to reflect the existence of axonal tracts and the degree of myelination [6], and the primary axis of water diffusion can be used to reconstruct the trajectories of major WM tracts [7–10]. The unique DTI contrasts are also useful for delineating WM structures in unmyelinated or demyelinated WM structures, which are difficult with conventional T1 and T2 MRIs. This has been demonstrated in embryonic mouse brains and demyelinated/dysmyelinated mouse brains [11–13]. It should be noted that the diffusion tensor model is a simplified model and can not resolve multiple intravoxel fiber directions. To overcome this problem, various data acquisition and postprocessing approaches have been proposed, such as diffusion spectrum imaging, Q-ball imaging and high angular resolution diffusion [14–18]. These approaches come, however, with a price in terms of imaging time, signal-to-noise ratio and difficulties in reducing motion and eddy-current-related artifacts.
In the following sections, applications of DTI in detecting major and subtle WM pathology in rodent CNS are reviewed, followed by several technical details that may be helpful in designing studies that involve DTI of the rodent brain and spinal cord.
DTI reveals normal & abnormal WM tracts
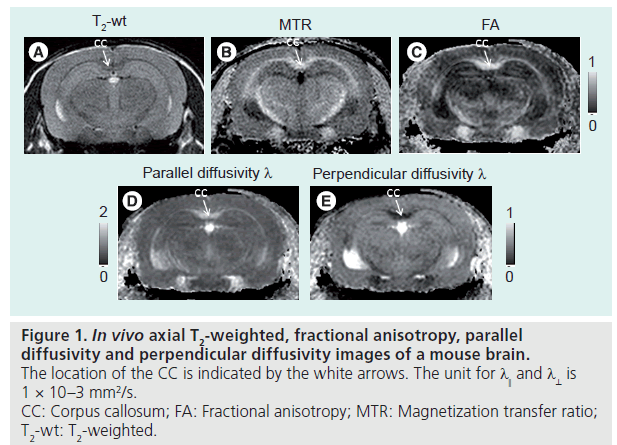
Several DTI contrasts are useful for studying WM pathology in rodent models of neurological diseases. Figure 1 shows the appearance of the mouse corpus callosum (CC) in T2-weighted, magnetization transfer ratio (MTR), and several DTI-derived images. The CC is the most prominent of the WM structures in these images. The T2-weighted and MTR images show that the CC has lower T2 and higher MTR than the surrounding cortex. The contrasts between gray and WM structures in these two images are related to the presence of myelin, as unmyelinated WM structures are often difficult to resolve from gray matter in T2 and MTR images. Fractional anisotropy (FA; a unit-less measure of diffusion anisotropy), parallel diffusivity (λ║, also called axial diffusivity), and perpendicular diffusivity (λ ┴, also called radial diffusivity) [19] are derived from the estimated diffusion tensor at each pixel. In the FA image, the CC is brighter than the cortex, which indicates higher diffusion anisotropy in the CC than in the cortex. Parallel and perpendicular diffusivities measure the extent of water diffusion along or perpendicular to the primary diffusion orientation (defined by the primary eigenvector of the estimated diffusion tensor). Since primary diffusion orientation in WM regions often coincide with the underlying trajectory of axonal tracts, parallel and perpendicular diffusivities are often referred to as diffusivity along or perpendicular to the axonal tracts, respectively. In a normal mouse brain (Figure 1), parallel diffusivity in the CC is higher than those in the cortex, while perpendicular diffusivity in the CC is lower than in the cortex. These results suggest that water diffusion in the CC is less restricted or hindered along the axonal fibers than in the cortex and more restricted or hindered perpendicular to the axonal fibers than in the cortex. It is necessary to note that parallel and perpendicular diffusivities can only be reliably obtained for the prolate tensor (λ1>>λ2 and λ3), with a clearly defined primary axis of water diffusion. In the mouse brain, most normal WM regions have a prolate shaped tensor, but special attention should be paid to regions with crossing or branching axonal fibers and injured WM regions to ensure the condition is satisfied.
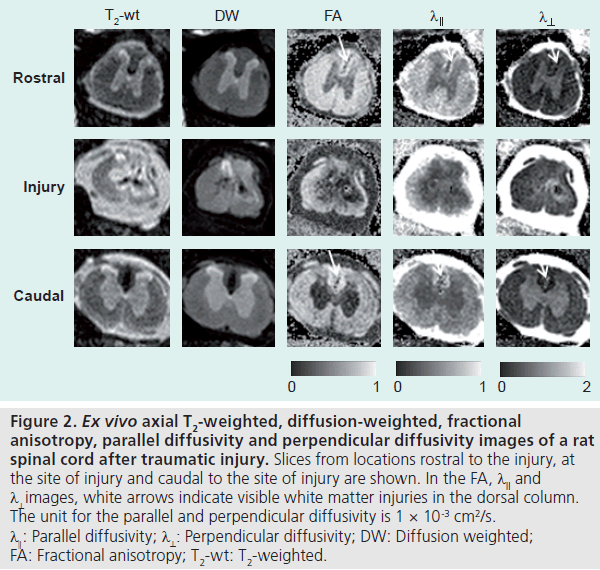
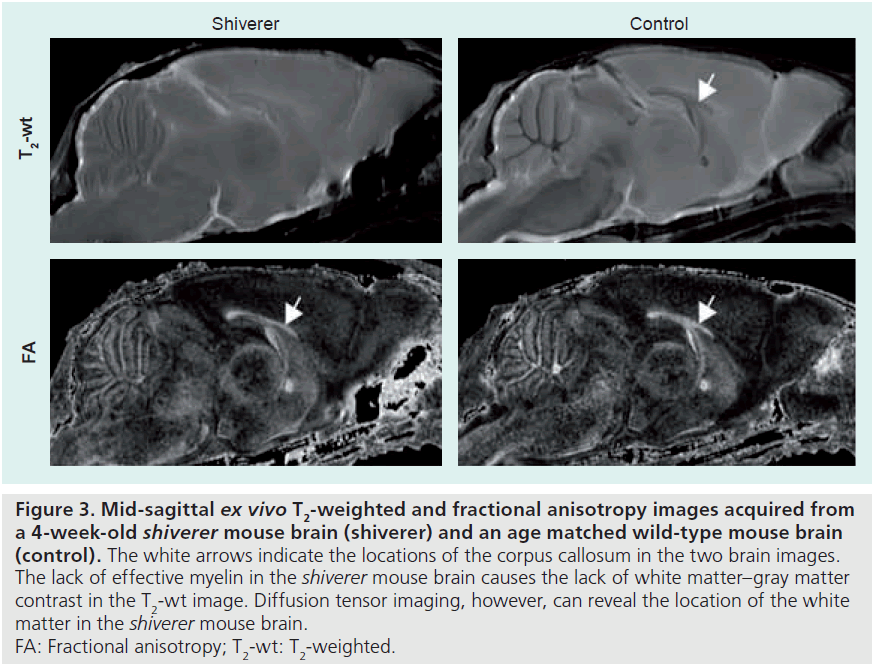
The above DTI-based measurements and DTI tractography can be used to monitor changes in WM tract morphology. In the peripheral nervous system, DTI tractography and FA have been used to study nerve degeneration and regeneration [20,21]. In the CNS, major brain or spinal cord injuries (e.g., trauma [22,23] or stroke [24,25] and brain tumor [26]) can lead to severe WM injury and can be detected by DTI and visualized by tractography. DTI has also been used to study the extent of WM injury. Figure 2 shows serial axial sections of a rat spinal cord after moderate spinal cord injury. Using DTI, the extent of the WM injury in the spinal cord and lesion volume can be easily quantified. Note that in the dorsal column WM rostral to the site of injury, it is difficult to define the area of WM injury in the T2-weighted and diffusionweighted images, while the lesion can be easily delineated in DTI data. DTI is also useful for studying WM abnormalities in embryonic and neonatal animal models, in which the lack of myelination makes it difficult for conventional MRI to visualize the WM tract. Wang et al. have demonstrated the use of DTI to study abnormal WM tract formation in a knockout mouse model at embryonic stage [27]. Figure 3 compares T2-weighted and diffusion tensor images of a shiverer mouse brain and a wild-type mouse brain. The shiverer mouse, which lacks effective myelination, is a commonly used mouse model of inherited myelin diseases. The T2-weighted image of the shiverer mouse shows limited gray matter and WM contrast in the brain, whereas the FA image provides satisfactory contrasts for delineation of major WM structures. This useful property of DTI facilitates the tracking of structures of interest in studies of mouse models of myelin diseases.
Figure 2.Ex vivo axial T2-weighted, diffusion-weighted, fractional anisotropy, parallel diffusivity and perpendicular diffusivity images of a rat spinal cord after traumatic injury.
Figure 3.Mid-sagittal ex vivo T2-weighted and fractional anisotropy images acquired from a 4-week-old shiverer mouse brain (shiverer) and an age matched wild-type mouse brain (control).
DTI contrasts of axon & myelin pathology
Although DTI is widely used today, the contrast mechanisms of DTI or diffusion MRI of WM tissues are still not fully understood. The primary reason is that the WM tissues have multiple structures capable of influencing diffusion MRI signals [6] and WM pathology often involves more than one structure. For example, axon and myelin are tightly coupled functionally and physiologically, and both axon membrane and myelin sheath can act as barriers to water diffusion perpendicular to the long axis of axons. Since injuries to the axon will inevitably lead to injuries to the myelin, and vice versa, it is difficult to separate the effect of axon and myelin on diffusion MRI signals. In addition, there are numerous miniature structures inside the axon that may affect water molecule diffusion, parallel or perpendicular to the long axis of axons [6]. Furthermore, the MRI signals we measured in diffusion MRI or DTI include contributions from both intracellular (axon) and extracellular (between axons) spaces [6]. All these factors may affect the sensitivity of DTI/diffusion MRI for detecting pathological changes, because these factors may counteract each other. For example, certain pathological conditions (e.g., axon degeneration or demyelination) may create new barriers for diffusion, as in axonal beading, while at the same time clear certain barriers, as in demyelination. It is necessary to examine the relationship between DTI signals and pathological changes carefully before using the DTI signals as substitute markers.
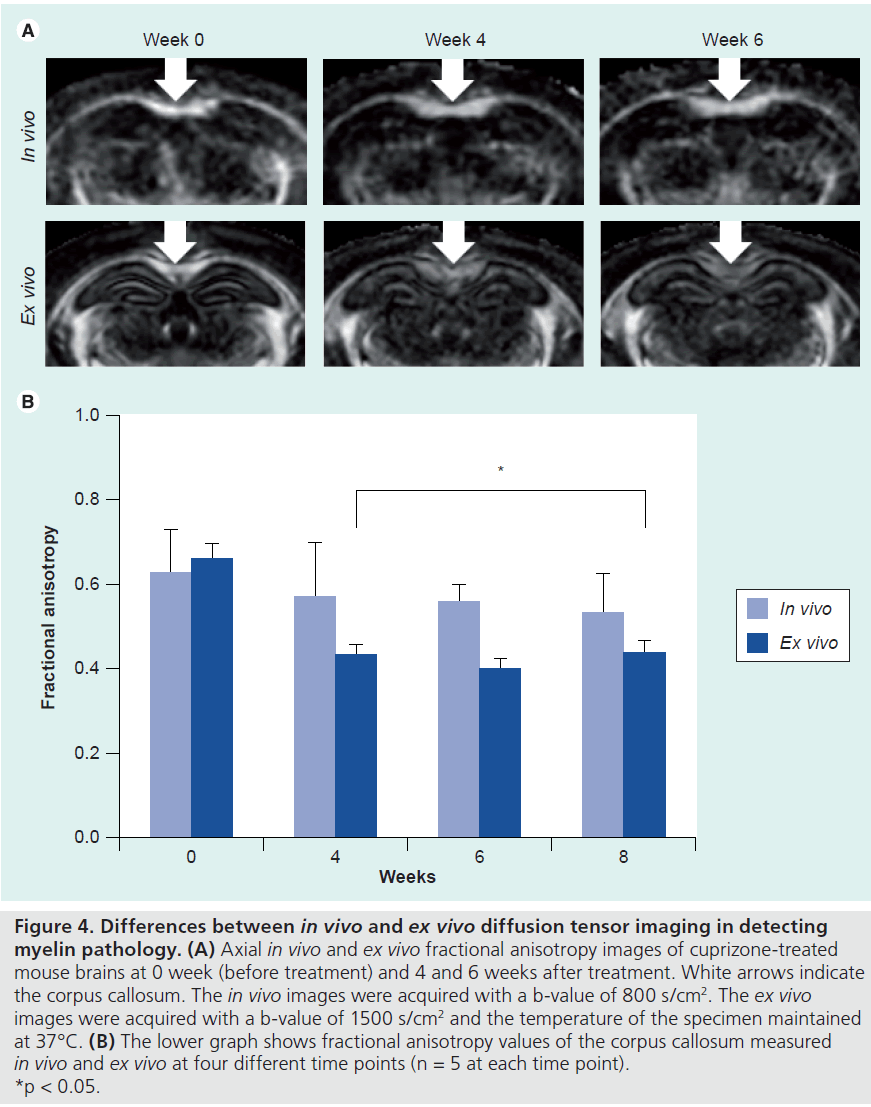
Figure 4.Differences between in vivo and ex vivo diffusion tensor imaging in detecting myelin pathology.
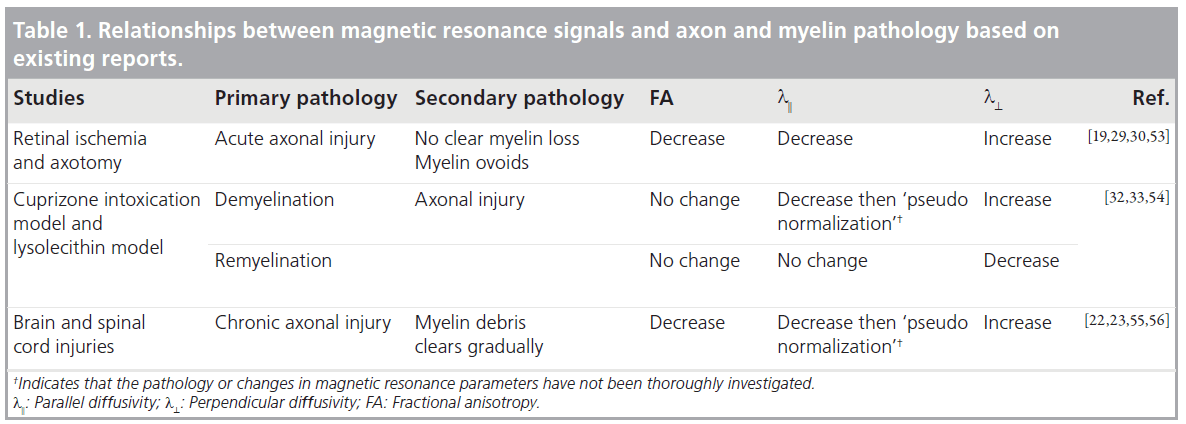
Several DTI-based measurements, such as the previously mentioned FA, parallel diffusivity and perpendicular diffusivity, can potentially be used to measure the degree of axon and myelin pathology. Although there is no animal model with pure axon pathology or pure myelin pathology, the relationships between these DTIbased measurements and pathology have been examined in several animal models with relatively simple pathology. Table 1 summarizes the existing evidence on the relationships between axon and myelin pathology and changes in DTI signals. FA was widely used for detecting WM pathology, but lacks specific information of the underlying pathology. The potential of parallel and perpendicular diffusivities in providing more specific information on axon and myelin integrity was first demonstrated by Song et al. [19,28]. Several studies suggest that parallel diffusivity is sensitive to certain axonal injuries. Using a mouse retinal ischemia model, Sun et al. demonstrated that parallel diffusivity can sensitively detect acute injury of myelinated axons in the optic nerve, while T2 MRI shows no apparent change, owing to limited disruptions in myelin at similar stages [29]. In a rat spinal cord axotomy model, Zhang et al. demonstrated that parallel diffusivity was able to detect WM pathology, primarily axon degeneration, at 3 days after axotomy [30]. Using the cuprizonetreated mouse model [31], in which the CC shows reliable demyelination and remyelination, Sun et al. showed that perpendicular diffusivity is sensitive to myelin injury [32]. It has also been demonstrated that FA, parallel and perpendicular diffusivities are significantly correlated with several histological measurements of axonal and myelin injury [33,34]. However, several tissues remain to be investigated. For example, several reports suggested that parallel diffusivity was not sensitive to injuries to unmyelinated WM tracts [35] and it showed a ‘pseudonormalization’ when active clearance of axon/myelin debris was present [22]. Furthermore, the specificity of perpendicular diffusivity to myelin injury has not been firmly established, as increases in parallel diffusivity were reported after injury in unmyelinated axons [35]. In models with more complex pathology, (e.g., spinal cord injury), Herrera et al. demonstrated that parallel diffusivity was not correlated with axonal damage [36]. Further studies on the relationships between the DTI parameters and axon and myelin pathology are necessary, and multiparameter (T1, T2, MTR and DTI) imaging may help to define the underlying pathology more accurately than any single modality.
Technical considerations
Imaging resolution is the key challenge in rodent DTI
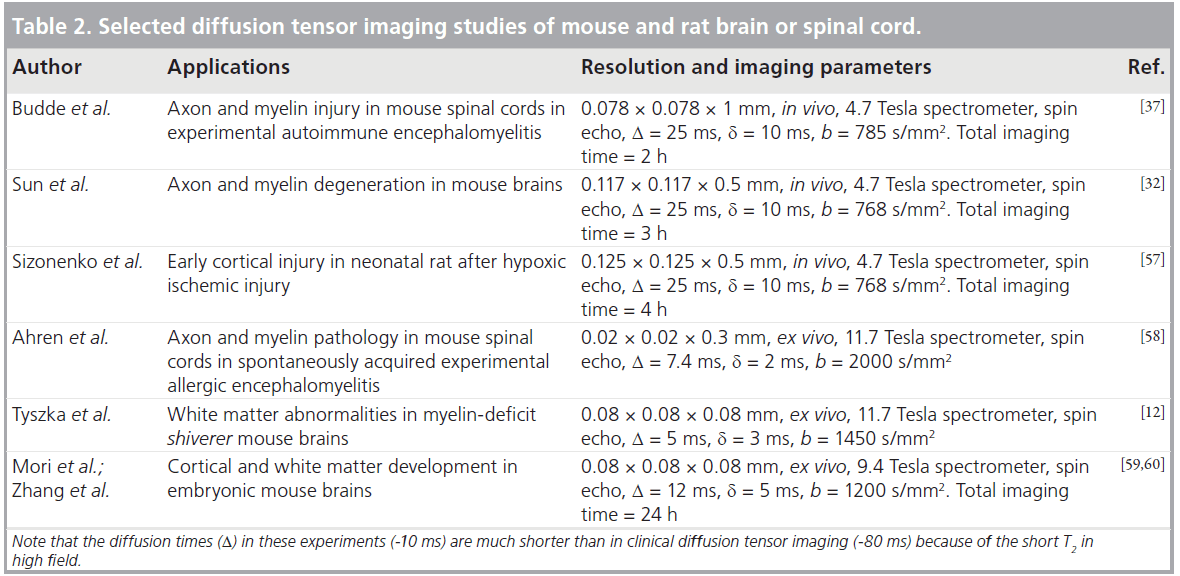
Imaging resolution is a key factor to consider in designing a study involving rodent DTI. A mouse brain is approximately 1000-times smaller than a human brain in term of the total volume. The current resolution of human brain DTI is approximately 1–2 mm per pixel. In order to keep relative resolution at the same level to resolve miniature rodent WM structures, a resolution of 0.1–0.2 mm per pixel is required. Table 2 lists several DTI experiments and their imaging parameters. The best resolution achieved for in vivo DTI is approximately 0.1 × 0.1 × 0.5 mm [37], and the best resolution achieved for ex vivo DTI is approximately 0.05 × 0.05 × 0.05 mm [38,39].
Once the target resolution is set, the primary technical challenge in DTI of the rodent brain is to maintain satisfactory signal-to-noise ratio at the target resolution. To achieve satisfactory signal-to-noise ratio, most mouse brain DTI experiments were performed on high field systems (>4.7 Tesla). The disadvantage of a strong magnetic field is that it shortens tissue T2 while lengthening tissue T1. High field systems (>4.7 Tesla) also have more severe field inhomogeneity problems than 1.5 Tesla and 3 Tesla systems. The short T2 and field inhomogeneity make implementation of echo planar imaging type of acquisition, commonly used for clinical DTI, difficult on high field systems. While most studies to date used spin echo or fast spin echo type of acquisition, mouse brain DTI experiments performed using multiple shot echo planar imaging have emerged and shown great potential [40].
In vivo DTI versus ex vivo DTI
Another issue that needs to be considered is the compatibility of in vivo and ex vivo DTI results. Both in vivo and ex vivo DTI have been used for studying rodent models. In vivo DTI can follow the same animal longitudinally, and the signals collected reflect axon and myelin microstructures in live animals. In vivo DTI, however, has limited imaging resolution and is susceptible to artifacts associated with subject motion. Ex vivo DTI, on the other hand, provides much higher resolution than in vivo DTI, and is free of motion artifacts. The major disadvantage of ex vivo DTI is that the signals from post-mortem specimens may not be accurate representations of the tissue structures in live animals, as fixation procedures may alter tissue microstructures, for example, the ratio of intracellular and extracellular space and membrane permeability, which will result in differences in DTI signals measured in vivo and ex vivo. Shepherd et al. compared the effects of two widely used aldehyde fixative solutions on diffusion MR signals acquired from dissected rat cortical slices and found significant increased membrane permeability and decreased extracellular space [41,42]. Sun et al. compared in vivo and ex vivo DTI data from normal and infracted mouse brains and found that while diffusivity values in fixed brain tissues were significantly reduced compared with live animals, relative anisotropy values in normal and injured WM structures remained unchanged after fixation [19,43]. It remained unclear whether it is true for anisotropy and diffusivity measurements in injured WM. Figure 4 compares in vivo and ex vivo FA measured in the CC of mice treated with cuprizone. While in vivo and ex vivo FA are similar in the CC of normal mice (0 week), there were significant differences between in vivo and ex vivo FA measured in demyelinated CC (4–8 weeks).
High-throughput analysis of DTI data
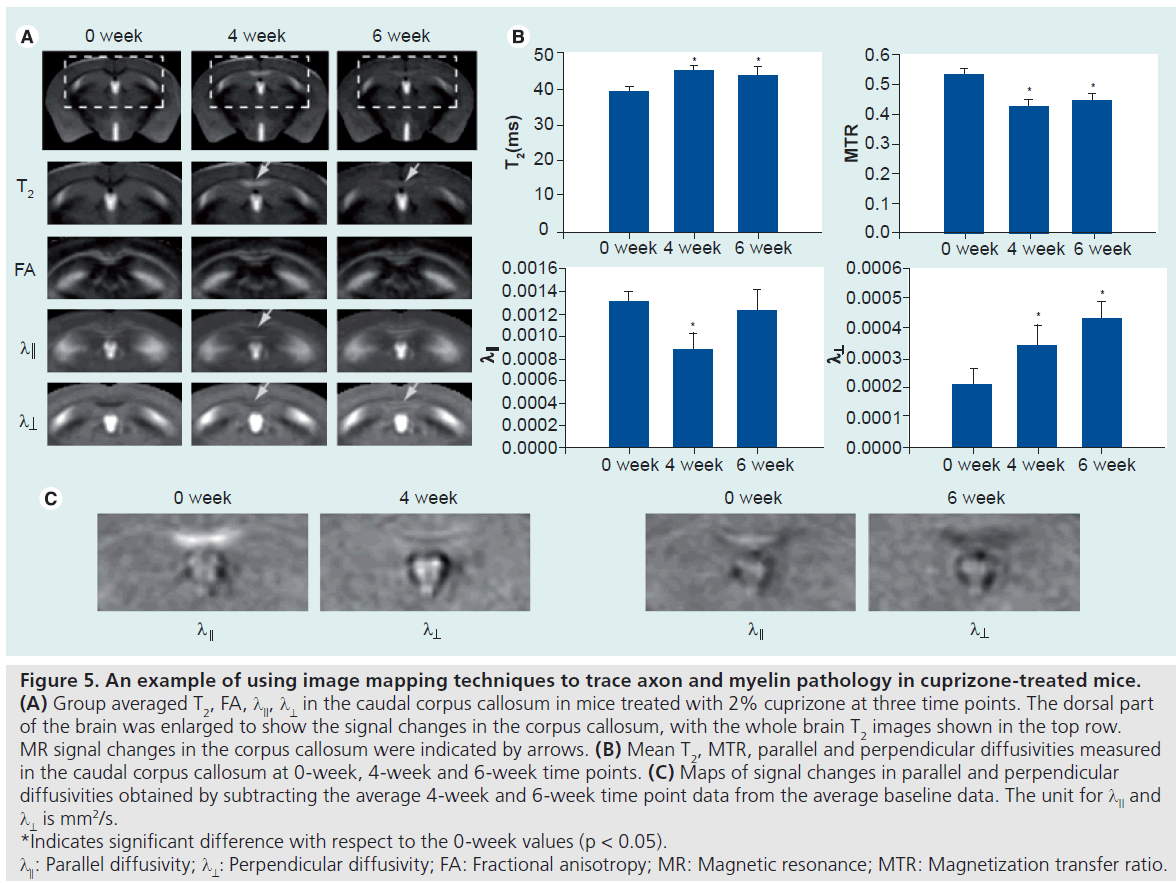
With modern MRI techniques, we can now acquire high-resolution images longitudinally [44,45]. Analyzing such data often involves delineating regions of interest in multiple subject data in order to examine changes in MR parameters or follow them over time. The procedure is time consuming and is susceptible to operator bias. One potential solution is to use image registration and mapping techniques and MRI-based rodent brain atlases to perform automated analysis. Several MRI-based mouse brain atlases and image registration techniques have been published [46–51]. These atlases can serve as the common platform for image analysis and integration of MRI and histological data for more detailed analysis. Our group has been working on the creation of a DTI-based mouse brain atlas [39] and its applications. Although direct mapping of diffusion tensor data is not straightforward and requires additional procedures to ensure the consistency of tensor data [52], diffusion tensor derived scalar measurements (e.g., mean diffusivity, FA, parallel and perpendicular diffusivities) can be transformed directly. The rich tissue contrast provided by DTI allows better delineation of various anatomical structures inside the brain than conventional T1 and T2 MRI. The rich tissue contrasts provided by DTI enable accurate mapping because the contrasts remain relatively consistent in demyelinated/dysmyelinated structures.
In 2006, Tyszka et al. demonstrated the use of image mapping techniques and DTI in studying the phenotypes of the shiverer mouse brain [12]. In their study, voxel-based analysis revealed changes in apparent diffusion constant, parallel and perpendicular diffusivities in major WM tracts and several other brain regions. Figure 5 shows an example of using DTI and image mapping techniques to trace demyeliantion and axonal injury in cuprizone-treated mice. In vivo T2, MT and diffusion tensor images were acquired and spatially normalized to a mouse brain atlas and averaged (n ≥ 5 at each time point). At the 4-week time point, a hyperintense T2 signal was found in the caudal CC. Parallel diffusivity in the same region showed a slight decrease, while perpendicular diffusivity showed a slight increase. At the 6-week time point, the T2 signal in the caudal CC reduced from its 4-week peak, but remained higher than the baseline level. Parallel diffusivity in the CC returned to its baseline level while perpendicular diffusivity remained elevated. No apparent change in FA was detected. As all images have been spatially normalized, values from individual mouse brain images can be obtained via common regions of interest defined in the atlas images. This approach ensured that the definitions of regions of interest were consistent for different subjects, and therefore removed operator bias. Changes detected in T2, MTR, parallel and perpendicular diffusivities in the caudal CC (Figure 5B) are consistent with existing reports. Histological data from existing reports show that there is progressive demyelination and axonal injury at the 4-week and 6-week time points, and inflammation at the 4-week time point (microglia data now shown). Figure 5C shows maps of difference in parallel and perpendicular diffusivities, between the baseline and the 4-week and 6-week time points. With DTI and image mapping techniques, it is possible to track the changes in MR signals at each pixel efficiently.
Figure 5.An example of using image mapping techniques to trace axon and myelin pathology in cuprizone-treated mice.
Conclusion
Even though DTI is now a routine procedure in clinics, DTI of rodent brains and spinal cords have just started in the last few years. This review describes the existing applications and technical considerations of DTI for study of WM injury in rodent models. With the recent availability of high field animal imaging instruments and advances in gradient and shimming systems, successful rodent DTI-based studies have been increasingly reported. Future advancements in this area will certainly benefit biomedical researchers in related fields.
Future perspective
In the next 5–10 years, DTI of rodent brain and the spinal cord will be able to achieve higher resolution with advanced imaging hardware, with ex vivo DTI likely to reach 20–30 μm, which is the approximate theoretical resolution limit. During the same time, our knowledge on the relationships between DTI signals and WM pathology will progress and result in more sensitive DTI-based markers for detecting and monitoring WM pathology.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
* of interest
References
- Basser P, Pierpaoli C: Microstructural and physiological features of tissues elucidated by quantitative-dfifusion-tensor MRI. J. Magn. Reson. B. 111, 209–219 (1996).
- Le Bihan D: Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 4(6), 469–480 (2003).
- Mori S, Zhang J: Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51(5), 527–539 (2006). & An introduction to diffusion tensor imaging (DTI) for people not familiar with MRI.
- Basser PJ, Mattiello J, LeBihan D: Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 103(3), 247–254 (1994).
- Basser PJ, Pierpaoli C: Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B. 111(3), 209–219 (1996).
- Beaulieu C: The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 15(7–8), 435–455 (2002). & Provides a detailed description of cellular structures that may be related to anisotropic water diffusion in the white matter.
- Mori S, Crain BJ, Chacko VP, van Zijl PCM: Three dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annal. Neurol. 45, 265–269 (1999).
- Xue R, van Zijl PCM, Crain BJ, Solaiyappan M, Mori S: In vivo threedimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn. Reson. Med. 42, 1123–1127 (1999).
- Conturo TE, Lori NF, Cull TS et al.: Tracking neuronal fiber pathways in the living human brain. Proc. Natl Acad. Sci. USA 96(18), 10422–10427(1999).
- Jones DK, Simmons A, Williams SC, Horsfield MA: Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn. Reson. Med. 42(1), 37–41 (1999).
- Mori S, Itoh R, Zhang J et al.: Diffusion tensor imaging of the developing mouse brain. Magn. Reson. Med. 46, 18–23 (2001).
- Tyszka JM, Readhead C, Bearer EL, Pautler RG, Jacobs RE: Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage 29(4), 1058–1065 (2006).
- Nair G, Tanahashi Y, Low HP, Billings- Gagliardi S, Schwartz WJ, Duong TQ: Myelination and long diffusion times alter diffusion-tensor-imaging contrast in myelin-deficient shiverer mice. Neuroimage. 28(1), 165–174 (2005).
- Frank LR: Anisotropy in high angular resolution diffusion-weighted MRI. Magn. Reson. Med. 45(6), 935–939 (2001).
- Frank LR: Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magn. Reson. Med. 47(6), 1083–1099 (2002).
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ: High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn. Reson. Med. 48(4), 577–582 (2002).
- Tuch DS, Reese TG, Wiegell MR, Wedeen VJ: Diffusion MRI of complex neural architecture. Neuron 40(5), 885–895 (2003).
- Wedeen V, Reese T, Tuch DS et al.: Mapping fiber orientation spectra in cerebral white matter with Fourier-transform diffusion MRI. Presented at: Annual Meeting of the International Society of Magnetic Resonance in Medicine (ISMRM). Denver, Colorodo, USA, 1–7 April 2000.
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH: Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20(3), 1714–1722 (2003).
- Lehmann HC, Zhang J, Mori S, Sheikh KA: Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp. Neurol. 223(1), 238–244 (2010).
- Takagi T, Nakamura M, Yamada M et al.: Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage 44(3), 884–892 (2009).
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D: Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 27(44), 11869–11876 (2007).
- Kozlowski P, Raj D, Liu J, Lam C, Yung AC, Tetzlaff W: Characterizing white matter damage in rat spinal cord with quantitative MRI and histology. J. Neurotrauma 25(6), 653–676 (2008).
- Stone BS, Zhang J, Mack DW, Mori S, Martin LJ, Northington FJ: Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann. Neurol. 64(5), 535–546 (2008).
- Ding G, Jiang Q, Li L et al.: Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J. Cereb. Blood Flow Metab. 28(8), 1440–1448 (2008).
- Asanuma T, Doblas S, Tesiram YA et al.: Diffusion tensor imaging and fiber tractography of C6 rat glioma. J. Magn. Reson. Imaging 28(3), 566–573 (2008).
- Wang Y, Zhang J, Mori S, Nathans J: Axonal growth and guidance defects in Frizzled3 knock-out mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J. Neurosci. 26(2), 355–364 (2006).
- Song SK, Yoshino J, Le TQ et al.: Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26(1), 132–140 (2005).
- Sun SW, Liang HF, Cross AH, Song SK: Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage 40(1), 1–10 (2008). & Studied changes in DTI signals following a chain of pathological events in the mouse optic nerve after retinal ischemia.
- Zhang J, Jones M, DeBoy CA et al.: Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J. Neurosci. 29(10), 3160–3171 (2009). & Compared acute and chronic axon and myelin pathology and DTI signals in a rat spinal cord model.
- Matsushima GK, Morell P: The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 11(1), 107–116 (2001).
- Sun SW, Liang HF, Trinkaus K et al.: Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 55(2), 302–308 (2006).
- DeBoy CA, Zhang J, Dike S et al.: High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain 130(Pt 8), 2199–2210 (2007).
- Budde MD, Xie M, Cross AH, Song SK: Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J. Neurosci. 29(9), 2805–2813 (2009). & Correlated DTI parameters with pathology in a mouse model of multiple sclerosis.
- Drobyshevsky A, Derrick M, Wyrwicz AM et al.: White matter injury correlates with hypertonia in an animal model of cerebral palsy. J. Cereb. Blood Flow Metab. 27(2), 270–281 (2007).
- Herrera JJ, Chacko T, Narayana PA: Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J. Neurosci. Res. 86(2), 443–447 (2008).
- Budde MD, Kim JH, Liang HF et al.: Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn. Reson. Med. 57(4), 688–695 (2007).
- Jiang Y, Johnson GA: Microscopic diffusion tensor imaging of the mouse brain. Neuroimage 50(2), 465–471 (2010).
- Aggarwal M, Zhang J, Miller MI, Sidman RL, Mori S: Magnetic resonance imaging and micro-computed tomography combined atlas of developing and adult mouse brains for stereotaxic surgery. Neuroscience 162(4), 1339–1350 (2009).
- Harsan LA, Paul D, Schnell S et al.: In vivo diffusion tensor magnetic resonance imaging and fiber tracking of the mouse brain. NMR Biomed. 23(7), 884–896 (2010).
- Shepherd TM, Thelwall PE, Stanisz GJ, Blackband SJ: Aldehyde fixative solutions alter the water relaxation and diffusion properties of nervous tissue. Magn. Reson. Med. 62(1), 26–34 (2009). & Describes changes in diffusion signals caused by chemical fixation.
- Thelwall PE, Shepherd TM, Stanisz GJ, Blackband SJ: Effects of temperature and aldehyde fixation on tissue water diffusion properties, studied in an erythrocyte ghost tissue model. Magn. Reson. Med. 56(2), 282–289 (2006).
- Sun SW, Neil JJ, Liang HF et al.: Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Magn. Reson. Med. 53(6), 1447–1451 (2005).
- Benveniste H, Kim K, Zhang L, Johnson GA: Magnetic resonance microscopy of the C57BL mouse brain. Neuroimage 11, 601–611 (2000).
- Natt O, Watanabe T, Boretius S, Radulovic J, Frahm J, Michaelis T: High-resolution 3D MRI of mouse brain reveals small cerebral structures in vivo. J. Neurosci. Methods 120(2), 203–209 (2002).
- Badea A, Ali-Sharief AA, Johnson GA: Morphometric analysis of the C57BL/6J mouse brain. Neuroimage 37(3), 683–693 (2007).
- Jacobs RE, Ahrens ET, Dickinson ME, Laidlaw D: Towards a microMRI atlas of mouse development. Comput. Med. Imaging Graph 23(1), 15–24 (1999).
- Ma Y, Hof PR, Grant SC et al.: A threedimensional digital atlas database of the adult C57BL/6J mouse brain by magnetic resonance microscopy. Neuroscience 135(4), 1203–1215 (2005).
- MacKenzie-Graham A, Lee EF, Dinov ID et al.: A multimodal, multidimensional atlas of the C57BL/6J mouse brain. J. Anat. 204(2), 93–102 (2004).
- Petiet AE, Kaufman MH, Goddeeris MM, Brandenburg J, Elmore SA, Johnson GA: High-resolution magnetic resonance histology of the embryonic and neonatal mouse: a 4D atlas and morphologic database. Proc. Natl Acad. Sci. USA 105(34), 12331–12336 (2008).
- Chan E, Kovacevic N, Ho SK, Henkelman RM, Henderson JT: Development of a high resolution three-dimensional surgical atlas of the murine head for strains 129S1/SvImJ and C57Bl/6J using magnetic resonance imaging and micro-computed tomography. Neuroscience 144(2), 604–615 (2007).
- Xu D, Mori S, Shen D, van Zijl PC, Davatzikos C: Spatial normalization of diffusion tensor fields. Magn. Reson. Med. 50(1), 175–182 (2003).
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK: Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage 32(3), 1195–1204 (2006).
- Xie M, Tobin JE, Budde MD et al.: Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J. Neuropathol. Exp. Neurol. 69(7), 704–716 (2010).
- Kim JH, Loy DN, Liang HF, Trinkaus K, Schmidt RE, Song SK: Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn. Reson. Med. 58(2), 253–260 (2007).
- Gulani V, Webb AG, Duncan ID, Lauterbur PC: Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn. Reson. Med. 45(2), 191–195 (2001).
- Sizonenko SV, Camm EJ, Garbow JR et al.: Developmental changes and injury induced disruption of the radial organization of the cortex in the immature rat brain revealed by in vivo diffusion tensor MRI. Cereb. Cortex 17(11), 2609–2617 (2007).
- Ahrens ET, Laidlaw DH, Readhead C, Brosnan CF, Fraser SE, Jacobs RE: MR microscopy of transgenic mice that spontaneously acquire experimental allergic encephalomyelitis. Magn. Reson. Med. 40(1), 119–132 (1998).
- Mori S, Itoh R, Zhang J et al.: Diffusion tensor imaging of the developing mouse brain. Magn. Reson. Med. 46(1), 18–23 (2001).
- Zhang J, Richards LJ, Yarowsky P, Huang H, van Zijl PC, Mori S: Three-dimensional anatomical characterization of the developing mouse brain by diffusion tensor microimaging. Neuroimage 20(3), 1639–1648 (2003).