Review Article - Imaging in Medicine (2010) Volume 2, Issue 2
Current advances in CT imaging of stroke
Jeremy L Rempel and Richard I Aviv†
Department of Neuroradiology, Sunnybrook Health Sciences Centre, 2075 Bayview Avenue, Toronto, M4N 3M5, ON, Canada
- *Corresponding Author:
- Richard I Aviv
Department of Neuroradiology
Sunnybrook Health Sciences Centre 2075
Bayview Avenue, Toronto, M4N 3M5 ON, Canada
Tel: +1 416 480 4372
Fax:+1 416 480 5855
E-mail: richard.aviv@sunnybrook.ca
Abstract
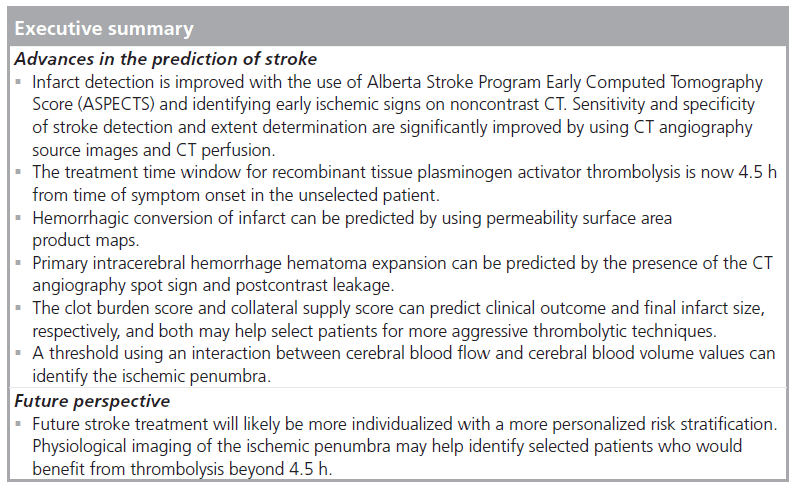
Stroke is the second most common cause of death in people over the age of 60. Imaging plays a pivotal role not only in stroke detection, but also in predicting infarct extent, hemorrhagic risk, tissue fate and clinical outcome. Noncontrast CT remains the modality of choice for investigation of acute stroke, with the use of the Alberta Stroke Program Early CT Score, CT angiography source images and CT perfusion significantly improving sensitivity. Hemorrhagic conversion risk may be predicted using permeability surface area product maps. The CT angiographic spot sign and postcontrast leakage visible on contrast CT can predict hematoma expansion in primary intracerebral hemorrhage. Clot burden and collateral blood-supply assessment can help identify patients who may benefit the most from more aggressive thrombolytic techniques. Physiological imaging of the ischemic penumbra may help identify selected patients that would benefit from thrombolysis beyond the current 4.5 h treatment window.
Keywords
clot burden; CT angiography; CT angiography spot sign; CT perfusion ; hemorrhagic conversion; penumbra; recombinant tissue plasminogen activator ; stroke
Stroke, brain attack, ischemic event and infarction are all terms used to describe the second most common cause of death in the USA in people over the age of 60 years and the fifth leading cause of death in people aged 15–59 years [1]. In those who survive the initial ischemic insult, 50% will have a persistent deficit and 20% will require transfer to a skilled nursing facility [2]. Imaging plays a pivotal role not only in the detection of infarction and intracranial hemorrhage, but also in determining the type of treatment and in predicting tissue fate and, ultimately, clinical outcome.
Advances in infarct detection
Noncontrast (NC)-CT remains the modality of choice for the acute investigation of acute stroke imaging [3]. Several early ischemic signs (EIS) exist, such as hypoattenuation, loss of gray–white matter definition, loss of basal ganglia outline [4], loss of the insular ribbon [5], cerebral swelling and cortical sulcal effacement [6]. Some of these findings may be detected as early as 45 min after onset of infarction; however, they can be very subtle [7]. Failure to detect these early subtle findings is not necessarily a clinical limitation, as this patient population is the intended target of thrombolysis. It is, however, important to quantify the extent of infarction, as larger infarcts are associated with higher thrombolysis-related complications.
To help quantify these findings, the Alberta Stroke Program Early CT Score (ASPECTS) was developed and has the advantage of high inter- and intraobserver agreement [8–10]. This scoring system is a ten-point score of the middle cerebral artery (MCA) territory, with a point lost for every area demonstrating hypoattenuation. It is unknown whether a critical threshold of infarct size exists below which thrombolytic treatment should not be administered. Studies have shown an increased risk of hemorrhage and poor clinical outcomes with an ASPECTS score of 7 or less [10,11]. A large meta-analysis found that the presence of EIS was associated with a three-times higher risk of poor outcome [12]. EIS affecting more than a third of the MCA territory in the European Cooperative Acute Stroke Study (ECASS) I study was associated with a trend to mortality with recombinant tissue plasminogen activator (rtPA) treatment [13]. Infarct volume of more than one third of the MCA territory has been shown to correspond well to an ASPECTS of over 7 [11]. However, increased mortality with EIS was not confirmed in ECASS II or reanalysis of the National Institute of Neurological Disorders and Stroke (NINDS) dataset [14–16]. The detection of EIS, therefore, remains key to the correct diagnosis of stroke and the extent may determine whether a patient receives thrombolysis treatment.
Unfortunately, the recognition of EIS remains generally poor with a sensitivity range reported between 30 [14] and 60% within 3-6 h, respectively [17]. A stroke or narrow window (window width and level of 35 Hounsfield units) improves sensitivity by increasing the distinction between gray- and white-matter density. Knowledge of infarction side does not appear to improve detection [18–20], whereas level of experience does [21]. NC-CT therefore may be negative in 40-60% of cases within 3 h of symptom onset.
The addition of CT angiography (CTA) provides valuable information relating to vessel occlusion and degree of collateralization; furthermore, it has also been shown to improve infarct detection [22–24]. By using CTA-source images (SI) reconstructed into 3‑mm slices, the sensitivity and specificity of MCA infarct detection, excluding small lacunar lesions, is 75-95 and 80-90%, respectively [25].
Visually, the contrast afforded by CTA-SI provides a greater demarcation between normal and abnormal tissue. Therefore, CTA-SI are superior in the determination of extent of infarction for all levels of experience [25]. Improved extent-detection explains the greater predictive value for final infarct size of CTA-SI [26]. CTA-SI hypoattenuation on older generation scanners was previously considered to be an estimate of cerebral blood volume as opposed to cytotoxic edema on NC-CT [23,27]. CTA-SI may visualize alterations in blood volume, which may not yet be associated with a threshold sufficient to result in NC-CT changes. The infarct detected on CTA-SI correlates well with the diffusion-weighted MRI abnormality and is an independent predictor of final infarct volume size in patients presenting within 6 h [23]. Applying ASPECTS scoring method to CTA-SI data and using a threshold of 7 or less has been demonstrated to be more sensitive than NC-CT [25] for final infarct prediction. Using this CTA-SI ASPECTS threshold may result in fewer patients being misclassified into groups that may result in inappropriate treatment compared with NC-CT.
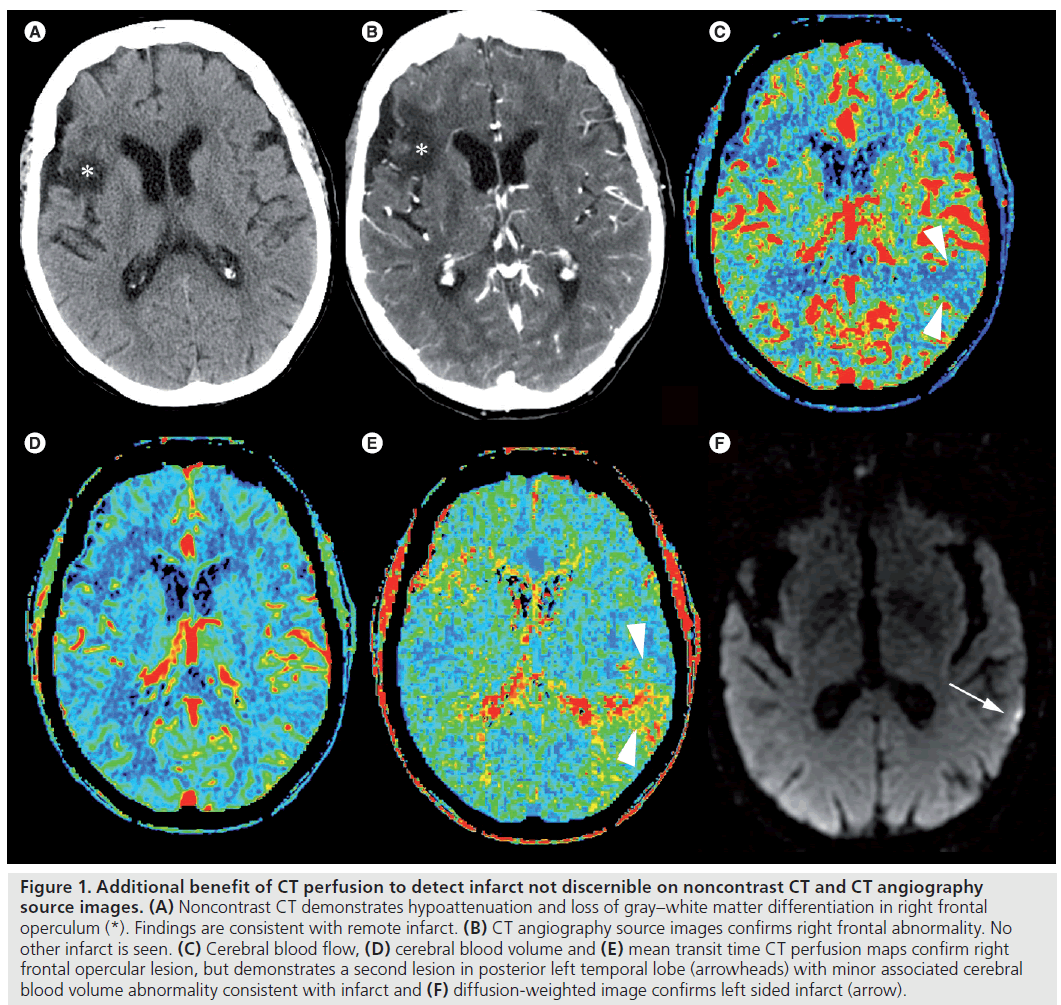
Computed tomography perfusion (CTP) data add the ability to differentiate between the infarct core and the ischemic penumbra. However, CTP data also have the ability to improve infarct detection [28–32]. The accuracy of CTP for stroke detection and extent determination is 72–86%. Sensitivities and specificities lie between 78 and 95%, respectively (Figure 1). False negatives are usually due to inadequate spacial coverage, lacunar infarcts or failure to detect multiple small emboli [33].
Figure 1. Additional benefit of CT perfusion to detect infarct not discernible on noncontrast CT and CT angiography source images. (A) Noncontrast CT demonstrates hypoattenuation and loss of gray–white matter differentiation in right frontal operculum (*). Findings are consistent with remote infarct. (B) CT angiography source images confirms right frontal abnormality. No other infarct is seen. (C) Cerebral blood flow, (D) cerebral blood volume and (E) mean transit time CT perfusion maps confirm right frontal opercular lesion, but demonstrates a second lesion in posterior left temporal lobe (arrowheads) with minor associated cerebral blood volume abnormality consistent with infarct and (F) diffusion-weighted image confirms left sided infarct (arrow).
Computed tomography perfusion ASPECTS scoring (applying the same criteria as NC‑CT ASPECTS but on CTP data) has been shown to be predictive of clinical outcome. Specifically, using cerebral blood volume (CBV) data and utilizing an ASPECTS threshold of 8 has been shown to predict patients who experience major neurologic improvement and have good clinical outcomes at 3 months (modified Rankin scale ≤2) [25,34]. Another study also reported a benefit of CBV ASPECTS over NC‑CT [35]. However, a lower threshold of 6–7 was used resulting in a higher false-negative rate of 13–18%.
Advances in extending the treatment time window
Thrombolytic therapy utilizing rtPA is currently the only approved medical therapy for the treatment of acute stroke if initiated within 4.5 h [36]. The NINDS study group was the first to report a significant benefit in patients who received rtPA, where patients were 30% more likely to have minimal or no disability at 3 months than were patients who received placebo [15]. However, the benefit of thrombolytic therapy is clearly time dependent, and may not end at the current 3–4.5 h time limit. Further evaluation of the NINDS data [37], as well as a large meta-analysis [38] of several large stroke studies [15,39–42], confirm that the benefit of thrombolytic therapy decreases with time from the onset of symptoms. However, both studies reported that this benefit likely extends beyond 3 h, but not likely to 6 h. In most countries, currently fewer than 2% of patient’s are treated with rtPA for acute stroke, primarily owing to delayed presentation to a stroke center [43].
Several earlier studies have investigated a treatment time window of 0–6 h after the onset of symptoms; however, these have failed to show a significant advantage with thrombolytic therapy beyond 3 h [39–41,44]. The Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) also looked at thrombolytic therapy 3–6 h after onset of symptoms and showed a trend towards lower infarct growth for patients receiving rtPA, but failed to show significance owing to small numbers [45]. Nevertheless, this study did show a significant association between reperfusion and improved clinical outcomes, suggesting reperfusion as a surrogate for clinical outcome.
The ECASS III investigators are the first to demonstrate a significant benefit from thrombolysis beyond 3 h and up to 4.5 h [36]. With a larger number of patients enrolled, an absolute benefit over the placebo group of 7.2% was demonstrated up to 4.5 h after treatment onset. A significantly higher incidence of symptomatic hemorrhage was demonstrated in the rtPA group; however, this was still quite rare (2.4%), with the incidence similar to other studies using a 3 h time window [15,40,46]. There was, however, no significant difference in mortality when the treatment window was extended to 4.5 h. Previously reported odds ratios for predicting a good clinical outcome following thrombolytic therapy clearly decrease with time, with an odds ratio of 2.81 for treatment within 0–90 min following symptom onset and falling to 1.55 for treatment 91–180 min following symptom onset [38]. The ECASS III investigators reported an odds ratio of 1.34 for patients treated 181–270 min following symptom onset. Although it now appears that thrombolytic therapy is beneficial in the unselected patient up to 4.5 h following symptom onset, patients should still be treated as soon as possible to gain the greatest benefit.
Physiological selection of patients may enable extension of the treatment time window. This approach would allow a selection of ideal patients within the traditional 4.5 h window but also include patients beyond conventional treatment time windows to be considered for treatment. The desmoteplase in acute stroke studies eloquently demonstrated this principle utilizing a visually determined 120% diffusionweighted MRI/perfusion-weighted MRI mismatch for patient enrollment up to 9 h. The use of a more fibrin-specific thrombolytic in this extended time period was associated with symptomatic hemorrhage rates comparable with studies shorter than 4.5 h. Despite a promising clinical efficacy in the Phase II studies [47,48], a subsequent Phase III study failed to show clinical benefit due to a variety of factors, including enrollment of patients without vessel occlusion, low placebo baseline National Institutes of Health Stroke Scale (NIHSS) scores and nondrug related, nonhemorrhagic deaths in the treatment group [49].
A number of questions remain to be answered before physiological imaging becomes a reality. There is some doubt in neurology circles as to how many patients may still demonstrate a penumbra in the extended time window. Anecdotally and experimentally, the ischemic penumbra is also demonstrated to decrease progressively with time from symptom onset, but is also shown to persist in up to 44% of patients imaged 18–24 h following symptom onset [50]. There is much optimism that appropriately selected subsets of acute stroke patients with persisting penumbra and a vessel occlusion may benefit from thrombolytic therapy well beyond the 4.5 h time window.
Advances in predicting hemorrhagic transformation of infarct risk & intracerebral hemorrhage expansion
An important function of NC‑CT is the detection of intracranial hemorrhage – a contraindication to thrombolysis. However, hemorrhagic transformation is seen in up to 43% of CT studies [15,40,47,51]. Hemorrhagic transformation is considered a complication of thrombolytic therapy; however, it is also part of the natural evolution of cerebral infarction. The ECASS investigators divided hemorrhage into a radiologic classification, where hemorrhagic ischemia (HI) described petechial hemorrhage without mass effect, and parenchymal hematoma (PH) denoted hematoma with mass effect. Both HI and PH are further sub-classified into whether hemorrahge involves less (HI1 and PH1) or more than 30% (HI2 and PH2) of the infarcted tissue.
Multiple risk factors are known for the development of hemorrhagic transformation, including baseline stroke severity [52,53], time to reperfusion [54], thrombolytic protocol violations [39,40], rtPA treatment [38,51,52], white matter disease burden [55], aspirin [51] and heparin use [56].
Disruption of blood–brain barrier integrity is considered focal to the development of hemorrhagic transformation. A two-phase CTP acquisition allows for permeability surface area product (PS) maps to be acquired [57]. PS is used to assess the rate of contrast agent extravasation from the intravascular to the extravascular space through a disrupted blood–brain barrier [58]. Contrast agent extravasation leads to prolonged enhancement of the tissue beyond the intravascular or first phase of a CTP study; therefore, requiring a two-phase acquisition for PS determination.
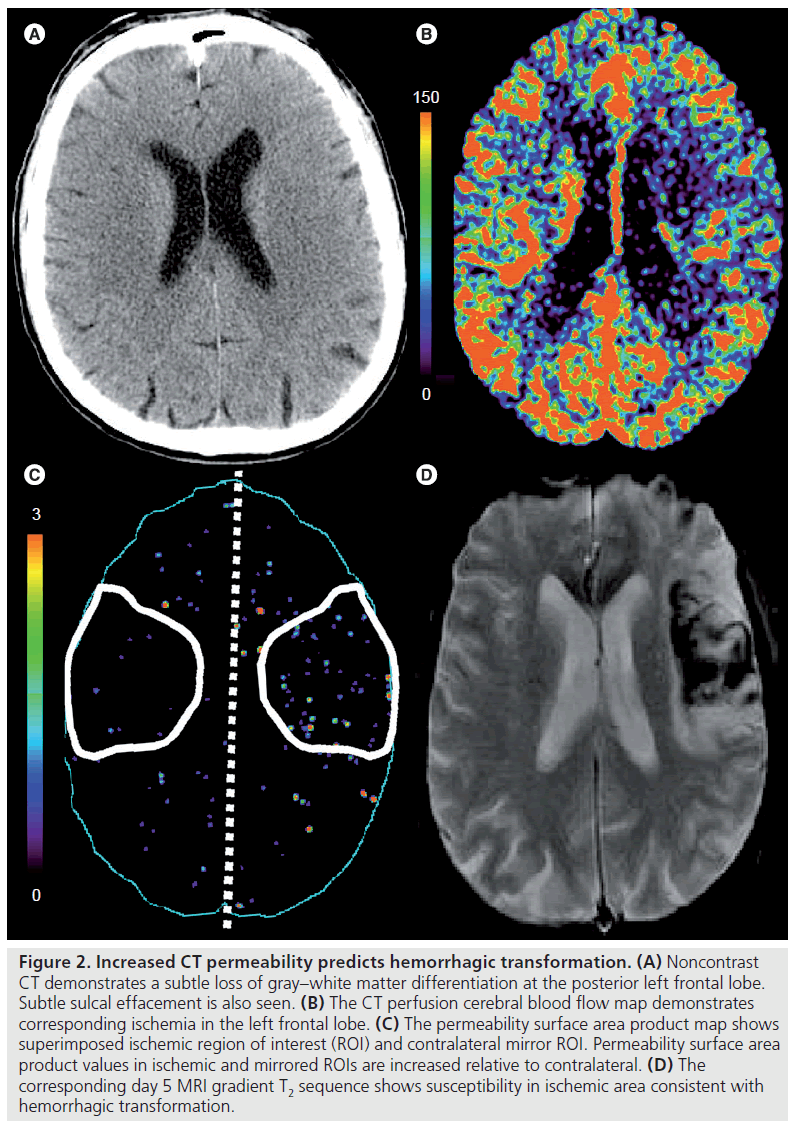
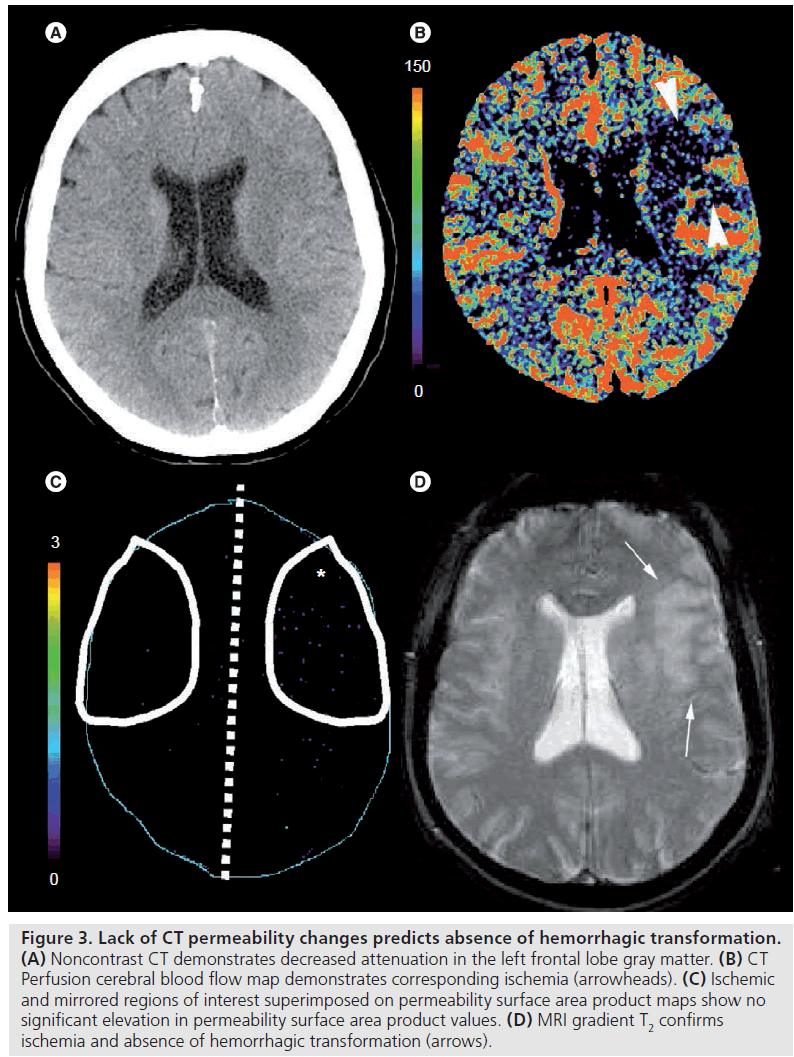
Aviv et al. demonstrated a significantly higher ischemic PS region in patients with hemorrhagic transformation compared with those without [57]. A PS threshold of 0.23 ml/min/100 g enabled differentiation between those patients undergoing hemorrhagic transformation (Figure 2) and those who did not (Figure 3). No significant difference in ischemic severity measured by cerebral blood flow (CBF) was found in the two groups. Patients treated with rtPA were more likely to have hemorrhagic transformation; however, there was no significant difference in PS between the rtPA- and non-rtPA-treated patients.
Figure 2. Increased CT permeability predicts hemorrhagic transformation. (A) Noncontrast CT demonstrates a subtle loss of gray–white matter differentiation at the posterior left frontal lobe. Subtle sulcal effacement is also seen. (B) The CT perfusion cerebral blood flow map demonstrates corresponding ischemia in the left frontal lobe. (C) The permeability surface area product map shows superimposed ischemic region of interest (ROI) and contralateral mirror ROI. Permeability surface area product values in ischemic and mirrored ROIs are increased relative to contralateral. (D) The corresponding day 5 MRI gradient T2 sequence shows susceptibility in ischemic area consistent with hemorrhagic transformation.
Figure 3. Lack of CT permeability changes predicts absence of hemorrhagic transformation. (A) Noncontrast CT demonstrates decreased attenuation in the left frontal lobe gray matter. (B) CT Perfusion cerebral blood flow map demonstrates corresponding ischemia (arrowheads). (C) Ischemic and mirrored regions of interest superimposed on permeability surface area product maps show no significant elevation in permeability surface area product values. (D) MRI gradient T2 confirms ischemia and absence of hemorrhagic transformation (arrows).
Primary intracerebral hemorrhage
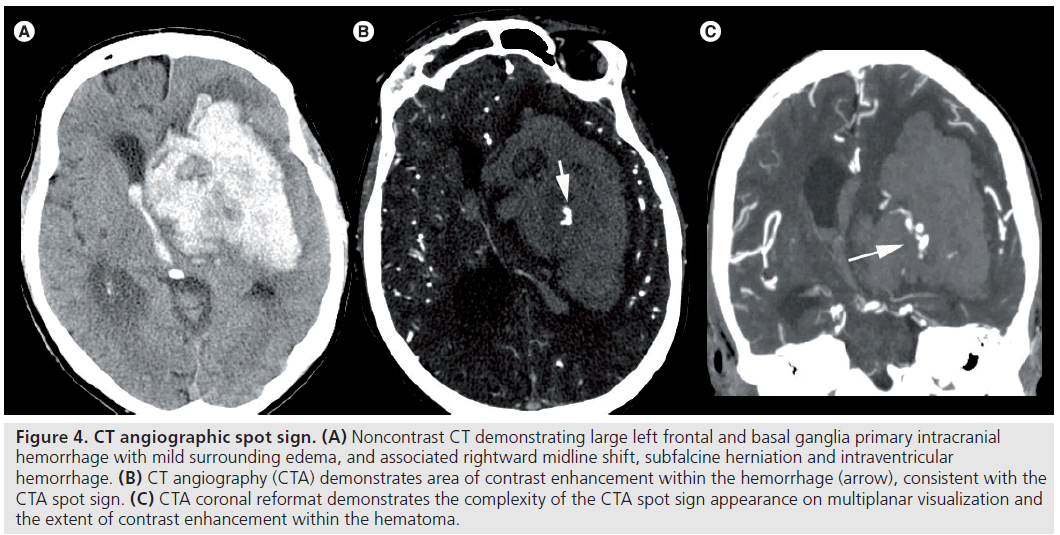
Intracerebral hemorrhage accounts for 10–30% of all strokes [59] with outcomes significantly worse than with ischemic stroke. Up to 50% mortality at 30 days has been documented [60], and hematoma size has been demonstrated to be one of the most important predictors of 30 day mortality [61]. Hematoma expansion is also highly predictive of neurological deterioration [62–64] and is an independent predictor of mortality and functional outcomes [65]. Clinical risk factors for hematoma expansion include hyperglycemia [64,66], hypertension [67] and anticoagulation [68–70]. The CTA spot sign has been described by several authors as a reliable marker of hematoma expansion and poor clinical outcome (Figure 4) [71–73]. This CTA spot sign is defined as one or more foci of contrast density within the hematoma – usually twice that of hematoma background – not communicating with vessels beyond the hematoma margin on CTA source images (Figure 5) [71].
Figure 4.CT angiographic spot sign. (A) Noncontrast CT demonstrating large left frontal and basal ganglia primary intracranial hemorrhage with mild surrounding edema, and associated rightward midline shift, subfalcine herniation and intraventricular hemorrhage. (B) CT angiography (CTA) demonstrates area of contrast enhancement within the hemorrhage (arrow), consistent with the CTA spot sign. (C) CTA coronal reformat demonstrates the complexity of the CTA spot sign appearance on multiplanar visualization and the extent of contrast enhancement within the hematoma.
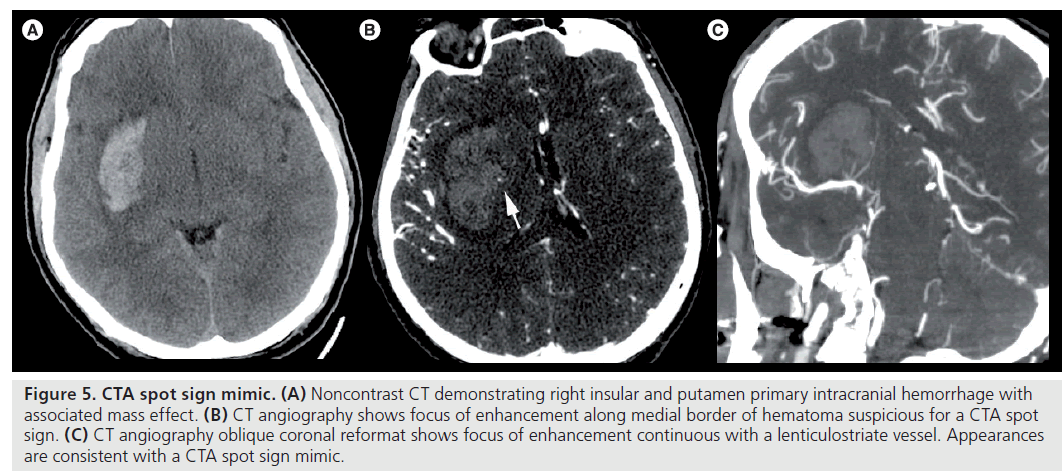
Figure 5.CTA spot sign mimic. (A) Noncontrast CT demonstrating right insular and putamen primary intracranial hemorrhage with associated mass effect. (B) CT angiography shows focus of enhancement along medial border of hematoma suspicious for a CTA spot sign. (C) CT angiography oblique coronal reformat shows focus of enhancement continuous with a lenticulostriate vessel. Appearances are consistent with a CTA spot sign mimic.
However, a small number of hematomas continue to expand in the absence of a CTA spot sign. Ederies et al. demonstrated contrast accumulation, termed postcontrast leakage (PCL), within a hematoma on postcontrast CT performed after initial CTA was associated with hematoma expansion [74]. Patients with PCL and a CTA spot sign tended to have larger absolute hematoma expansion and demonstrated larger initial hematoma volumes. However, in 60% of patients demonstrating only PCL, significant hematoma expansion was also documented. Recombinant factor VIIIa is a treatment option for reducing hematoma expansion, and is currently under investigation within a multicenter NINDS funded study, the CTA Spot Sign for Predicting and Treating Intracerebral Hemorrhage Growth (STOP-IT) study. Recombinant factor VIIIa is associated with thrombotic risks and it is expected that the CTA spot sign may help identify patients who would benefit the most from this type of treatment.
Advances in understanding clot burden & vascular recanalization
Recanalization is the main target of stroke treatment strategies to reduce tissue at risk and reverse neurologic deficits. Revascularization first depends on recanalization of the primary arterial occlusive lesion, but also on reperfusion of the distal vascular bed [75,76].
The success of recanalization is dependent upon the clot composition, thrombolytic technique, clot burden and location, and collateral supply [77–81]. Larger, more proximal clot is harder to treat and leads to worse clinical outcomes compared with smaller, more distally located clot [78,81–83]. This has been demonstrated indirectly with patient’s having a hyperattenuated middle cerebral artery sign, larger final infarct volumes and worse functional outcomes [80]. Larger, more proximal clot burden involving the distal internal carotid and the middle cerebral artery have been demonstrated to have even lower early recanalization rates and worse neurologic improvement rates compared with middle cerebral artery occlusion alone [84].
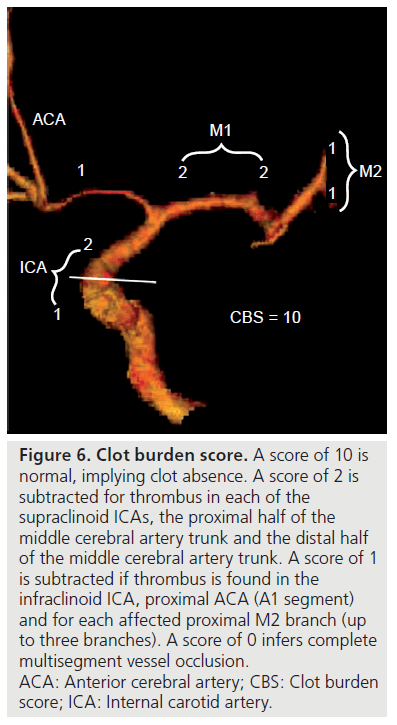
The concept of clot burden has been indirectly examined using the hyperattenuated arterial sign on NC‑CT [78,85,86], and more directly via conventional angiography [87]. CTA provides a widely available and rapid assessment of intracranial and cervical circulation, allowing for the evaluation of the extent of clot. A grading system or clot-burden score has been developed, using a scale of 0–10 (Figure 6) [88]. A score of 10 is normal, implying clot absence. A score of 2 is subtracted for thrombus in each of the supraclinoid internal carotid arteries (ICAs), the proximal half of the MCA trunk, and the distal half of the MCA trunk. A score of 1 is subtracted if thrombus is found in the infraclinoid ICA, proximal anterior cerebral artery (A1 segment) and for each affected proximal M2 branch (up to two branches). A score of 0 infers complete multisegment vessel occlusion.
Figure 6.Clot burden score. A score of 10 is normal, implying clot absence. A score of 2 is subtracted for thrombus in each of the supraclinoid ICAs, the proximal half of the middle cerebral artery trunk and the distal half of the middle cerebral artery trunk. A score of 1 is subtracted if thrombus is found in the infraclinoid ICA, proximal ACA (A1 segment) and for each affected proximal M2 branch (up to three branches). A score of 0 infers complete multisegment vessel occlusion. ACA: Anterior cerebral artery; CBS: Clot burden score; ICA: Internal carotid artery.
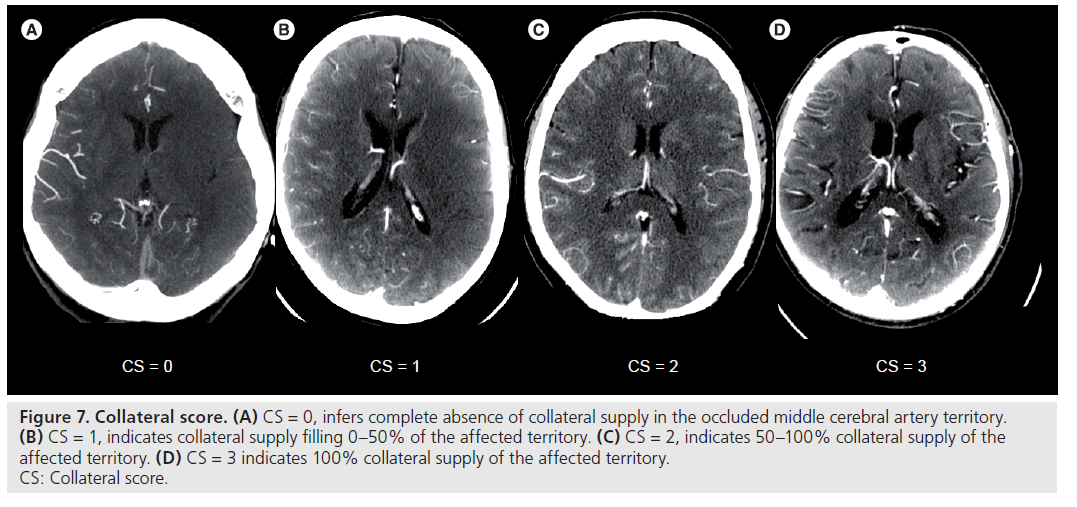
Collateral blood supply through peripheral leptomeningeal sources is also important and has been demonstrated to correlate with smaller final infarct volumes [77,89]. Evaluation of collateral blood supply can be a challenge, often due to the small vessel size and complex routes [90]. A grading system of collateral blood flow using conventional angiography demonstrated a significant correlation between the degree of collateralization and favorable outcomes [91,92]. The presence of collateral circulation was the only radiologic predictor of favorable outcomes in one study [92]. A grading system using multidetector CTA has also been developed (Figure 7), which grades the degree of leptomeningeal collateral blood supply in the MCA territory [93]. A score of 0 infers complete absence of collateral supply in the occluded MCA territory. A score of 1 indicates collateral supply filling 0–50% of the affected territory, and a score of 2 indicates 50–100% collateral supply of the affected territory. A score of 3 indicates 100% collateral supply of the affected territory.
Figure 7.Collateral score. (A) CS = 0, infers complete absence of collateral supply in the occluded middle cerebral artery territory. (B) CS = 1, indicates collateral supply filling 0–50% of the affected territory. (C) CS = 2, indicates 50–100% collateral supply of the affected territory. (D) CS = 3 indicates 100% collateral supply of the affected territory. CS: Collateral score.
Increasingly, CTA is being used in the primary assessment of patients presenting with acute stroke. The goal is to gather as much information as possible, particularly pertaining to vascular anatomy and to the site and degree of vascular occlusion prior to thrombolytic therapy. The clot burden and collateral blood supply scoring systems provide additional information in the prediction of stroke outcome. The clot burden score is an independent predictor of clinical and radiologic outcomes in acute MCA territory stroke [88]. Patient’s with smaller clot burden are more likely to have smaller baseline infarcts, lower baseline NIHSS scores, achieve good clinical outcomes and have smaller final infarct sizes. Following intravenous rtPA therapy, patients with smaller clot-burden also demonstrate higher revascularization rates [88]. A clot burden score threshold of less than 6 has a modest sensitivity (73%) and specificity (64.6%) for predicting good clinical outcomes. These patients had higher baseline ASPECTS, smaller CBV and CBF volumes, higher collateral blood supply score and smaller final infarct size.
The collateral blood supply score, although not able to independently predict clinical outcomes, was able to predict final infarct size [88]. In the absence of adequate collateral flow, irreversible neuronal damage occurs within minutes [94]. Collateral supply helps to prevent or limit the degree of infarction until recanalization allows ischemic penumbra reperfusion [92]. It therefore follows that good collateral blood supply can predict smaller infarct volumes.
Increasingly advanced imaging techniques are being used to stratify patient risk for stroke management strategies [47,95]. Proximal clot location is increasingly felt to be an important determinant in outcome [78,96–98], with patients with larger clot burdens having larger infarction volumes and poor clinical outcomes [99,100]. Multiple recanalization techniques are available, including intravenous and intra-arterial thrombolysis, mechanical thrombectomy devices, and low-frequency pulsed-waved transcranial Doppler sonography [82,83,101,102].
Recanalization rates do vary depending on location and extent of thrombus. Occlusion length has been demonstrated to be an important factor in the efficacy and complication rate of mechanical thrombectomy [81]. More invasive techniques offer the benefit of higher recanalization rates, however, at the expense of increased complications, such as parenchymal hemorrhage [103] or vessel injury/rupture [104]. Recanalization rates utilizing intravenous rtPA are only 9% for combined ICA and MCA occlusions, compared with 39% for isolated MCA occlusions [84]. Patient stratification utilizing tools, such as clot burden scores, ASPECTS, NIHSS scores and CTP data, can help identify patients with a larger thrombus extent and, therefore, help select patients who may benefit from more aggressive recanalization strategies.
Advances in imaging thresholds & predictors of tissue fate
The term ischemic penumbra is defined as a region of hypoperfused, electrically silent and functionally impaired but viable tissue [94]. This penumbra or tissue at risk of infarction has been a primary goal of neuroimaging techniques to identify. rtPA is currently the only drug approved for acute stroke treatment in North America. By clot lysis, this medication works to restore blood flow to ischemic regions, thereby potentially salvaging this at-risk tissue.
Early MRI studies identified tissue surrounding the diffusion abnormality as the ischemic penumbra using diffusion–perfusion imaging. Diffusion restriction was considered irreversibly infarcted tissue, and the remaining perfusion abnormality the ischemic penumbra [105].
This diffusion–perfusion model, however, is likely to be too simplistic. Firstly, the perfusion abnormality often overestimates final infarct volume [106], thereby overestimating the tissue at risk. Secondly, the diffusion abnormality is not necessarily irreversibly infarcted tissue, with some studies demonstrating reversibility with early revascularization [107,108]. Using these concepts, Kidwell et al. developed a modified model of the ischemic penumbra in which the penumbra includes the diffusion–perfusion mismatch region (minus the region of benign oligemia) as well as a portion of the initial diffusion abnormality itself [109].
Despite advances in MRI imaging techniques, CT remains the most widely available and used imaging modality in acute stroke. CTP data utilizing CBF and CBV maps have also been demonstrated to be able to identify the ischemic penumbra [29,30,110]. A CBF threshold of 25 ml/min/100 g has been correlated with tissue progressing to infarction in the absence of recanalization [111,112]. CBV values tend to remain constant or even slightly increase in the ischemic penumbra and fall in areas of infarction. Utilizing CBF and CBV thresholds in isolation to identify the ischemic penumbra have not been highly accurate, prompting the use of logistic regression and multivariate analysis to identify ways of increasing the sensitivity and specificity of penumbra identification. One such method identified an interaction between CBF and CBV that provided maximum separation between penumbra and infarcted tissue in gray matter. Any combination of CBF and CBV values above the derived threshold of 31.3 were classified as penumbra, and below this value as infarction. The model demonstrated a sensitivity, specificity and overall accuracy of 97.0, 97.2 and 97.1%, respectively, for infarct detection [112]. The model focuses on the CBF and CBV alterations to characterize the ischemic penumbra, and the matched decrease in both parameters in areas of infarction [110,113]. Given that infarct CBF values are slightly lower, and CBV values much lower in infarcted than penumbral tissue, the product of these two values maximizes the separation between these two entities.
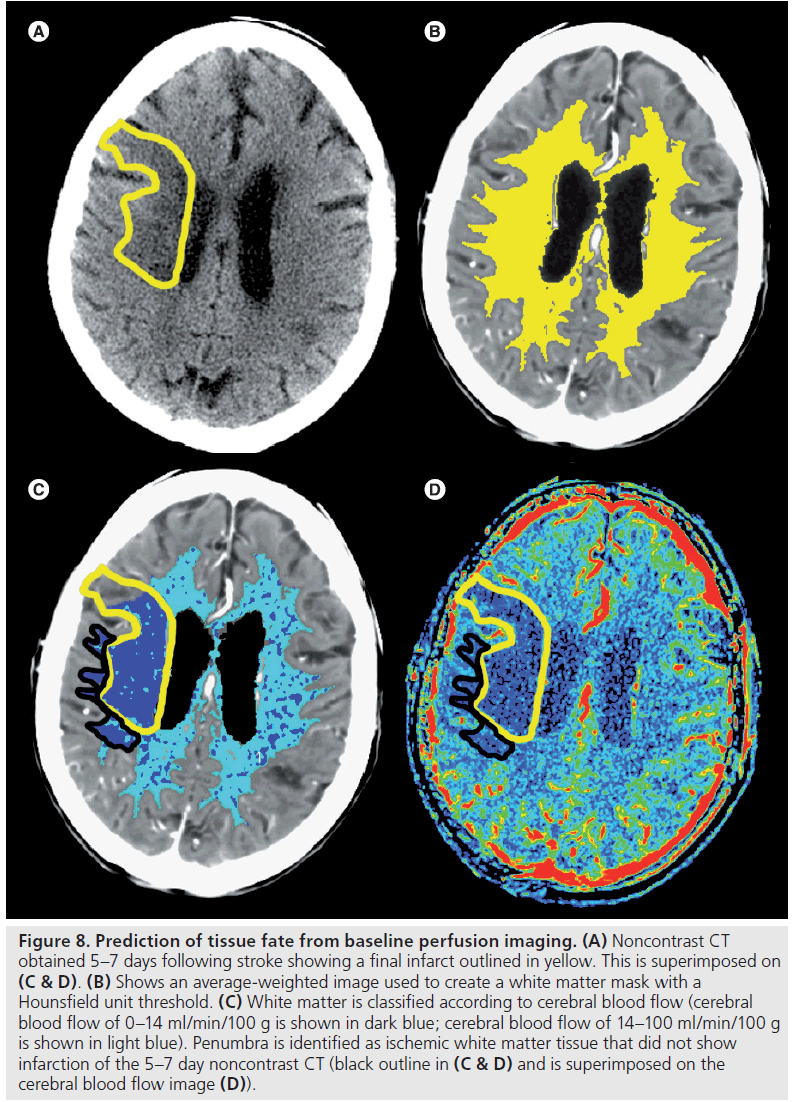
Perfusion and diffusion thresholds for infarction are likely to be different between gray and white matter [114–118]. A CBF threshold of 14 ml/min/100 g was shown to correlate well with final white matter infarct volume in the absence of recanalization [116,118,119]. The same model described for identifying gray matter penumbra has also been applied to white matter. Using logistic regression analysis, the threshold of 8.14 was identified for determining infarction from ischemic penumbra in white matter with a sensitivity, specificity and overall accuracy of 95, 94 and 95%, respectively (Figure 8) [118]. The same authors determined thresholds using CBV and CBF values in isolation. A CBV threshold of 0.82 ml/100 g resulted in a sensitivity and specificity for final infarct of 76 and 88%, respectively; however, there was significant overlap between the penumbra and final infarct regions. Using a CBF threshold resulted in slightly higher sensitivity and specificity of 81 and 91%, respectively; however, significant overlap between the penumbra and final infarct regions was still observed.
Figure 8.Prediction of tissue fate from baseline perfusion imaging. (A) Noncontrast CT obtained 5–7 days following stroke showing a final infarct outlined in yellow. This is superimposed on (C & D). (B) Shows an average-weighted image used to create a white matter mask with a Hounsfield unit threshold. (C) White matter is classified according to cerebral blood flow (cerebral blood flow of 0–14 ml/min/100 g is shown in dark blue; cerebral blood flow of 14–100 ml/min/100 g is shown in light blue). Penumbra is identified as ischemic white matter tissue that did not show infarction of the 5–7 day noncontrast CT (black outline in (C & D) and is superimposed on the cerebral blood flow image (D)).
An observed increased CBV in the ischemic penumbra can be explained by direct cerebral autoregulatory responses to maintain CBF by dilating precapillary vessels in response to the decreased perfusion pressure [120]. However, reduced CBV in infarcted tissue is not completely understood [22,29,110,113]. A possible explanation for a matched decrease in CBF and CBV involves failure of autoregulation in response to severe hypoperfusion [120]. Metabolic mechanisms, such as neuronal death resulting in significantly elevated extracellular potassium concentrations and vasoconstriction, have also been proposed [121].
Conclusion
There have been many advances in the imaging of acute stroke in recent years. The identification of stroke on NC‑CT has improved by identifying EIS and systematically assessing for stroke by using ASPECTS. Sensitivity and specificity have further improved by using CTA‑SI and CTP data to identify the presence of infarction.
In the unselected patient, thrombolytic therapy with rtPA improves clinical outcomes up to 4.5 h following symptom onset. It remains to be determined whether a selected patient with an identifiable ischemic penumbra may benefit beyond this treatment time window.
Hemorrhagic conversion risk can be predicted by assessing the blood–brain barrier using a two-phase CTP acquisition and acquiring PS data. In cases of primary intracerebral hemorrhage, hematoma expansion can be predicted by using the CTA spot sign and PCL. Assessing the extent of clot burden and degree of collateral blood supply not only aids in the prediction of clinical outcomes, but helps select patients who may benefit from more aggressive thrombolytic therapies, such as intra-arterial rtPA or mechanical thrombectomy.
Cerebral blood flow thresholds for both gray and white matter that can predict the final volume of infarction in the absence of recanalization have been identified. Using the interactions between CBF and CBV data allow accurate determinations of the ischemic penumbra and salvageable brain tissue if recanalization occurs.
Acute stroke imaging assessment now offers a wealth of information to the clinician by identifying the presence and extent of infarction, predicting hemorrhagic transformation and hematoma expansion, selecting patients for more aggressive thrombolysis and identifying the presence of ischemic penumbra, ultimately, predicting clinical outcomes. This information may help promote a shift from treating the unselected stroke patient to individualizing stroke therapy and selecting patients who may benefit from aggressive thrombolysis beyond the current treatment time window.
Future perspective
Acute stroke imaging will continue to evolve in the coming years. This will include a more widespread use and implementation of the advanced imaging techniques described. A standard NC‑CT will no longer be acceptable as an acute stroke workup. With the extensive information that comes from the use of CTA‑SI and CTP, the detection and evaluation of the extent of infarction will be much more accurate. Using PS maps more routinely will allow the prediction of hemorrhagic conversion, thus allowing risk stratification prior to thrombolytic treatment.
Physiological imaging using CBF and CBV thresholds will allow an accurate assessment of the ischemic penumbra regardless of the time from symptom onset. Future work will no doubt study thrombolytic therapy in patients who demonstrate a persistent penumbra beyond the 4.5 h treatment time window. These selected patients will hopefully benefit from thrombolysis well beyond the current time constraints, thus opening thrombolytic therapy to a much larger patient population.
Additional thrombolytic agents will also likely be approved for clinical use. More fibrin specific agents, such as desmoteplase, may show clinical benefit specifically in the extended treatment time window.
Extending the treatment time window will also allow more routine intra-arterial treatment options for acute stroke. The use of current and new thrombolytic agents and continued evolution of mechanical thrombectomy devices will surely be seen.
Acute stroke imaging and treatment will continue to grow and evolve; however, the effective implementation and management of this information will likely be an ongoing challenge for most institutions. Acute stroke teams not only require easy access to advanced stroke imaging and stroke neurology care, but also the continued support from emergency department personnel and medical transport personnel, not to mention anesthesia and angiography support. The effectiveness of an acute stroke team will be dependent upon its weakest point and will serve as an ongoing focus of improvement for successful stroke treatment.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as: * of interest * of considerable interest
References
- Mackay J, Mensah GA: The Atlas ofHeart Disease and Stroke. World Health Organization and Centers for Disease Control, Geneva, Switzerland (2004).
- Public health and aging: hospitalizations for stroke among adults aged =65 years – United States, 2000. MMWR Morb. Mortal. Wkly Rep. 52(25), 586–589 (2003).
- Adams RJ, Albers G, Alberts MJ et al.: Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke 39(5), 1647–1652 (2008).
- Tomura N, Uemura K, Inugami A et al.: Early CT finding in cerebral infarction: obscuration of the lentiform nucleus. Radiology 168(2), 463–467 (1988).
- Truwit CL, Barkovich AJ, Gean-Marton A, Hibri N, Norman D: Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology 176(3), 801–806 (1990).
- Na DG, Kim EY, Ryoo JW et al.: CT sign of brain swelling without concomitant parenchymal hypoattenuation: comparison with diffusion- and perfusion-weighted MR imaging. Radiology 235(3), 948–992 (2005).
- Kucinski T, Koch C, Grzyska U et al.: The predictive value of early CT and angiography for fatal hemispheric welling in acute stroke. AJNR Am. J. Neuroradiol. 19(5), 839–846 (1998).
- Coutts SB, Demchuk AM, Barber PA et al.: Interobserver variation of ASPECTS in real time. Stroke 35(5), e103–e105 (2004).
- Pexman JH, Barber PA, Hill MD et al.: Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. Am. J. Neuroradiol. 22(8), 1534–1542 (2001).
- Barber PA, Demchuk AM, Zhang J, Buchan AM: Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 355(9216), 1670–1674 (2000).
- Dzialowski I, Hill MD, Coutts SB et al.: Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke 37(4), 973–978 (2006).
- Wardlaw JM, Mielke O: Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment – systematic review. Radiology 235(2), 444–453 (2005).
- Hacke W, Kaste M, Fieschi C et al.: Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 274(13), 1017–1025 (1995).
- Patel SC, Levine SR, Tilley BC et al.: Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA 286(22), 2830–2838 (2001).
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group: Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 333(24), 1581–1588 (1995).
- Demchuk AM, Hill MD, Barber PA et al.: Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke 36(10), 2110–2115 (2005).
- von Kummer R, Bourquain H, Bastianello S et al.: Early prediction of irreversible brain damage after ischemic stroke at CT. Radiology 219(1), 95–100 (2001).
- Tomsick TA, Brott TG, Chambers AA et al.: Hyperdense middle cerebral artery sign on CT: efficacy in detecting middle cerebral artery thrombosis. AJNR Am. J. Neuroradiol. 11(3), 473–477 (1990).
- von Kummer R, Holle R, Gizyska U et al.: Interobserver agreement in assessing early CT signs of middle cerebral artery infarction. Am. J. Neuroradiol. 17(9), 1743–1748 (1996).
- Wardlaw JM, Dorman PJ, Lewis SC, Sandercock PA: Can stroke physicians and neuroradiologists identify signs of early cerebral infarction on CT? J. Neurol. Neurosurg. Psychiatry 67(5), 651–653 (1999).
- von Kummer R: Effect of training in reading CT scans on patient selection for ECASS II. Neurology 51(3 Suppl. 3), S50–S52 (1998).
- Lev MH, Segal AZ, Farkas J et al.: Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke 32(9), 2021–2028 (2001).
- Schramm P, Schellinger PD, Fiebach JB et al.: Comparison of CT and CT angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke 33(10), 2426–2432 (2002).
- Ezzeddine MA, Lev MH, McDonald CT et al.: CT angiography with whole brain perfused blood volume imaging: added clinical value in the assessment of acute stroke. Stroke 33(4), 959–966 (2002).
- Aviv RI, Shelef I, Malam S et al.: Early stroke detection and extent: impact of experience and the role of computed tomography angiography source images. Clin. Radiol. 62(5), 447–452 (2007).
- Coutts SB, Lev MH, Eliasziw M et al.: ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke 35(11), 2472–2476 (2004).
- Hamberg LM, Hunter GJ, Kierstead D et al.: Measurement of cerebral blood volume with subtraction three-dimensional functional CT. Am. J. Neuroradiol. 17(10), 1861–1869 (1996).
- Ueda T, Yuh WTC, Maley JE et al.: Outcome of acute ischemic lesions evaluated by diffusion and perfusion MR imaging. Am. J. Neuroradiol. 20(6), 983–989 (1999).
- Wintermark M, Reichhart M, Cuisenaire O et al.: Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusionweighted magnetic resonance imaging in acute stroke patients. Stroke 33(8), 2025–2031 (2002).
- Eastwood JD, Lev MH, Wintermark M et al.: Correlation of early dynamic CT perfusion imaging with whole-brain MR diffusion and perfusion imaging in acute hemispheric stroke. Am. J. Neuroradiol. 24(9), 1869–1875 (2003).
- Schramm P, Schellinger PD, Klotz E et al.: Comparison of perfusion computed tomography and computed tomography angiography source images with perfusionweighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours’ duration. Stroke 35(7), 1652–1658 (2004).
- Hopyan J, Ciarallo A, Dowlatshahi D et al.: Incremental benefit of CT perfusion for certainty of stroke diagnosis over unenhanced CT and CT angiographic source images. Radiology (2010) (In Press).
- Wintermark M, Fischbein NJ, Smith WS et al.: Accuracy of dynamic perfusion CT with deconvolution in detecting acute hemispheric stroke. Am. J. Neuroradiol. 26(1), 104–112 (2005).
- Aviv RI, Mandelcorn J, Chakraborty S et al.: Alberta Stroke Program Early CT Scoring of CT perfusion in early stroke visualization and assessment. Am. J. Neuroradiol. 28(10), 1975–1980 (2007). & Using CT perfusion data improves the sensitivity and specificity of both stroke detection and extent of infarction and is predictive of good clinical outcome using an Alberta Stroke Program Early CT Score threshold of 8.
- Parsons MW, Pepper EM, Chan V et al.: Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann. Neurol. 58(5), 672–679 (2005).
- Hacke W, Kaste M, Bluhmki E et al.: Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 359(13), 1317–1329 (2008). & Clinical outcomes are significantly improved with recombinant tissue plasminogen activator treatment up to 4.5 h following symptom onset. Hemorrhage risks are similar to studies using 3 h time window.
- Marler JR, Tilley BC, Lu M et al.: Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology 55(11), 1649–1655 (2000).
- Hacke W, Donnan G, Fieschi C et al.: Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 363(9411), 768–774 (2004).
- Hacke W, Kaste M, Fieschi C et al.: Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352(9136), 1245–1251 (1998).
- Hacke W, Kaste M, Fieschi C et al.: Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 274(13), 1017–1025 (1995).
- Clark WM, Wissman S, Albers GW et al.: Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 282(21), 2019–2026 (1999).
- Clark WM, Albers GW, Madden KP, Hamilton S: The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 31(4), 811–816 (2000).
- Albers GW, Olivot JM: Intravenous alteplase for ischaemic stroke. Lancet 369(9558), 249–250 (2007).
- Savitz SI, Lew R, Bluhmki E, Hacke W, Fisher M: Shift analysis versus dichotomization of the modified Rankin scale outcome scores in the NINDS and ECASS-II trials. Stroke 38(12), 3205–3212 (2007).
- Davis SM, Donnan GA, Parsons MW et al.: Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebocontrolled randomised trial. Lancet Neurol. 7(4), 299–309 (2008).
- Wahlgren N, Ahmed N, Davalos A et al.: Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 369(9558), 275–282 (2007).
- Hacke W, Albers G, Al-Rawi Y et al.: The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a Phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 36(1), 66–73 (2005).
- Furlan AJ, Eyding D, Albers GW et al.: Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 37(5), 1227–1231 (2006).
- Hacke W, Furlan AJ, Al-Rawi Y et al.: Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 8(2), 141–150 (2009).
- Darby DG, Barber PA, Gerraty RP et al.: Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke 30(10), 2043–2052 (1999).
- Larrue V, von Kummer RR, Muller A, Bluhmki E: Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European–Australasian Acute Stroke Study (ECASS II). Stroke 32(2), 438–441 (2001).
- Larrue V, von Kummer R, del Zoppo G, Bluhmki E: Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 28(5), 957–960 (1997).
- Tong DC, Adami A, Moseley ME, Marks MP: Relationship between apparent diffusion coefficient and subsequent hemorrhagic transformation following acute ischemic stroke. Stroke 31(10), 2378–2384 (2000).
- Molina CA, Alvarez-Sabin J, Montaner J et al.: Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke 33(6), 1551–1556 (2002).
- Neumann-Haefelin T, Hoelig S, Berkefeld J et al.: Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke 37(10), 2463–2466 (2006).
- Kase CS, Furlan AJ, Wechsler LR et al.: Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology 57(9), 1603–1610 (2001).
- Aviv RI, d’Esterre CD, Murphy BD et al.: Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology 250(3), 867–877 (2009). & Hemorrhagic conversion risk can be predicted using a two-phase CT perfusion study to assess blood–brain barrier integrity using permeability surface area product maps.
- Lee T-Y: Functional CT: physiological models. Trends Biotechnol. 20, S3–S10 (2002).
- Broderick JP, Adams HP Jr, Barsan W et al.: Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 30(4), 905–915 (1999).
- Broderick JP, Brott T, Tomsick T, Huster G, Miller R: The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N. Engl. J. Med. 326(11), 733–736 (1992).
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G: Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 24(7), 987–993 (1993).
- Leira R, Davalos A, Silva Y et al.: Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology 63, 461–467 (2004).
- Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T: Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 27(10), 1783–1787 (1996).
- Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T: Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke 28(12), 2370–2375 (1997).
- Davis SM, Broderick J, Hennerici M et al.: Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66(8), 1175–1181 (2006).
- Becker KJ, Baxter AB, Bybee HM et al.: Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke 30(10), 2025–2032 (1999).
- Ohwaki K, Yono E, Nagashima H et al.: Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 35, 1353–1367 (2004).
- Toyoda K, Okada Y, Minematsu K et al.: Antiplatelet therapy contributes to acute deterioration of intracerebral hemorrhage. Neurology 65(7), 1000–1004 (2005).
- Yasaka M, Minematsu K, Naritomi H, Sakata T, Yamaguchi T: Predisposing factors for enlargement of intracerebral hemorrhage in patients treated with warfarin. Thromb. Haemost. 89(2), 278–283 (2003).
- Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J: Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 63(6), 1059–1064 (2004).
- Wada R, Aviv RI, Fox AJ et al.: CT angiography spot sign predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 38(4), 1257–1262 (2007). & The CT angiography spot sign can predict hematoma expansion in primary intracerebral hemorrhage.
- Goldstein JN, Fazen LE, Snider R et al.: Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 68(12), 889–894 (2007).
- Kim J, Smith A, Hemphill JC 3rd et al.: Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. Am. J. Neuroradiol. 29, 520–525 (2008).
- Ederies A, Demchuk A, Chia T et al.: Postcontrast CT extravasation is associated with hematoma expansion in CTA spot sign negative patients. Stroke 40(5), 1672–1676 (2009).
- Khatri P, Neff J, Broderick JP et al.: Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke 36(11), 2400–2403 (2005).
- Soares BP, Tong E, Hom J et al.: Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization. A proof of concept using CT in acute ischemic stroke patients. Stroke 41(1) e34–e40 (2009).
- Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C: Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke 20(6), 735–740 (1989).
- Somford DM, Nederkoorn PJ, Rutgers DR et al.: Proximal and distal hyperattenuating middle cerebral artery signs at CT: Different prognostic implications. Radiology 223(3), 667–671 (2002).
- Rosenthal ES, Schwamm LH, Roccatagliata L et al.: Role of recanalization in acute stroke outcome: rationale for a CT angiogram-based “benefit of recanalization” model. Am. J. Neuroradiol. 29(8), 1471–1475 (2008).
- Mattle HP, Arnold M, Georgiadis D et al.: Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke 39(2), 379–383 (2008).
- Gralla J, Burkhardt M, Schroth G et al.: Occlusion length is a crucial determinant of efficiency and complication rate in thrombectomy for acute ischemic stroke. Am. J. Neuroradiol. 29(2), 247–252 (2008).
- del Zoppo GJ, Higashida RT, Furlan AJ et al.: PROACT: a Phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in acute cerebral thromboembolism. Stroke 29(1), 4–11 (1998).
- del Zoppo GJ, Poeck K, Pessin MS et al.: Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann. Neurol. 32(1), 78–86 (1992).
- Kim YS, Garami Z, Mikulik R, Molina CA, Alexandrov AV: Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/middle cerebral artery occlusion and isolated middle cerebral artery occlusion. Stroke 36(4), 869–871 (2005).
- Tomsick T, Brott T, Barsan W et al.: Thrombus localization with emergency cerebral CT. AJNR Am. J. Neuroradiol. 13(1), 257–263 (1992).
- Manelfe C, Larrue V, von Kummer R et al.: Association of hyperdense middle cerebral artery sign with clinical outcome in patients treated with tissue plasminogen activator. Stroke 30(4), 769–772 (1999).
- Qureshi AI, Alkawi A, Hussein HM, Divani AA: Angiographic analysis of intravascular thrombus volume in patients with acute ischemic stroke. J. Endovasc. Ther. 14(4), 475–482 (2007).
- Tan IY, Demchuk AM, Hopyan J et al.: CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. Am. J. Neuroradiol. 30(3), 525–531 (2009). & Clot burden score and collateral blood supply score can predict clinical outcomes and final infarct size, respectively, and may help select patients for more aggressive thrombolysis.
- Hendrikse J, Hartkamp MJ, Hillen B, Mali WP, van der Grond J: Collateral ability of the circle of Willis in patients with unilateral internal carotid artery occlusion: border zone infarcts and clinical symptoms. Stroke 32(12), 2768–2773 (2001).
- Liebeskind DS: Collateral circulation. Stroke 34(9), 2279–2284 (2003).
- Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP: Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke 35(6), 1340–1344 (2004).
- Kucinski T, Koch C, Eckert B et al.: Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology 45(1), 11–18 (2003).
- Tan JC, Dillon WP, Liu S et al.: Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann. Neurol. 61(6), 533–543 (2007).
- Astrup J, Siesjo BK, Symon L: Thresholds in cerebral ischemia – the ischemic penumbra. Stroke 12(6), 723–725 (1981).
- Albers GW, Thijs VN, Wechsler L et al.: Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann. Neurol. 60(5), 508–517 (2006).
- Smith WS, Tsao JW, Billings ME et al.: Prognostic significance of angiographically confirmed large vessel intracranial occlusion in patients presenting with acute brain ischemia. Neurocrit. Care 4(1), 14–17 (2006).
- Torres-Mozqueda F, He J, Yeh IB et al.: An acute ischemic stroke classification instrument that includes CT or MR angiography: the Boston Acute Stroke Imaging Scale. Am. J. Neuroradiol. 29(6), 1111–1117 (2008).
- Linfante I, Llinas RH, Selim M et al.: Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke 33(8), 2066–2071 (2002).
- Bastianello S, Pierallini A, Colonnese C et al.: Hyperdense middle cerebral artery CT sign. Comparison with angiography in the acute phase of ischemic supratentorial infarction. Neuroradiology 33(3), 207–211 (1991).
- Kaste M, Waltimo O: Prognosis of patients with middle cerebral artery occlusion. Stroke 7(5), 482–485 (1976).
- Flint AC, Duckwiler GR, Budzik RF, Liebeskind DS, Smith WS: Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI Part I trials. Stroke 38(4), 1274–1280 (2007).
- Molina CA, Ribo M, Rubiera M et al.: Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke 37(2), 425–429 (2006).
- Furlan A, Higashida R, Wechsler L et al.: Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 282(21), 2003–2011 (1999).
- Smith WS, Sung G, Starkman S et al.: Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36(7), 1432–1438 (2005).
- Baird AE, Benfield A, Schlaug G et al.: Enlargement of human cerebral ischemic lesion volumes measured by diffusionweighted magnetic resonance imaging. Ann. Neurol. 41(5), 581–589 (1997).
- Parsons MW, Yang Q, Barber PA et al.: Perfusion magnetic resonance imaging maps in hyperacute stroke: relative cerebral blood flow most accurately identifies tissue destined to infarct. Stroke 32(7), 1581–1587 (2001).
- Kidwell CS, Saver JL, Mattiello J et al.: Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/ perfusion magnetic resonance imaging. Ann. Neurol. 47(4), 462–469 (2000).
- Chalela JA, Kang DW, Luby M et al.: Early magnetic resonance imaging findings in patients receiving tissue plasminogen activator predict outcome: insights into the pathophysiology of acute stroke in the thrombolysis era. Ann. Neurol. 55(1), 105–112 (2004).
- Kidwell CS, Alger JR, Saver JL: Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke 35(11 Suppl. 1), 2662–2665 (2004).
- Wintermark M, Reichhart M, Thiran JP et al.: Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann. Neurol. 51(4), 417–432 (2002).
- Hossmann KA: Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 36(4), 557–565 (1994).
- Murphy BD, Fox AJ, Lee DH et al.: Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke 37(7), 1771–1777 (2006).
- Koenig M, Kraus M, Theek C et al.: Quantitative assessment of the ischemic brain by means of perfusion-related parameters derived from perfusion CT. Stroke 32(2), 431–437 (2001).
- Koga M, Reutens DC, Wright P et al.: The existence and evolution of diffusion– perfusion mismatched tissue in white and gray matter after acute stroke. Stroke 36(10), 2132–2137 (2005).
- Arakawa S, Wright PM, Koga M et al.: Ischemic thresholds for gray and white matter: a diffusion and perfusion magnetic resonance study. Stroke 37(5), 1211–1216 (2006).
- Bristow MS, Simon JE, Brown RA et al.: MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J. Cereb. Blood Flow Metab. 25(10), 1280–1287 (2005).
- Falcao AL, Reutens DC, Markus R et al.: The resistance to ischemia of white and gray matter after stroke. Ann. Neurol. 56(5), 695–701 (2004).
- Murphy BD, Fox AJ, Lee DH et al.: White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology. 247(3), 818–825 (2008). & Ischemic penumbra can be identified using CT perfusion data and using a threshold derived from the interaction between cerebral blood flow and cerebral blood volume values.
- Simon JE, Bristow MS, Lu H et al.: A novel method to derive separate gray and white matter cerebral blood flow measures from MR imaging of acute ischemic stroke patients. J. Cereb. Blood Flow Metab. 25(9), 1236-1243 (2005).
- Powers WJ, Grubb RL Jr, Raichle ME: Physiological responses to focal cerebral ischemia in humans. Ann. Neurol. 16(5), 546-552 (1984).
- Kuschinsky W, Wahl M: Local chemical and neurogenic regulation of cerebral vascular resistance. Physiol. Rev. 58(3), 656-689 (1978).