Review Article - Interventional Cardiology (2021)
Cardiac myxoma as cytokine producing tumour: A review
- Corresponding Author:
- Yoichi Ajiro
Department of Psychiatry Division of Clinical Research,
National Hospital Organization Yokohama Medical Centre,
3-60-2 Harajuku, Totsuka-ku, Yokohama-shi, Kanagawa-ken,
245-8575,
Japan,
E-mail: you617bacchus@gmail.com
Received date: March 29, 2021 Accepted date: April 13, 2021 Published date: April 20, 2021
Abstract
Cardiac myxoma is the most common primary cardiac tumour. Many cytokines participate in the pathophysiology and growth of cardiac myxoma. Inflammatory cytokines, including interleukin-1, interleukin-4, interleukin-6, interleukin-8, interleukin-12, tissue necrosing factor-α, and interferon-γ, contribute to the development of inflammation and inflammation-related symptoms and further affect tumour growth. Growth factors, including vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor 1, and epidermal growth factor, contribute to angiogenesis and tumour growth and interfere with the inflammatory response. Recently, we reported a cardiac myxoma whose cells were positive for interleukin-1β and hemopoietic factor granulocyte colony-stimulating factor in addition to interleukin-6. This review summarises the current knowledge of cardiac myxomas as cytokine-producing tumours.
Keywords
Cardiac myxoma • Carney complex • Cytokine
Introduction
Cardiac myxoma is the most common primary cardiac tumour. This tumour possess a wide range of clinical presentations that may mimic a variety of neoplastic and non-neoplastic conditions [1]. The most common symptoms are systemic embolism, congestive heart failure, and nonspecific constitutional symptoms, including myalgia, muscle weakness, arthralgia, fever, weight loss, fatigue, and some skin manifestations [1,2]. Many cytokines are reported to be related to the symptoms and growth of cardiac myxoma [1,3]. Recently, we reported a cardiac myoma accompanying afebrile neutrophilic dermatosis with Granulocyte Colony Stimulating Factor (G-CSF), interleukin-1β (IL-1β), and IL-6 positive myxoma cells [4]. This review summarises the recent knowledge regarding cytokines related to cardiac myxoma (Table 1).
| Reference | Study type | Cytokines |
|---|---|---|
| Ajiro Y 2020 [4] | Case report (sporadic) | Il-6, IL-1β, G-CSF |
| Parissis JT 1996 [7] | Clinical study | Il-6 |
| Lin JN 2011 [8] | Case report (sporadic) | Il-6, TNFα, IL-4, IL-12 p70 interferon-γ, |
| Mendoza CE 2001 [9] | Clinical study | Il-6 |
| Jourdan M 1990 [11] | Clinical stud | Il-6 |
| Endo A 2002 [13] | Clinical study | Il-6 |
| Visoiu IS 2018 [14] | Case report (Carney) | Il-6 (comments in Discussion) |
| Nishio Y 2005 [15] | Case report (sporadic) | Il-6 |
| Bushnell JR 2011 [16] | Case report (sporadic) | Il-6 |
| Salobir B 2001 [17] | Case report (sporadic) | Il-6 (comments in Discussion) |
| Sumino H 1997 [18] | Case report (sporadic) | Il-6 |
| Soeparwata R [19] | Clinical study | Il-6, TNFα, IL-1β, GM-CSF |
| Aguilar C 2021 [20] | Case report (sporadic) | Il-6 |
| Ezerioha N 2015 [21] | Case report (sporadic) | Il-6 |
| Zhang T 2003 [22] | Clinical study | MCP1(CCL2) |
| Shi P 2015 [23] | Basic research | CCL3 |
| Sakamoto H 2004 [24] | Basic research | IL-8(CXCL8), CXCL1, VEGF growth-related oncogene-α |
| Burns ER 1982 [25] | Case report (sporadic) | erythropoietin |
| Liu CC 2010 [28] | Basic research | TNFα |
| Kono T 2000 [29] | Clinical study | VEGF |
| Fujisawa H 2002 [30] | Clinical study | bFGF |
| Wu XL 2013 [31] | Basic research | IGF1 |
| Huo Y 2016 [32] | Basic research | IGF1 |
Abbreviations: IL: Interleukin; G-CSG: Granulocyte Colony Stimulating Factor; TNF: Tumour Necrosis Factor; GM-CSF: Granulocyte Macrophage Colony Stimulating Factor; MCP-1: Monocyte Chemoattractant Protein-1; CCL: C-C motif chemokine; CXCL: C-X-C motif chemokine; VEGF: Vascular Endothelial Growth Factor; bFGF: basic Fibroblast Growth Factor; IGF-1: Insulin-like Growth Factor.
Table 1: Literature regarding cytokines in cardiac myxoma.
Cytokines of Myxoma
Interleukins and myxoma
Interleukins (ILs) are a group of cytokines first to be expressed by leukocytes and were later found to be synthesised by many other cells, including monocytes, macrophages, endothelial cells, and helper CD4 T lymphocytes. ILs have paracrine and autocrine functions and exhibit multiple biological activities [5].
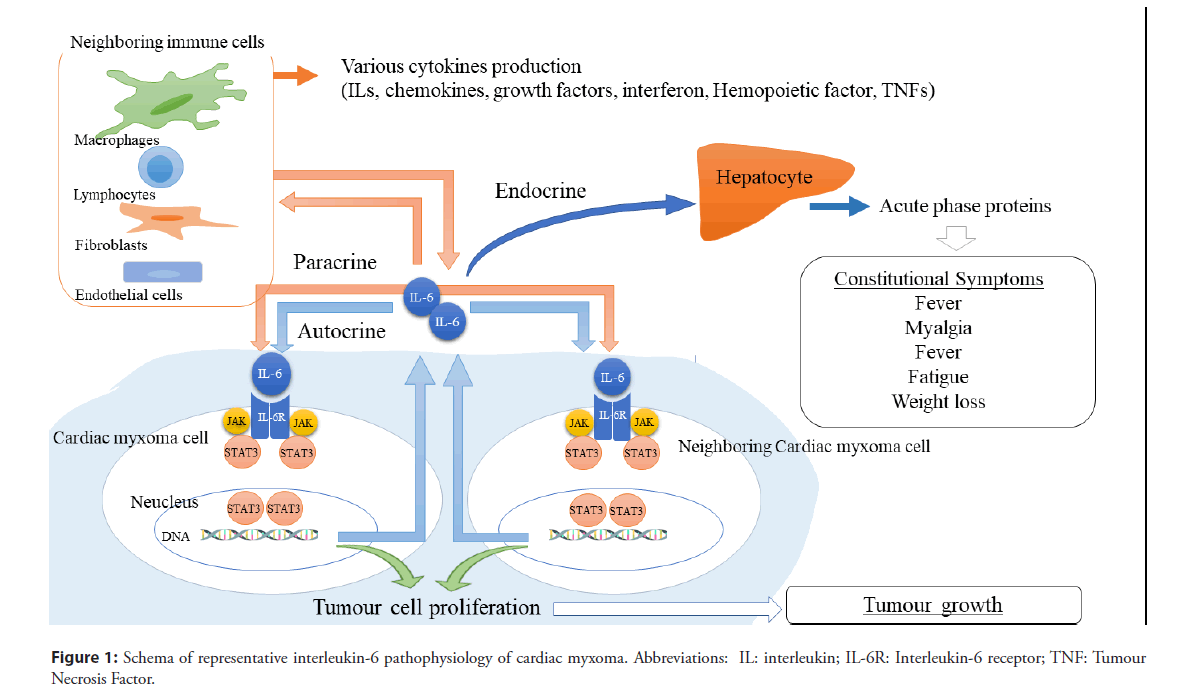
IL-6, the most commonly reported cytokine in cardiac myxoma [1,3,6-9], is recognised as one of the most prominent pro-inflammatory cytokines [10]. IL-6 also exerts multiple biological activities, such as regulation of immunological responses and hematopoiesis, and stimulation of some malignant and non-malignant cell growth [10-12]. Moreover, IL-6 has paracrine, endocrine, and autocrine growth functions [10,12]. IL-6 exerts its effects through (1) the IL-6 receptor (IL-6R), (2) IL-6 and IL-6R complex associates with a second receptor protein, glycoprotein 130 kDa (gp130), and (3) gp130 dimerises and initiates multiple intracellular signalling pathways, such as the Janus kinase (JAK)/ Signal Transducer and Activator of Transcription (STAT) pathway, rat sarcoma proto oncogene (ras)/Mitogen-Activated Protein Kinase (MAPK) pathway, and phosphatidylinositol-3 kinase (PI3K)/Akt pathway [10] (Figure 1).
Figure 1: Schema of representative interleukin-6 pathophysiology of cardiac myxoma. Abbreviations: IL: interleukin; IL-6R: Interleukin-6 receptor; TNF: Tumour Necrosis Factor.
Cardiac myxoma produces IL-6, and IL-6 is expressed in neoplasms [1,3,7]. IL-6 production and many definitive and possible pathophysiology in cardiac myxoma has been reported: constitutional symptoms and elevated C-reactive protein [8,9,13,14], autoimmune diseases [11,15-17], lymphoadenopathy [6], hypothyroidism [18], hypertrophy [6], endothelial dysfunction and atherosclerosis with intercellular adhesion molecule 1 expression [14], tumour growth [6,9,19], recurrence [9], remote metastasis [6], embolization [6,20], cerebral embolization relevant to IL-6 and matrix metalloproteinase-2 (MMP-2) [20], and cerebral aneurysmal [21].
Other inflammatory cytokines, such as tissue necrosis factor-α (TNF-α), IL-1, IL-8, IL-12, and interferon-γ, have also been reported with regard to cardiac myxoma. Lin et al. reported elevated levels of both serum inflammatory and anti-inflammatory cytokines in a large cardiac myxoma with leucocytosis [8]. Soeparwata et al. reported elevated TNF-α and/or IL-1β levels in addition to IL-6 in a large myxoma [19]. Serologically elevated serum IL-1β levels and histologically IL-1β-positive cardiac myxoma cells have been reported [4,19].
Chemokines and cardiac myxoma
Chemokines are a family of small cytokines that have chemotaxis and play various roles, including inflammation, tumour growth, and regulation of basal leukocyte migration. In cardiac myxoma, the monocyte chemotactic protein-1 (MCP-1), also known as CC chemokine ligand 2 (CCL2) by structural chemokine nomenclature, was reported to be involved in tumour growth and angiogenesis [3]. Zhang et al. reported that MCP-1 is expressed together with thymidine phosphorylase in cardiac myxoma cells. MCP-1 is known to contribute to angiogenesis in many tumour types. Their study demonstrated a similar function of MCP-1 in cardiac myxoma, as understood from the corelation between high microvessel and macrophage counts, with high MCP-1 and thymidine phosphorylase expression [22]. Shi et al. reported that CC Chemokine Receptor 5 (CCR5) and Astrocyte Elevated Gene- 1 (AEG-1) proteins were highly expressed in cardiac myxoma tissue and closely correlated with tumour size, which would be mediated through CCL3/CCR5-induced epithelial-to-mesenchymal transition [23]. Production of IL-8, CXC chemokine ligand 8 (CXCL8), and growth-related oncogene-α, and CXCL1, in cultured myxoma cells has been reported [24].
Interleukins and myxoma
Interferons were initially identified as factors that suppress viral infections. It has been shown to exhibit growth inhibitory, immunomodulatory, and many other activities, in addition to antiviral properties. Lin et al. demonstrated elevated serum interferon-γ in a large cardiac myxoma patient with leucocytosis [8].
Hemopoietic factor and cardiac myxoma
Hemopoietic factors are cytokines that promote blood cell differentiation and proliferation. Regarding cardiac myxoma, Burns et al. showed elevated serum erythropoietin levels in a cardiac myxoma with erythrocytosis [25]. Moreover, Soeparwata et al. reported an elevated serum granulocyte macrophage colony stimulating factor (GM-CSF) in a large cardiac myxoma [19]. We demonstrated G-CSF-positive myxoma cells in a cardiac myxoma with leucocytosis and afebrile neutrophilic dermatosis [4]. The elevated IL-4, Th2 cytokine, is also reported in cardiac myxoma [8]. Although there is no direct description of hemopoietic cytokines, thrombocytosis and leucocytosis in an atrial myxoma with a PRKAR1A gene mutation and hypereosinophilia in an atrial myxoma have been reported [26,27], suggesting the involvement of GM-CSF, IL-3, IL-5, IL-11, or thrombopoietin.
TNF Family
The TNF family is known to be a cytotoxic or lymphotoxin factor that induces apoptosis in cells. Elevated serum TNF-α, which is also known as an inflammatory cytokine, has been reported in large cardiac myxoma with leukocytosis [8,19]. Liu et al. also reported Fas-mediated apoptosis in connection with TNF-α in cardiac myxoma [28].
Growth Factor
Growth factor is a general term for endogenous proteins to promote the proliferation and differentiation of specific cells. Among various growth factors, Vascular Endothelial Growth Factor (VEGF), basic Fibroblast Growth Factor (bFGF), Insulinlike Growth Factor 1 (IGF-1), and Epidermal Growth Factor (EGF) have been reported to be involved in angiogenesis and tumour growth in cardiac myxoma [3]. Kono et al. showed the expression of VEGF and VEGF messenger RNA in cardiac myxoma tissue and showed a correlation with tumour size and microvessel density, suggesting the induction of angiogenesis for tumour growth [3,29]. Sakamoto et al. demonstrated the presence of VEGF and its receptors, VEGF receptor-1 and -2, in the cytoplasm of myxoma cells, and that myxoma cells secrete large amounts of VEGF. They also demonstrated that myxoma cell proliferation was enhanced by VEGF in a dose-dependent manner, and cell proliferation was inhibited in a dose-dependent manner by a neutralising VEGF antibody. They concluded that cardiac myxoma cells possess a VEGF-autocrine system that could contribute to the malignant potential of myxomas through direct stimulation of tumour cell growth as well as through induction of| angiogenesis [24]. Fujisawa et al. revealed bFGF and its receptor expression, particularly around microvessels, appearing as a ring structure of cardiac myxoma, suggesting a possible role for tumour angiogenesis and proliferative activity [3,30]. Wu et al. demonstrated the proliferative effect of IGF-1 on cardiac myxoma cells by negatively regulating the protein/lipid phosphatase and tensin homolog deleted on chromosome ten (PTEN)/pleckstrin homology domain leucine-rich repeat phosphatase 2 (PHLPP2) signalling pathway [31]. In addition, Huo et al. also demonstrated the proliferative effect of IGF-1 on cardiac myxoma cells and further enhanced the expression of EGF receptor and MMP-9 by regulating Myocyte Enhancer Factor 2D (MEF2D) [32].
Cytokine-Relevant Clinical Manifestations of Cardiac Myoma
The pathophysiology of clinical manifestations in cardiac myxoma is explained, at least in part, by the cytokines by cardiac myxoma. Symptoms of cardiac myxoma, such as fever, weight loss, arthralgia, myalgia, muscle weakness, fatigue, and some skin manifestations, may be induced by many cytokines [1,2,4,8-10,13,33-36]. Moreover, cytokine storms elicited by viral infections, such as COVID-19, resemble symptoms such as fever, myalgia, and arthralgia [35,37,38]. In tumour growth, including the recurrence and metastasis, hematopoietic and growth factors and inflammatory cytokines are reported to be involved [1,3,6,9,19, 22,23,29].
It is important to note the polyfunctional and multiplicity characteristics of cytokines, including its action in an autocrine, paracrine, and endocrine manner. When our myxoma case was considered, nuclear factors for the IL-6 gene function as promoters of G-CSF for IL-1β response [39]; IL-1β promotes IL-6 production and neutrophil activation via macrophage stimulation [40,41]; stimulated macrophages further produce cytokines including IL-1β [22,40,41]; the resultant activated cytokines would cause neutrophilic dermatosis in remote areas of the heart [4]. In addition, once neoplasms, including cardiac myxoma, produce cytokines, their effects will be enhanced and spread to the whole body owing to the cytokine characteristics of selfenhancement and a lack of inhibitory feedback due to neoplasm. The pathogenesis of cardiac myxoma has not been fully elucidated due to its complexity. Understanding the pathophysiology of cardiac myxoma will further elucidate the therapeutic target for inhibiting cardiac myxoma growth and controlling cytokinerelated clinical manifestations.
Genetic and Tumorigenesis Consideration
The protein kinase cAMP-dependent type 1 regulatory subunit α (PRKAR1A) gene mutations are found in familial cardiac myxoma associated with Carney complex [42], whereas most nonfamilial cardiac myxoma do not show mutations [43]. PRKAR1A gene, acting as a tumour suppressor gene, encodes the regulatory subunit of cAMP-dependent protein kinase A. The mutations of PRKAR1A gene cause PRKAR1α haplo insufficiency and reduction of protein with predisposition to tumorigenesis [1,3]. The relevancy between PRKAR1A gene disorder and cytokines has not been investigated profoundly. Barely, elevated serum IL-6 level and IGF-1 protein and mRNA in cardiac myxoma associated with Carney complex are reported [44,45]. Considering the fact that the cytokine relevancies are shown regardless of familial or sporadic cardiac myxoma, the cytokine network activation is considered to be common pathophysiology not related to genetic disposition in cardiac myxoma.
For tumorigenesis, environmental mutagens, genetic predisposition, and acquired susceptibility from life style factors should be considered [46]. As life style factors, infection may play a role in tumorigenesis [46], and nutrition has an important influence on the risk of developing cancer [47]. The inflammation due to infection increases proliferating cells which are more sensitive to the induction of DNA damage and thereby are more likely to propagate the mutagenic events into daughter cells than dormant cells [48,49]. Nutrition can also exert tumorigenesis through providing mutagens and, at least in part, through stimulating inflammation [47,50]. From this perspective, cytokines would function not only growth promoter but also tumorigenesis as acquired susceptibility factor in cardiac myxoma.
Interestingly, Puntila et al. reported a 4-year-old-boy with cardiac myxoma which was found out by close continuous observation from infancy of Carney complex family member with PRKAR1A gene mutation [51]. This report implies that the acquired factor other than ageing will contribute, at least in part, to tumorigenesis of cardiac myxoma. In addition, this report also gives an idea that the well-designed prospective observation of cytokine measurements on Carney family members would illuminate role of cytokines not only on tumour growth but also tumorigenesis of cardiac myxoma. Progress in understanding the complex pathophysiology of cardiac myxoma will serve to explore novel therapeutic approaches.
Conclusion
Various cytokines, including interleukins, chemokines, interferon-γ, TNF-α, growth factors, and hematopoietic factors, play an important role in the pathogenesis of cardiac myxoma. Progress in understanding the complex pathophysiology of cardiac myxoma will serve to explore novel therapeutic approaches.
Conflict of Interest
The author declares that there is no conflict of interest regarding the publication of this article.
Funding
None
Acknowledgement
We thank Editage for English editing.
References
- Bartoloni GAP. Cardiac myoma. In: Cardiac tumor pathology. Current clinical pathology: Hummana Press; Cristina Basso MV, Gaetano Thiene, editors, 31-44. (2013).
- Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 80(3): 159-172 (2001).
- Amano J, Kono T, Wada Y, et al. Cardiac myxoma: Its origin and tumor characteristics. Ann Thorac Cardiovasc Surg. 9(4): 215-221 (2003).
- Ajiro Y, Nino H, Ueda T, et al. Granulocyte-colony stimulating factor and interleukin-1β-positive cardiac myxoma accompanying neutrophilic dermatosis. JTCVS Techniques. 1: 69-71 (2020).
- Vaillant JAA, Qurie A. Interleukin. StatPearls. (2021).
- Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 45(2): 183-193 (2004)
- Parissis JT, Mentzikof D, Georgopoulou M, et al. Correlation of interleukin-6 gene expression to immunologic features in patients with cardiac myxomas. J Interferon Cytokine Res. 16(8): 589-593 (1996).
- Lin JN, Lai CH, Lu LF, et al. Fever of unknown origin from a left atrial myxoma: An immunologic basis and cytokine association. South Med J. 104(5): 360-362 (2011)
- Mendoza CE, Rosado MF, Bernal L. The role of interleukin-6 in cases of cardiac myxoma. Clinical features, immunologic abnormalities, and a possible role in recurrence. Tex Heart Inst J. 28(1): 3-7 (2001).
- Rose-John S. Interleukin-6 signalling in health and disease. F1000Res. 9: (2020).
- Jourdan M, Bataille R, Seguin J, et al. Constitutive production of interleukin-6 and immunologic features in cardiac myxomas. Arthritis Rheum. 33(3): 398-402 (1990).
- Morishima A, Marui A, Shimamoto T, et al. A case of interleukin-6-producing cardiac myxoma resembling multicentric castleman's disease. J Thorac Cardiovasc Surg. 138(2): 499-501 (2009).
- Endo A, Ohtahara A, Kinugawa T, et al. Characteristics of cardiac myxoma with constitutional signs: A multicenter study in japan. Clin Cardiol. 25(8): 367-370 (2002).
- Visoiu IS, Constantinescu C, Patrascu N, et al. Advanced atherosclerosis with leriche syndrome, in a patient with carney complex. Maedica (Bucur). 13(2): 147-151 (2018).
- Nishio Y, Ito Y, Iguchi Y, et al. Mpo-anca-associated pseudovasculitis in cardiac myxoma. Eur J Neurol. 12(8): 619-620 (2005).
- Bushnell JR, Weston C, Karamadoukis L. An unusual presentation of atrial myxoma with haematuria and proteinuria. NDT Plus. 4(2): 124-125 (2011).
- Salobir B, Sabovic M, Kozelj M. Increased levels of antiphospholipid antibodies in a woman with left atrial myxoma and systemic embolisms. Lupus. 10(11): 815-817 (2001).
- Sumino H, Kanda T, Kobayashi I, et al. Reduced serum t3 level in a patient with nodular goiter and cardiac myxoma. J Med. 28(5-6): 319-324 (1997).
- Soeparwata R, Poeml P, Schmid C, et al. Interleukin-6 plasma levels and tumor size in cardiac myxoma. J Thorac Cardiovasc Surg. 112(6): 1675-1677 (1996).
- Aguilar C, Carbajal T, Beltran BE, et al. Cerebral embolization associated with parenchymal seeding of the left atrial myxoma: Potential role of interleukin-6 and matrix metalloproteinases. Neuropathology. 41(1): 49-57 (2021).
- Ezerioha N, Feng W. Intracardiac myxoma, cerebral aneurysms and elevated interleukin-6. Case Rep Neurol. 7(2): 152-155 (2015).
- Zhang T, Koide N, Wada Y, et al. Significance of monocyte chemotactic protein-1 and thymidine phosphorylase in angiogenesis of human cardiac myxoma. Circ J. 67(1): 54-60 (2003).
- Shi P, Fang C and Pang X. Astrocyte elevated gene-1 regulates ccl3/ccr5-induced epithelial-to-mesenchymal transition via erk1/2 and akt signaling in cardiac myxoma. Oncol Rep. 34(3): 1319-1326 (2015).
- Sakamoto H, Sakamaki T, Sumino H, et al. Production of endothelin-1 and big endothelin-1 by human cardiac myxoma cells-implications of the origin of myxomas. Circ J. 68(12): 1230-1232 (2004).
- Burns ER, Schulman IC, Murphy MJ. Hematologic manifestations and etiology of atrial myxoma. Am J Med Sci. 284(2): 17-22 (1982).
- Massobrio L, Nasti S, Martinuzzi C, et al. Mutation analysis of prkar1a gene in a patient with atrial myxoma. Clin Lab. 62(4): 731-734 (2016).
- Fernandes GC, Pajares AW, Amboss N, et al. Right atrial myxoma with peripheral eosinophilia: Eosinophilia in cardiac myxoma. J Card Surg. 35(2): 507-510 (2020).
- Liu CC, Jung SM, Orlandi A, et al. The fas-mediated apoptotic pathway in cardiac myxoma. Int J Surg Pathol. 18(6): 493-498 (2010).
- Kono T, Koide N, Hama Y, et al. Expression of vascular endothelial growth factor and angiogenesis in cardiac myxoma: A study of fifteen patients. J. Thorac Cardiovasc. Surg. 119(1): 101-107 (2000).
- Fujisawa H, Koide N, Kono T, et al. Expression of basic fibroblast growth factor and its receptor-1 in cardiac myxoma. J Cardiovasc Surg (Torino). 43(5): 589-594 (2002).
- Wu XL, Yang DY, Tan DJ, et al. Inhibitory effect of atorvastatin on the cell growth of cardiac myxomas via the pten and phlpp2 phosphatase signaling pathway. Oncol Rep. 30(2): 757-762 (2013).
- Huo Y, Zhao Q, Wang C, et al. The involvement of myocyte enhancer factor 2d in regulating tumor biology of cardiac myxoma. Tumour Biol. 37(4): 5405-5411 (2016).
- Wallenius V, Wallenius K, Ahrén B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 8(1): 75-79 (2002).
- Hövels-Gürich HH, Seghaye MC, Amo-Takyi BK, et al. Cardiac myxoma in a 6-year-old child--constitutional symptoms mimicking rheumatic disease and the role of interleukin-6. Acta Paediatr. 88(7): 786-788 (1999).
- Nakamura K, Saito K, Hara Y, et al. Severe epidemic myalgia with an elevated level of serum interleukin-6 caused by human parechovirus type 3: A case report and brief review of the literature. BMC Infect Dis. 18(1): 381 (2018).
- Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 34(12): 809-824 (2004).
- Schett G, Manger B, Simon D, et al. Covid-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol. 16(8): 465-470 (2020).
- Chow A, Her Z, Ong EK, et al. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 203(2): 149-157 (2011).
- Shannon MF, Coles LS, Fielke RK, et al. Three essential promoter elements mediate tumour necrosis factor and interleukin-1 activation of the granulocyte-colony stimulating factor gene. Growth Factors. 7(3): 181-193 (1992).
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: Back to the future. Immunity. 39(6): 1003-1018 (2013).
- Afonina IS, Müller C, Martin SJ, et al. Proteolytic processing of interleukin-1 family cytokines: Variations on a common theme. Immunity. 42(6): 991-1004 (2015).
- Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase a type i-alpha regulatory subunit in patients with the carney complex. Nature Genetics. 26(1): 89-92 (2000).
- Fogt F, Zimmerman RL, Hartmann CJ, et al. Genetic alterations of carney complex are not present in sporadic cardiac myxomas. Int J Mol Med. 9(1): 59-60 (2002).
- Yokomuro H, Yoshihara K, Watanabe Y, et al. The variations in the immunologic features and interleukin-6 levels for the surgical treatment of cardiac myxomas. Surg Today. 37(9): 750-753 (2007).
- Bandettini WP, Karageorgiadis AS, Sinaii N, et al. Growth hormone and risk for cardiac tumors in carney complex. Endocr Relat Cancer. 23(9): 739-746 (2016).
- Au WW. Life style factors and acquired susceptibility to environmental disease. Int J Hyg Environ Health. 204(1): 17-22 (2001).
- Key TJ, Bradbury KE, Perez-Cornago A, et al. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ. 368: m511 (2020).
- Gentile JM. Schistosome related cancers: A possible role for genotoxins. Environ Mutagen. 7(5): 775-785 (1985).
- Bartsch H, Ohshima H, Pignatelli B, et al. Endogenously formed n-nitroso compounds and nitrosating agents in human cancer etiology. Pharmacogenetics. 2(6): 272-277 (1992).
- Karczewski J, Śledzińska E, Baturo A, et al. Obesity and inflammation. Eur Cytokine Netw. 29(3): 83-94 (2018).
- Puntila J, Hakala T, Salminen J, et al. Positive genetic test led to an early diagnosis of myxoma in a 4-year-old boy. Interact Cardiovasc Thorac Surg. 5(5): 662-663 (2006).
Journal Metrics:
| Impact Factor | 1.34 |
| Scimago Journal Rank (SJR) | 123 |
| SJR Total Cites | 15 |
| Source Normalized Impact per Paper (SNIP) | 0.144 |
| h-index (2023) | 12 |
| PubMed NLM ID: | 10148499 |
| Google Scholar h5 index: | 6 |
| Iindex Copernicus Value: | 105.52 |
Google Scholar citation report
Citations : 1400
Interventional Cardiology received 1400 citations as per Google Scholar report