Review Article - Imaging in Medicine (2010) Volume 2, Issue 1
Advances in imaging for liver cancer radiation therapy
Catherine Coolens & Laura A Dawson†Department of Radiation Oncology, Princess Margaret Hospital, University of Toronto, Toronto, ON, Canada
- Corresponding Author:
- Laura A Dawson
Department of Radiation Oncology
Princess Margaret Hospital
University of Toronto, Toronto, ON, Canada
Tel: +1 416 946 2125
Fax: +1 416 946 6566
E-mail: laura.dawson@rmp.uhn.on.ca
Abstract
Primary and metastatic liver cancers are a leading global cause of morbidity in an increasing number of patients. An important role is emerging for local hepatic therapies that can eradicate liver tumors with minimal morbidity, especially in patients not suitable for surgery or established ablative therapies. Radiation technology development has allowed for high doses of radiation to be precisely delivered to liver tumors while preserving liver function and sparing critical organs. Imaging of the liver for precise tumor localization, tumor motion assessment and guidance of radiation treatment is paramount in this process. In this article, an overview is provided describing how advances in liver imaging and their integration with the radiation therapy process have made it possible for high-dose radiation therapy to be delivered safely to liver cancer patients and become a viable treatment option.
Keywords
imaging ▪ liver cancer ▪ radiation therapy
Radiotherapy for liver cancer treatment
Primary liver cancer (hepatocellular carcinoma [HCC] and intrahepatic cholangiocarcinoma) and liver metastases are a leading global cause of morbidity in an increased number of patients [101]. Surgical resection of hepatic metastasis can cure selected patients with isolated liver metastases from colorectal cancer. Fong et al. [1] demonstrated a 30% 5-year survival and Scheele [2] a 40% 5-year survival and 30% freedom of disease in patients that have curative resection of colorectal liver metastases. Chemotherapy can downstage tumors making some unresectable patients resectable, and the 5-year survival of resected patients has shown improvement in recent studies (50–60% in some patients) [3]. However, most patients with liver metastases never become surgical candidates (<20%) owing to tumor location, tumor size or medical inoperability. Ablative therapies can control tumors less than 4 cm in diameter [4], but larger tumors, those adjacent to large vessels and cancers with vascular involvement are generally not eligible for standard local therapies. Fewer patients with HCC and intrahepatic cholangiocarcinoma are suitable for resection owing to near universal coexistence of underlying liver disease. An important role is emerging for local hepatic therapies that can provide ablation of the tumor but with minimal morbidity, especially in patients not suitable for surgery or other established ablative therapies.
Radiation technology development in recent years has made it possible for high doses of X-ray radiation to be precisely delivered to tumors while preserving liver function and sparing critical organs surrounding the lesion. Often the most limiting factor to escalating the tumor dose is the liver tolerance to external beam irradiation, which depends on the volume treated and fractionation schedule. The relationship between radiation dose and volume of liver irradiated and risk of radiationinduced liver disease was explored using normal tissue complication probability models analyzing a cohort of over 200 patients with hepatic malignancies treated in Michigan (MI, USA) [5]. This analysis demonstrates that tumoricidal doses of radiation can be safely delivered while a small effective volume of liver is irradiated. Several Phase II studies have demonstrated sustained liver cancer control from focal radiation therapy for primary and metastatic liver cancer, with evidence of a dose–response relationship for tumor control [5–13]. A challenge in delivering precise radiation therapy is that the liver can move substantially with breathing, with amplitudes of motion up to 30 mm (mean: 15 mm) during relaxed respiration [14–17]. This poses significant challenges for planning and delivery of precision high-dose radiation treatment. Therefore, within the radiotherapy framework, imaging of liver metastatic disease for precise tumor localization, tumor motion assessment and guidance of radiation therapy delivery is paramount.
Particle beam irradiation (e.g., proton or recently carbon ion therapy) takes advantage of the different physical characteristics of incident particle beams within the body [18], such that there is less dose deposited to normal tissues surrounding the target volume. Susceptibility to the aforementioned uncertainties, however, are even greater with particle beam irradiation and availability of these technologies is very limited.
Imaging for target definition
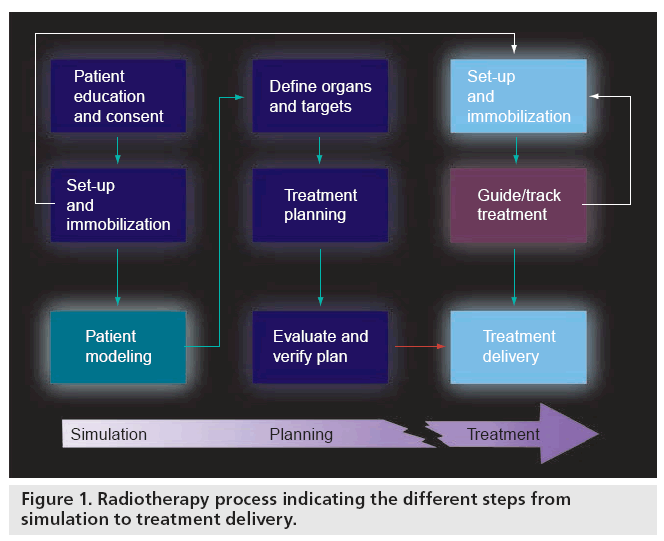
An illustration of the different key steps in the radiotherapy process is given in Figure 1. In order to design a patient’s radiotherapy treatment, a simulation is carried out of the interaction (energy transfer or dose distribution) between the MV X-ray beams and the patient anatomy in the treatment position. Commercial systems (called treatment planning systems) are available to perform these dose calculations and create a treatment ‘plan’ for delivery on the linear accelerator or treatment unit. Visualization of the 3D patient anatomy can be achieved with CT or MRI and, although MRI provides better softtissue contrast, the calculation of dose requires tissue electron density information that can only be obtained from a CT acquisition.
Figure 1: Radiotherapy process indicating the different steps from simulation to treatment delivery.
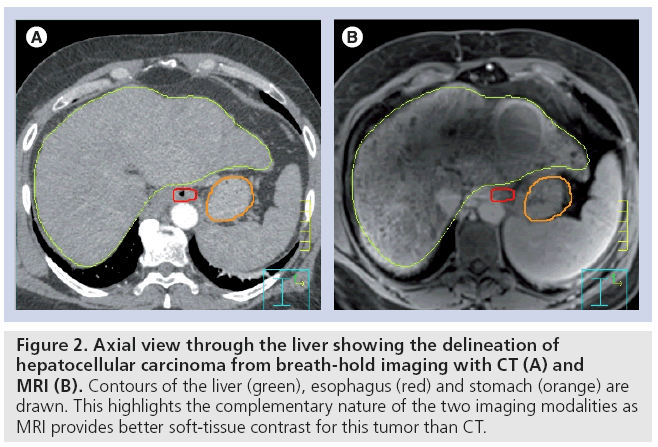
However, owing to the lack of CT soft-tissue contrast of liver metastases, iodine-based contrast agents are required for precise target definition. Considering that malignant liver tumors often derive most of their blood supply from the hepatic artery rather than the portal vein, the diagnostic assessment of liver tumors usually involves multiphase studies to fully exploit these differences in the kinetics of contrast enhancement and the sources of blood supply. Ideally, a triphasic contrast CT obtained during breathhold in the radiation therapy treatment position is used as the patient model. If possible, contrast magnetic resonance (MR) images will be acquired to provide complimentary information to aid the target definition process as MRI can offer high soft-tissue contrast resolution (Figure 2). Voroney et al. compared differences in MRI- and CT-derived liver cancer volumes from HCC patients and found them to be clinically important for a significant proportion of patients [19]. As the liver deforms easily with breathing, there can be differences in liver shape between CT and MRI. The use of nonrigid image registration of the CT and MR liver volumes reduced residual differences in CT and MR tumor delineations, but substantial differences remained in a minority of patients. The number of tumor foci was different on CT versus MRI in five patients with HCC; MRI showed more foci in three patients, CT in two. Furthermore, the median percentage of tumor surface area that differed by more than 5 mm was 26% (1–86%). Median percentage concordance volumes were 81% (77–86%) in metastases, 77% (60–88%) in HCC and 64% (25–85%) in cholangiocarcinoma.
Figure 2: Axial view through the liver showing the delineation of hepatocellular carcinoma from breath-hold imaging with CT (A) and MRI (B). Contours of the liver (green), esophagus (red) and stomach (orange) are drawn. This highlights the complementary nature of the two imaging modalities as MRI provides better soft-tissue contrast for this tumor than CT.
In addition to standard gadolinium-enhanced MRI, diffusion-weighted (DW) imaging is showing promise in liver lesion detection and delineation because the diffusion of water molecules along a field gradient reduces the MR signal. In areas of lower diffusion, the signal loss is less intense and the display from these areas is brighter. The use of a bipolar gradient pulse and suitable pulse sequences permits the acquisition of DW images (images in which areas of rapid proton diffusion can be distinguished from areas with slow diffusion). Parikh et al. retrospectively compared DW MRI with standard breath-hold T2-weighted MRI for focal liver lesion (FLL) detection and characterization for 53 patients with at least one FLL of 1 cm or greater in diameter [20]. Overall detection rate was significantly higher for DW (87.7%) versus T2-weighted (70.1%) imaging (p < 0.001). FLL characterization was not significantly different between DW (89.1%) and T2-weighted (86.8%) imaging.
By visualizing data from multiple imaging modalities and multiple phases of enhancement with image registration and fusion techniques, now available in commercial radiation planning (i.e., simulation) systems, the tumor delineation for radiation therapy planning should be more accurate, and the risk of a marginal miss reduced.
Imaging for liver motion evaluation
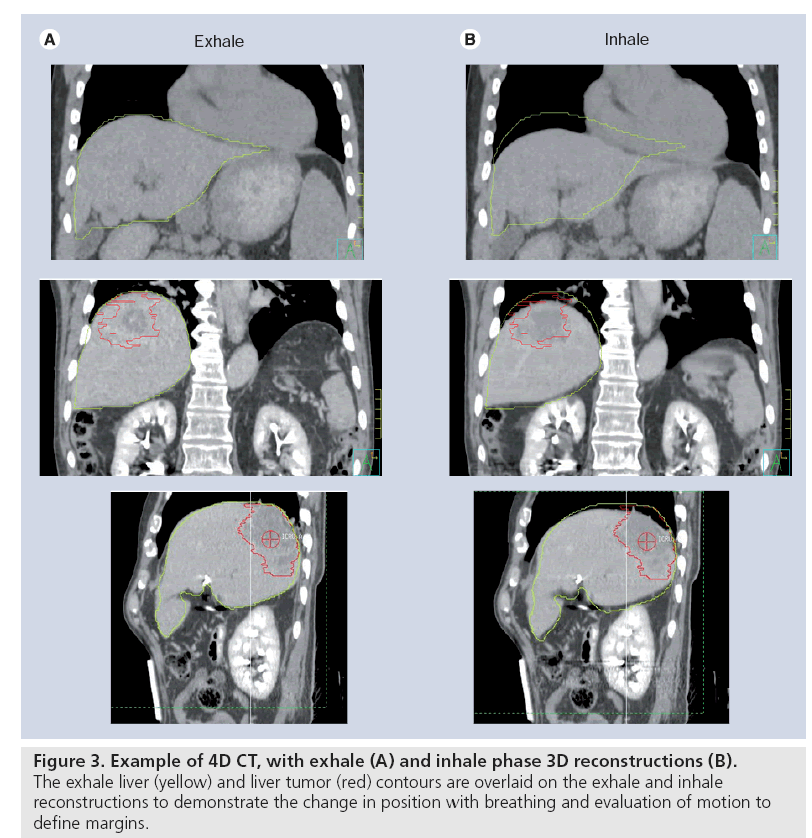
Evaluation of breathing motion is crucial in liver cancer radiation therapy, so that the motion can be considered at the time of planning to ensure that the radiation will be delivered as planned despite the presence of motion and/or to decide whether immobilization or reduction of motion is required for treatment. Different imaging modalities have been used to investigate liver motion, including fluoroscopy of inserted fiducial markers, cine MRI [21] and 4D CT [22,23]. CT scans acquired during free breathing can contain substantial artifacts that lead to a propagation of errors in tumor delineation and estimates of tumor control and toxicities. 4D CT, or respiratory-correlated CT, can reduce such artifacts (Figure 3). 4D CT scans are based on cine-CT imaging at each table position during approximately one breathing cycle before moving to the next table position and repeating the process. By time-stamping the axial slices per phase of breathing, the data can then retrospectively be sorted by time, therefore creating a 4D CT (i.e., 3D data over time) [24–26]. Radiotherapy institutions often rely on retrospectively correlated 4D CT obtained prior to radiation therapy delivery to assess respiratory motion of liver tumors for appropriate margin design at the time of radiation therapy planning and/or for respiratory-correlated treatment delivery. Commercial software typically bins 4D CT scans into ten phases, as a balance between sampling rate and computational cost. This technique, based on retrospective resorting of time-correlated axial scanning, is still subject to artifacts as different parts of the volume are scanned at different times; this also enhances the susceptibility to variability in breathing motion [27–29]. A similar technique exists for 4D helical CT by means of retrospective interpolation of the projection data but this is equally, if not more, prone to breathing variations [22]. Some groups have aimed to overcome these artifacts by using breathing control measures [30], nonrigid registrations [31,32] or predictive algorithms linked to internal anatomy [33]. Although the use of 4D CT to visualize the target volume for HCC has been described [34–36], it is challenging to precisely synchronize the individual vascular contrast kinetics with the 4D CT acquisition and, unless contrast agent is present in all reconstructed CT phases, visualizing the motion of the liver tumor itself may be difficult. A study by Coolens and Hawkins has investigated tumor volume and motion characterization in colorectal cancer liver metastases based on fluoroscopy, contrast-enhanced breath-hold CT, 4D CT and breath-hold and cine MRI in 15 patients [37,38]. In most cases, the tumor could not be visualized on fluoroscopy, and there was uncertainty in tumor delineation on all 4D CT reconstructions owing to a lack of contrast. Nevertheless, 4D CT and cine MRI were informative regarding the degree of liver motion that occurred during breathing. Despite the relatively low patient sample (n = 15), the results suggest that 4D CT underestimated the motion and fluoroscopy overestimated the motion as compared with 2D and cine MRI.
Figure 3: Example of 4D CT, with exhale (A) and inhale phase 3D reconstructions (B). The exhale liver (yellow) and liver tumor (red) contours are overlaid on the exhale and inhale reconstructions to demonstrate the change in position with breathing and evaluation of motion to define margins.
Most studies rely on surrogates for evaluating liver tumor motion such as the diaphragm, matched whole liver contours or radioopaque fiducial markers inserted into the liver [16,34,35,39–42]. Xi et al. reported a mean diaphragm displacement of 11.1 ± 5.7 mm based on 4D CT scans for ten patients [34]. Other studies included fiducial markers positioned in the tumor vicinity to estimate the motion during radiation therapy delivery, using fluoroscopy and 4D CT imaging [40,43–45]. The average amplitude of tumor motion was around 4 ± 4 mm (range: 1–12), 9 ± 5 mm (range: 2–19) and 5 ± 3 mm (range: 2–12) in the left–right, cranial–caudal and anterior–posterior direction, respectively.
Recent CT technology developments have seen the release of a 320-slice CT scanner, providing a 16 cm cranio–caudal coverage within 0.35 s. Using this volumetric scanning mode dynamically could provide a ‘true’ 4D assessment of liver and liver tumor motion as the volume within a single rotation can be acquired in a shorter time than the temporal resolution of a typical conventional 4D CT [46]. This offers a new outlook on motion management that could support an improvement in target delineation and liver tumor motion assessment. Practice regarding organ motion management can be varied and a report by the AAPM Task Group 76 on 4D CT recommended some standardization including common nomenclature [47]. Following these, this article uses the term ‘motion amplitude’ as the maximal motion displacement between inhale and exhale, whereas liver displacement refers to the shift in mean liver position with respect to the bony anatomy.
Another strategy to measure motion is to use imaging obtained at the time of radiation therapy delivery. Cone-beam CT (CBCT) refers to the acquisition of a CT scan while the patient is on the treatment couch by rotating a fixed kV X-ray source and flat panel detector, bolted on the treatment gantry [35,48–50].
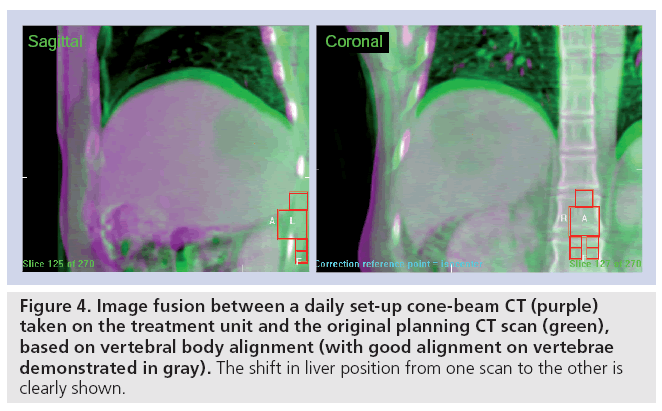
Case et al. evaluated a total of 314 4D CBCT data for patients with liver cancer undergoing highly conformal radiation therapy [51]. In the medial–lateral, cranial–caudal and anterior– posterior directions, the average liver breathing amplitude of motion was 1.4 mm (range: 0.1–5.7), 9.0 mm (range: 0.1–18.8) and 5.1 mm (range: 0.2–12.1) for patients treated during free breathing and 2.2 mm (range: 0–7.0), 6.7 mm (range: 0.4–14.0) and 3.3 mm (range: 0.1–10.5) for those treated with abdominal compression, as a means of reducing liver motion, respectively. Changes in the mean liver position relative to the vertebral bodies day-today were significantly larger than changes in the amplitude of breathing, providing motivation for imaging the liver at the time of delivery of each radiation therapy fraction to ensure the liver tumor is positioned as planned. It has been shown that these systematic errors in liver position have a larger impact than typically smaller random set-up errors in radiotherapy treatment delivery accuracy (Figure 4) [52]. The design of appropriate treatment margins may be based on population-based analysis of motion, or more appropriately, individualized margins based on a specific patient’s motion, and are beneficial for reducing normal tissue exposure and potential dose escalation
Figure 4: Image fusion between a daily set-up cone-beam CT (purple) taken on the treatment unit and the original planning CT scan (green), based on vertebral body alignment (with good alignment on vertebrae demonstrated in gray). The shift in liver position from one scan to the other is clearly shown.
Considering that MRI offers the advantage of improved tissue visualization compared with CT, the premise of using MRI to evaluate liver tumor motion directly is justified. A challenge in advancing MR implementation, however, is increasing its acquisition speed while maintaining sufficient signal-to-noise ratio and spatial resolution, particularly when scanning areas such as the abdomen that may require breathholding to avoid motion artifacts. To overcome this issue, multiple 2D cine MRI has been used to evaluate motion by positioning reference image frames through the middle of the tumor in one or more orthogonal planes [16,21]. As the liver may deform during breathing, more detailed volumetric motion information would benefit the radiation therapy planning and delivery process. Recently a novel MRI reconstruction technique has been developed for the acquisition of fast dynamic, 3D MRI [53]. This allows for 4D MRI visualization of the entire liver, including hepatic tumors, without the additional cost of patient dose associated with CT imaging. Without additional hardware adaptations the temporal scan resolution with this technique is approximately 60 Hz. Developments on the availability of MRI in the radiation treatment room could further the use of this modality at the time of treatment to provide improved soft-tissue visualization and image guidance of the radiation beams (see Future perspective).
Motion management strategies for liver radiation therapy
Depending on the in-room (i.e., in the treatment room) imaging available, different strategies have been designed to control and/or mitigate target motion, the oldest and most common one being to increase the target volume irradiated to account for motion. However, more patients with unresectable intrahepatic malignancies could receive higher doses if a smaller volume were required to be irradiated around the tumor volume, and thus there is motivation for other methods to reduce liver motion.
More advanced motion compensation methods include treating during active [15] or passive repeat (generally 15–30 s) breath-holds [54]; respiratory gating of the radiation therapy beam to irradiated tissues during a small phase interval of the breathing cycle [30,41,55–57]; and tumor tracking so that the radiation therapy beam moves as the liver moves [16,58,59]. A key component in selecting a compensation method is the need for continuity throughout the entire radiation therapy process. In other words, if a patient is treated in breath-hold, the planning and simulation CT scan should also be acquired during breath-hold. Breath-hold can reduce the volume of normal tissues irradiated and possibly facilitate dose escalation to liver cancers [60,61]; however, some patients cannot tolerate repeat breath-holds. This may be due to pulmonary disease, poor communication or a lack of reproducibly positioning of the liver with repeat breathholds. One can therefore distinguish motion management approaches into breath-hold and free-breathing treatment approaches.
Eccles et al. examined the reproducibility of using a breath-hold method during treatment to minimize liver motion [62]. Although intrafraction reproducibility was excellent in the majority of the screened patients, the interfraction reproducibility (between daily treatment fractions) was far worse, indicating ‘baseline shifts’ in liver position day-to-day, despite the use of breath-hold, suggesting image guidance is required during radiation therapy even when breath-hold liver immobilization is used. Further studies confirmed the average interfraction variability (standard variation) ranged from 3.4 to 4.4 mm [20–22].
Regardless of the motion management strategy used, shifts in the average liver position dayto- day occur, thus imaging at the time of each radiation treatment to account for these shifts in liver position is necessary [51].
Image guidance for liver radiation therapy
External immobilization and verification of bony anatomy using X-ray imaging are the current standard of care to ensure reproducible patient positioning during radiotherapy delivery. As was shown in Figure 1, image guidance must be used to align the patient, from the reference state at the time of treatment to the reference state at the time of planning. Imaging the tumor immediately prior to or during radiation therapy delivery can improve the positioning of the radiation beams to the tumor, and ensure that radiation therapy is more likely to be delivered as planned. Most modern radiotherapy centers performing conformal radiation therapy have in-room imaging to guide the treatment process. The standard imaging method is MV X-ray imaging of the treatment beam using a flat panel aSi portal imager that is attached to the linear accelerator (linac) at an opposing angle from the beam gantry, resulting in a 2D image. This can be extended to 3D by rotating the portal imager around the patient and doing an MV CT reconstruction [48]. More recently, kV volumetric imaging has been added to the imaging possibilities on a linac by bolting an additional kV X-ray tube and associated flat panel X-ray detector at a 90° angle to the treatment beam. This allows for real-time fluoroscopy during radiation delivery as well as CBCT volumetric imaging before and after treatment to allow for soft-tissue volumetric image guidance (e.g., using the liver or fiducials in the liver to position the liver tumor relative to the radiation therapy beams) [63]. Alternative in-room imaging methods include the use of a diagnostic quality CT on rails in the treatment room that moves into place before and after treatment [64,65], dual kV X-ray systems attached at 30° angles to the treatment gantry [66,67], and mounting a linac on a ring gantry for both treatment and MV CT acquisition (tomotherapy) [68–70]. All these technologies have been used to improve radiation therapy delivery for liver cancers.
For breath-hold liver cancer radiation therapy, image guidance can be performed using 2D coronal and sagital kV images at breathhold or using volumetric 3D images obtained at the breath-hold position. Typically, patients cannot hold their breath for the full duration of the 3D CBCT acquisition (60 s) and therefore the acquisition must be obtained in increments, allowing the patient to breathe between segments of image acquisition. In this approach, the soft-tissue liver can then be aligned to the reference image.
To account for shifts in liver position that may occur when patients are treated during free breathing, with abdominal compression and or with gating (delivering radiation when the tumor is in a known position) or tracking (following the tumor while irradiating), verification imaging at the time of radiation therapy delivery is also required. With gating, only the gated position and surrogacy must be verified; however, with tracking, the correlation between the surrogate and the entire breathing pattern must be verified as breathing is inherently irregular, and thus there is rationale for more frequent imaging during an individual radiation treatment with tracking compared with gating [16,40,71]. This is especially true if only a small imaging sample is being used to evaluate individual patient breathing motion [28]. In addition, the relationship of the surrounding anatomy with the tumor must be verified to ensure that the normal tissue dose is not substantially different than was planned.
Surrogates for liver tumor, such as fiducial markers inserted to aid in image guidance, have been investigated to facilitate tumor positioning at the time of radiation therapy delivery [66]. Other intrahepatic radio-opaque landmarks that may be used for tumor surrogates during image guidance include tumor calcifications, surgical clips or lipiodol from prior treatment with trans-catheter hepatic arterial chemoembolization.
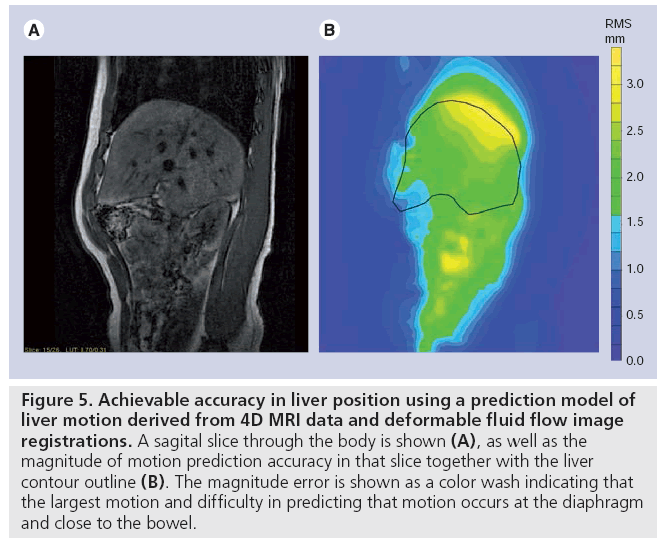
Another approach for deriving the tumor position during radiation therapy delivery is indirect motion monitoring with the use of external infrared markers on the patient’s abdomen or chest [43,44]. Correlation of tumor motion (via imaging) with abdominal surface motion is critical. Although it has been shown that there is a correlation between internal and external, intrafraction, diaphragm motion, external markers may not be continuously synchronized with tumor motion [28,71–78], and the relationship between external markers and actual intrahepatic tumor motion is not necessarily linear owing to deformation of the liver during breathing. To this extent Coolens et al. investigated the feasibility of applying deformable liver motion models from 4D MRI to predict free-breathing liver positions using an external marker, so that respiratory gating could be applied during freebreathing radiation therapy [79]. The results showed that for 15 patients, even without breathing coaching, the overall liver motion could be predicted to within 3 mm (Figure 5). This suggests that, if the breathing baseline is maintained, the model can cope with interfraction motion differences and reduce them to within the set-up accuracy. Despite the successful implementation of using fluid registration to build a motion model, further improvements are needed in terms of MR scan resolution (both temporal and spatial) and sensitivity to image distortions and artifacts to allow for real-time image guidance and motion management within the liver (see Future perspective).
Figure 5: Achievable accuracy in liver position using a prediction model of liver motion derived from 4D MRI data and deformable fluid flow image registrations. A sagital slice through the body is shown (A), as well as the magnitude of motion prediction accuracy in that slice together with the liver contour outline (B). The magnitude error is shown as a color wash indicating that the largest motion and difficulty in predicting that motion occurs at the diaphragm and close to the bowel.
Imaging for treatment monitoring
Further improvement of therapeutic benefit from liver radiation therapy will depend on the possibility to evaluate liver tissue characteristics beyond morphology and assess individual tumor response as well as onset of normal tissue complications during and/or following treatment. This would allow better identification of which patients are likely to benefit from radiation therapy, which could facilitate individualization of therapy based on early markers of outcomes occurring during a radiation therapy course. Diffusion MR and perfusion CT have been investigated as possible imaging early biomarkers for response [80,81]. This is an exciting and active area of research, but is in its infancy in liver cancer patients.
Future perspective
Without further improvements in soft-tissue imaging techniques, the use of contrast agents for tumor visualization will remain critical for liver target delineation during radiation therapy. Current preclinical research in this area focuses on the development of novel and improved imaging contrast agents with the future goal of improving target delineation and avoiding multiple contrast injections. As repeat contrast injections are linked to increased risk of adverse reactions, the latter objective is desirable if daily liver tumor visualization for radiation image guidance is to be achieved. In the preclinical setting, Zheng et al. developed a multimodel contrast agent based on liposome encapsulation specifically for the radiotherapy context [82]. It allows for a single injection of liposomes containing both CT and MRI contrast molecules that have a sufficiently long half-life to be present in the body for a typical 6-week radiation therapy treatment process, so that in theory, a tumor could be visualized using different modalities of imaging throughout the duration of the radiation course; this would aid in the precision of radiation therapy delivery. This novel contrast ‘agent’ has not yet been investigated in liver cancer patients.
Recent improvements in multislice CT now allow for volumetric imaging at 0.35 s per rotation owing to 2D solid-state detectors covering 160 mm. Potential extrapolation of this technology to in-room CBCT technology would improve efficiency and quality of verification imaging occurring during a radiation therapy course. Further developments in CT are to be expected from advances in dual-energy imaging for improved soft-tissue contrast visualization.
To fully exploit the soft-tissue visualization with MRI, faster imaging acquisition methods need to be found. The imaging speed can be increased by using stronger and faster gradients, but this, in turn, will reduce the signal-tonoise ratio and spatial resolution. An alternative means of increasing the acquisition speed of MRI is to use parallel imaging, which employs an array of multiple receiver coils with distinct spatial sensitivities. Within the radiotherapy context, however, new research is looking at overcoming the practical difficulties of using MRI for real-time image guidance during treatment delivery. One such challenge is the fact that the magnetic field affects the secondary electrons produced by an X-ray beam and, hence, will impact the resulting dose distribution. A prototype system is being built to investigate the feasibility of simultaneous irradiation and MRI with millimeter resolution [83].
Considering the drive towards real-time and online imaging for treatment verification during a radiation therapy course for liver cancer, there is an opportunity within the radiotherapy community for improved collaborations with diagnostic and interventional imaging to facilitate the inclusion of state-of-the-art diagnostic imaging into the radiation therapy process. With such collaborations, we expect that more precise and accurate radiation therapy can be delivered, which should potentially improve outcomes and reduce toxicity for patients with liver cancer.
Financial & competing interests disclosure
L Dawson has received grants for liver cancer radiation therapy research from Cancer Care Ontario, NCIC, CIHR, Bayer (present) and Elekta (prior). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.

Conclusion
References
- Fong Y, Blumgart LH, Cohen AM: Surgical treatment of colorectal metastases to the liver. CA Cancer J. Clin. 45(1), 50–62 (1995).
- Scheele J, Altendorf-Hofmann A, Grube T, Hohenberger W, Stangl R, Schmidt K: [Resection of colorectal liver metastases. What prognostic factors determine patient selection?]. Chirurg 72(5), 547–560 (2001).
- Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S: Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann. Surg. Oncol. 13(5), 668–676 (2006).
- Thanos L, Mylona S, Galani P, Pomoni M, Pomoni A, Koskinas I: Overcoming the heat-sink phenomenon: successful radiofrequency thermal ablation of liver tumors in contact with blood vessels. Diagn. Interv. Radiol. 14(1), 51–56 (2008).
- Dawson LA, Mcginn CJ, Normolle D et al.: Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 18(11), 2210–2218 (2000).
- Greco C, Catalano G, Di Grazia A, Orecchia R: Radiotherapy of liver malignancies. From whole liver irradiation to stereotactic hypofractionated radiotherapy. Tumori 90(1), 73–79 (2004).
- Herfarth KK, Debus J, Lohr F et al.: Stereotactic single-dose radiation therapy of liver tumors: results of a Phase I/II trial. J. Clin. Oncol. 19(1), 164–170 (2001).
- Herfarth KK, Debus J, Wannenmacher M: Stereotactic radiation therapy of liver metastases: update of the initial Phase-I/II trial. Front. Radiat. Ther. Oncol. 38, 100–105 (2004).
- Ben-Josef E, Normolle D, Pan C et al.: A Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 23(34), 8739–8747 (2005).
- Lee MT, Kim JJ, Dinniwell R et al.: Phase I study of individualized stereotactic body radiotherapy of liver metastases. J. Clin. Oncol. 27(10), 1585–1591 (2009).
- Tse RV, Hawkins M, Lockwood G et al.: Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J. Clin. Oncol. 26(4), 657–664 (2008).
- Rusthoven KE, Kavanagh BD, Cardenes H et al.: Multi-institutional Phase I/II trial of stereotactic body radiation therapy for liver metastases. J. Clin. Oncol. 27(10), 1572–1578 (2009).
- Hoyer M, Roed H, Traberg Hansen A et al.: Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 45(7), 823–830 (2006).
- Balter JM, Dawson LA, Kazanjian S et al.: Determination of ventilatory liver movement via radiographic evaluation of diaphragm position. Int. J. Radiat. Oncol. Biol. Phys. 51(1), 267–270 (2001).
- Shimizu S, Shirato H, Xo B et al.: Threedimensional movement of a liver tumor detected by high-speed magnetic resonance imaging. Radiother. Oncol. 50(3), 367–370 (1999).
- Shirato H, Seppenwoolde Y, Kitamura K, Onimura R, Shimizu S: Intrafractional tumor motion: lung and liver. Semin. Radiat. Oncol. 14(1), 10–18 (2004).
- Mori S, Hara R, Yanagi T et al.: Fourdimensional measurement of intrafractional respiratory motion of pancreatic tumors using a 256 multislice CT scanner. Radiother. Oncol. 92(2), 231–237(2009).
- Kato H, Tsujii H, Miyamoto T et al.: Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int. J. Radiat. Oncol. Biol. Phys. 59(5), 1468–1476 (2004).
- Voroney JP, Brock KK, Eccles C, Haider M, Dawson LA: Prospective comparison of computed tomography and magnetic resonance imaging for liver cancer delineation using deformable image registration. Int. J. Radiat. Oncol. Biol. Phys. 66(3), 780–791 (2006).
- Parikh T, Drew SJ, Lee VS et al.: Focal liver lesion detection and characterization with diffusion-weighted MR imaging: comparison with standard breath-hold T2-weighted imaging. Radiology 246(3), 812–822 (2008).
- Kirilova A, Lockwood G, Choi P et al.: Three-dimensional motion of liver tumors using cine-magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 71(4), 1189–1195 (2008).
- Hussain SM, Semelka RC: Hepatic imaging: comparison of modalities. Radiol. Clin. North Am. 43(5), 929–947, ix (2005).
- Cai J, Read PW, Baisden JM, Larner JM, Benedict SH, Sheng K: Estimation of error in maximal intensity projection-based internal target volume of lung tumors: a simulation and comparison study using dynamic magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 69(3), 895–902 (2007).
- Pan T, Lee TY, Rietzel E, Chen GT: 4D-CT imaging of a volume influenced by respiratory motion on multislice CT. Med. Phys. 31(2), 333–340 (2004).
- Rietzel E, Pan T, Chen GT: Four-dimensional computed tomography: Image formation and clinical protocol. Med. Phys. 32(4), 874–889 (2005).
- Keall PJ, Starkschall G, Shukla H et al.: Acquiring 4D thoracic CT scans using a multislice helical method. Phys. Med. Biol. 49(10), 2053–2067 (2004).
- Yamamoto T, Langner U, Loo BW Jr, Shen J, Keall PJ: Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 72(4), 1250–1258 (2008).
- Evans PM, Coolens C, Nioutsikou E: Effects of averaging over motion and the resulting systematic errors in radiation therapy. Phys. Med. Biol. 51(1), N1–N7 (2006).
- Coolens C, Evans PM, Seco J et al.: The susceptibility of IMRT dose distributions to intrafraction organ motion: an investigation into smoothing filters derived from four dimensional computed tomography data. Med. Phys. 33(8), 2809–2818 (2006).
- Kini VR, Vedam SS, Keall PJ, Patil S, Chen C, Mohan R: Patient training in respiratory-gated radiotherapy. Med. Dosim. 28(1), 7–11 (2003).
- Mcclelland JR, Blackall JM, Tarte S et al.: A continuous 4D motion model from multiple respiratory cycles for use in lung radiotherapy. Med. Phys. 33(9), 3348–3358 (2006).
- Schmidt-Richberg A, Handels H, Ehrhardt J: Integrated segmentation and non-linear registration for organ segmentation and motion field estimation in 4D CT data. Methods Inf. Med. 48(4), 344–349 (2009).
- Li R, Lewis JH, Cervino LI, Jiang SB: 4D CT sorting based on patient internal anatomy. Phys. Med. Biol. 54(15), 4821–4833 (2009).
- Xi M, Liu MZ, Deng XW et al.: Defining internal target volume (ITV) for hepatocellular carcinoma using fourdimensional CT. Radiother. Oncol. 84(3), 272–278 (2007).
- Guckenberger M, Sweeney RA, Wilbert J et al.: Image-guided radiotherapy for liver cancer using respiratory-correlated computed tomography and cone-beam computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 71(1), 297–304 (2008).
- Beddar AS, Briere TM, Balter P et al.: 4D-CT imaging with synchronized intravenous contrast injection to improve delineation of liver tumors for treatment planning. Radiother. Oncol. 87(3), 445–448 (2008).
- Coolens C, Ockwell C, Hawkins M: Volume and motion definition in helical CT, 4DCT and MR imaging in upper gastrointestinal radiotherapy planning. Presented at: 9th Biennial ESTRO Meeting. Barcelona, Spain, 8–13 September 2007.
- Hawkins M, Coolens C, Ockwell C, Tait D: Results from a Phase I partial liver radiotherapy trial for patients with unresectable liver metastases. Presented at: ECCO 15 – 34th ESMO Multidisciplinary Congress. Berlin, Germany, 20–24 September 2009.
- Cai J, Read PW, Sheng K: The effect of respiratory motion variability and tumor size on the accuracy of average intensity projection from four-dimensional computed tomography: an investigation based on dynamic MRI. Med. Phys. 35(11), 4974–4981 (2008).
- Kitamura K, Shirato H, Seppenwoolde Y et al.: Tumor location, cirrhosis, and surgical history contribute to tumor movement in the liver, as measured during stereotactic irradiation using a real-time tumor-tracking radiotherapy system. Int. J. Radiat. Oncol. Biol. Phys. 56(1), 221–228 (2003).
- Wagman R, Yorke E, Ford E et al.: Respiratory gating for liver tumors: use in dose escalation. Int. J. Radiat. Oncol. Biol. Phys. 55(3), 659–668 (2003).
- Blackall JM: Respiratory motion in image-guided interventions of the liver. PhD Thesis, University College London, UK (2002).
- Beddar AS, Kainz K, Briere TM et al.: Correlation between internal fiducial tumor motion and external marker motion for liver tumors imaged with 4D-CT. Int. J. Radiat. Oncol. Biol. Phys. 67(2), 630–638 (2007).
- Krishnan S, Briere TM, Dong L et al.: Daily targeting of liver tumors: screening patients with a mock treatment and using a combination of internal and external fiducials for image-guided respiratory-gated radiotherapy. Med. Phys. 34(12), 4591–4593 (2007).
- Xi M, Liu MZ, Zhang L et al.: How many sets of 4DCT images are sufficient to determine internal target volume for liver radiotherapy? Radiother. Oncol. 92(2), 255–259 (2009).
- Coolens C, Breen S, Purdie T et al.: Characterisation of a 320-slice CT scanner for perfusion assessment in radiotherapy. Med. Phys. 36(11), 5120–5127 (2009).
- Keall PJ, Mageras GS, Balter JM et al.: The management of respiratory motion in radiation oncology report of AAPM task group 76. Med. Phys. 33(10), 3874–3900 (2006).
- Groh BA, Siewerdsen JH, Drake DG, Wong JW, Jaffray DA: A performance comparison of flat-panel imager-based mV and kV cone-beam CT. Med. Phys. 29(6), 967–975 (2002).
- Letourneau D, Wong JW, Oldham M et al.: Cone-beam-CT guided radiation therapy: technical implementation. Radiother. Oncol. 75(3), 279–286 (2005).
- Hawkins MA, Brock KK, Eccles C, Moseley D, Jaffray D, Dawson LA: Assessment of residual error in liver position using kV cone-beam computed tomography for liver cancer high-precision radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 66(2), 610–619 (2006).
- Case RB, Sonke JJ, Moseley DJ, Kim J, Brock KK, Dawson LA: Inter- and intrafraction variability in liver position in non-breath-hold stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 75(1), 302–308 (2009).
- Van Herk M: Errors and margins in radiotherapy. Semin. Radiat. Oncol. 14(1), 52–64 (2004).
- White MJ, Hawkes DJ, Melbourne A et al.: Motion artifact correction in free-breathing abdominal MRI using overlapping partial samples to recover image deformations. Magn. Reson. Med. 62(2), 440–449 (2009).
- Nelson C, Starkschall G, Baiter P et al.: Respiration-correlated treatment delivery using feedback-guided breath-hold: a technical study. Med. Phys. 32(1), 175–181 (2005).
- Kubo HD, Hill BC: Respiration gated radiotherapy treatment: a technical study. Phys. Med. Biol. 41(1), 83–91 (1996).
- Hugo GD, Agazaryan N, Solberg TD: An evaluation of gating window size, delivery method, and composite field dosimetry of respiratory-gated IMRT. Med. Phys. 29(11), 2517–2525 (2002).
- Shen S, Duan J, Fiveash JB et al.: Validation of target volume and position in respiratory gated CT planning and treatment. Med. Phys. 30(12), 3196–3205 (2003).
- Choi BO, Choi IB, Jang HS et al.: Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer 8, 351 (2008).
- Chung YW, Han DS, Paik CH et al.: Localized esophageal ulcerations after cyberknife treatment for metastatic hepatic tumor of colon cancer. Korean J. Gastroenterol. 47(6), 449–453 (2006).
- Kubas A, Chapet O, Merle P, Lorchel F, d’Hombres A, Mornex F: [Dosimetric impact of breath-hold in the treatment of hepatocellular carcinoma by conformal radiation therapy]. Cancer Radiother. 13(1), 24–29 (2009).
- Ten Haken RK, Balter JM, Marsh LH, Robertson JM, Lawrence TS: Potential benefits of eliminating planning target volume expansions for patient breathing in the treatment of liver tumors. Int. J. Radiat. Oncol. Biol. Phys. 38(3), 613–617 (1997).
- Eccles C, Brock KK, Bissonnette JP, Hawkins M, Dawson LA: Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 64(3), 751–759 (2006).
- Jaffray DA: Kilovoltage volumetric imaging in the treatment room. Front. Radiat. Ther. Oncol. 40, 116–131 (2007).
- Owen R, Kron T, Foroudi F et al.: Comparison of CT on rails with electronic portal imaging for positioning of prostate cancer patients with implanted fiducial markers. Int. J. Radiat. Oncol. Biol. Phys. 74(3), 906–912 (2009).
- Ma CM, Paskalev K: In-room CT techniques for image-guided radiation therapy. Med. Dosim. 31(1), 30–39 (2006).
- Wurm Re, Gum F, Erbel S et al.: Image guided respiratory gated hypofractionated stereotactic body radiation therapy (H-SBRT) for liver and lung tumors: initial experience. Acta Oncol. 45(7), 881–889 (2006).
- Kitamura K, Shirato H, Shimizu S et al.: Registration accuracy and possible migration of internal fiducial gold marker implanted in prostate and liver treated with real-time tumor-tracking radiation therapy (RTRT). Radiother. Oncol. 62(3), 275–281 (2002).
- Lee IJ, Seong J, Lee CG et al.: Early clinical experience and outcome of helical tomotherapy for multiple metastatic lesions. Int. J. Radiat. Oncol. Biol. Phys. 73(5), 1517–1524 (2009).
- Mcintosh A, Hagspiel KD, Al-Osaimi AM et al.: Accelerated treatment using intensitymodulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer 115(21), 5117–5125 (2009).
- Rochet N, Sterzing F, Jensen A et al.: Helical tomotherapy as a new treatment technique for whole abdominal irradiation. Strahlenther. Onkol. 184(3), 145–149 (2008).
- Coolens C, Webb S, Shirato H, Nishioka K, Evans PM: A margin model to account for respiration-induced tumour motion and its variability. Phys. Med. Biol. 53(16), 4317–4330 (2008).
- Gierga DP, Brewer J, Sharp GC, Betke M, Willett CG, Chen GT: The correlation between internal and external markers for abdominal tumors: implications for respiratory gating. Int. J. Radiat. Oncol. Biol. Phys. 61(5), 1551–1558 (2005).
- Yan H, Zhu G, Yang J et al.: The investigation on the location effect of external markers in respiratory-gated radiotherapy. J. Appl. Clin. Med. Phys. 9(2), 2758 (2008).
- Yan H, Yin FF, Zhu GP, Ajlouni M, Kim JH: The correlation evaluation of a tumor tracking system using multiple external markers. Med. Phys. 33(11), 4073–4084 (2006).
- Onimaru R, Shirato H, Fujino M et al.: The effect of tumor location and respiratory function on tumor movement estimated by real-time tracking radiotherapy (RTRT) system. Int. J. Radiat. Oncol. Biol. Phys. 63(1), 164–169 (2005).
- Korreman S, Mostafavi H, Le QT, Boyer A: Comparison of respiratory surrogates for gated lung radiotherapy without internal fiducials. Acta Oncol. 45(7), 935–942 (2006).
- Ionascu D, Jiang SB, Nishioka S, Shirato H, Berbeco RI: Internal–external correlation investigations of respiratory induced motion of lung tumors. Med. Phys. 34(10), 3893–3903 (2007).
- Gierga DP, Brewer J, Sharp GC, Betke M, Willett CG, Chen GTY: The correlation between internal and external markers for abdominal tumors: implications for respiratory gating. Int. J. Radiat. Oncol. Biol. Phys. 61(5), 1551–1558 (2005).
- Coolens C, White MJ, Crum WR et al.: Feasibility of free-breathing respiratory gated liver radiotherapy with MRI-derived models. Clin. Oncol. (R. Coll. Radiol.) 19(3), S13 (2007).
- Eccles CL, Haider EA, Haider MA, Fung S, Lockwood G, Dawson LA: Change in diffusion weighted MRI during liver cancer radiotherapy: preliminary observations. Acta Oncol. 48(7), 1034–1043 (2009).
- Cao Y, Pan C, Balter JM et al.: Liver function after irradiation based on computed tomographic portal vein perfusion imaging. Int. J. Radiat. Oncol. Biol. Phys. 70(1), 154–160 (2008).
- Zheng J, Liu J, Dunne M, Jaffray DA, Allen C: In vivo performance of a liposomal vascular contrast agent for CT and MR-based image guidance applications. Pharm. Res. 24(6), 1193–1201 (2007).
- Raaymakers BW, Lagendijk JJW, Overweg J et al.: Integrating a 1.5 T MRI scanner with a 6 mV accelerator: proof of concept. Phys. Med. Biol. 12, N229 (2009).
- Seer: Surveillance, Epidemiology and End Results Program (2008) http://seer.cancer.gov
• Excellent review of variability in liver tumor motion amplitude, and baseline shifts that may occur during each radiation therapy fraction.
• Original paper describing retrospective 4D CT imaging concepts and testing the process on phantoms, animals and humans.
• Respiratory motion degrades anatomic position reproducibility during imaging, necessitates larger volumes to be irradiated and can introduce errors during radiation delivery. The aim of this research was to develop, test and clinically implement a method to acquire 4D CT scans using a multislice helical method to reduce some of these limitations.
• First to use nonrigid image registration techniques to evaluate the impact of systematic errors on radiotherapy dose distributions if 4D CT was not used to estimate liver organ motion prior to radiation delivery.
• Describes the use of simple orthogonal 2D image guidance for liver cancer radiation therapy, with the diaphragm as a surrogate for the liver tumor, in patients immobilized with breath-hold. The residual 3D error in liver position was less than 5 mm in the majority of cases. In addition, this is the first paper describing how breath-hold kV cone-beam CT could be used for image guidance for liver cancer patients.
• Correlated cone-beam CT is used to analyze shifts in liver position during liver radiation therapy. Intrafraction shifts were found to be very small (<3 mm) in the great majority of patients; however, shifts in liver position day-to-day (i.e., interfraction shifts) were far larger (>8 mm in some cases), providing motivation for image guidance during radiation therapy for patients treated during free breathing or with abdominal compression to reduce liver motion amplitude during breathing.
• Reproducibility of the liver immobilized with repeat breath-holds was measured in this paper. Intrafraction shifts (occurring during one radiation therapy fraction) were found to be very small (<3 mm); however, shifts in liver position day-to-day (i.e., interfraction shifts) were far larger, providing motivation for image guidance during radiation therapy for patients with breath-hold liver immobilization during radiation therapy.
• Aims to quantify the correlation between external respiratory signals and abdominal tumor motion in four patients with liver tumors. Although tumor motion generally correlated well with external fiducial marker motion, relatively large underlying tumor motion can occur compared with externalmarker motion and variations in the tumor position were seen for a given marker position. Treatment margins should be determined on the basis of a detailed understanding of an individual’s tumor motion, as opposed to relying only on external-marker information.
Website