Case Report - International Journal of Clinical Rheumatology (2022) Volume 17, Issue 3
Systemic Lupus Erythematosus Presenting With Two Different Neurologic Entities, Guillain Barre Syndrome And Posterior Reversible Encephalopathy Syndrome: Case Report
Firdevs Uluta ÅŸ*,
Department of Medical Genetics, Pamukkale University Faculty of Medicine, Denizli, Turkey
- *Corresponding Author:
- Firdevs Uluta ÅŸ
Department of Medical Genetics, Pamukkale University Faculty of Medicine, Denizli, Turkey
E-mail: firdevsulutas1014@gmail.com
Received: 03-May-2020, Manuscript No. FMIJCR-20-10252; Editor assigned: 04-May-2020, PreQC No. FMIJCR-20-10252(PQ); Reviewed: 18-May-2020, QC No. FMIJCR-20-10252; Revised: 01-Mar-2022, Manuscript No. FMIJCR-20-10252(R); Published: 08-Mar-2022, DOI: 10.37532/1758-4272.2022.17(3).057-060
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease. Neuropsychiatric manifestations of SLE are quite complex and heterogeneous. Coexistence of Guillain Barre Syndrome (GBS) and Posterior Reversible Encephalopathy Syndrome (PRES) in SLE patients has not been reported recently. Because their of rare association and clinical diversity, we aimed to write this case diagnosed with SLE who initially presented with GBS and developed PRES at follow-up. Although the patient was promptly diagnosed and all of the early supportive and immunusuppressive treatment modalities were applied in the intensive care unit, we did not prevent his death. We hope that this case could raise awareness for clinicians of atypical rare presentations of neuropsychiatric involvement in SLE.
Keywords: guillain barre syndrome • posterior reversible encephalopathy syndrome • systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an immune-mediated, lifelong disease. Vasculitis of small vessels, deposition of immune complexes, and autoantibody production in various organs play a role in the disease pathogenesis. Neuropsychiatric manifestations of SLE, including headaches, seizures, psychosis and delirium, are quite heterogeneous and may occur at the onset of lupus or later in the course of disease. The frequency of neurologic involvement ranges between 12%-95% in SLE patients [1]. Peripheral nervous system involvement is in less than 10% of all nervous system manifestations [2]. The simultaneous prevalence of SLE with Guillain Barre Syndrome (GBS) has been reported to be between 0.6% and 1.7% whereas the prevalence of posterior reversible encephalopathy syndrome (PRES) is 0.69% in SLE [3]. The coexistence of PRES and GBS in SLE patients has not been reported in the past. Prompt diagnosis and treatment of patients can be delayed due to this rare coalescence and clinical diversity. Also, many confusing clinical conditions including hypertensive and uremic encephalopathy, delirium, use of cytotoxic drugs, ischemic or haemorrhagic cerebrovascular events, and infections may have added to the neurological involvement [4]. Although it’s difficult to find overlapping effects of predisposing factors in these patients, making the distinction of the above clinical conditions is very important for treatment modalities. In the literature, this is the first educational case where GBS and PRES occurred together in a SLE patient.
Case Report
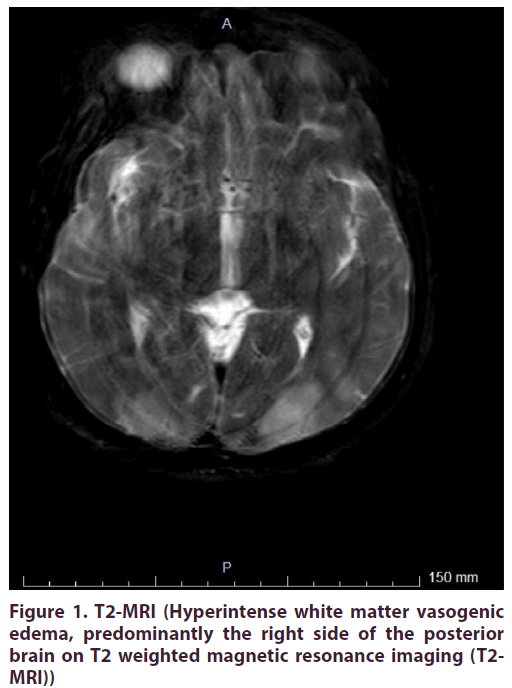
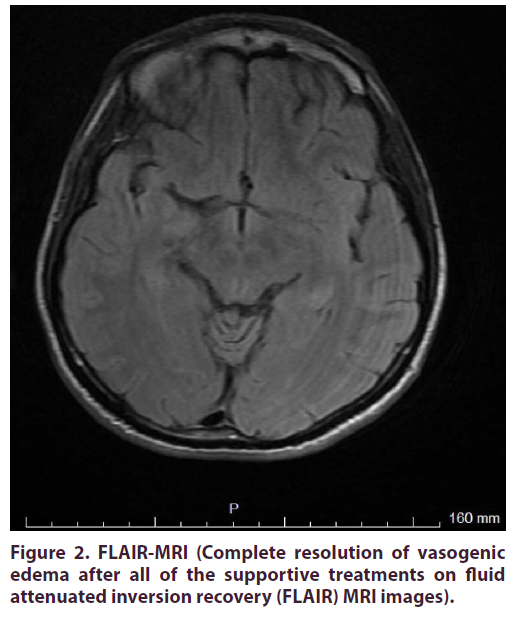
A 39 year-old man with a previous history of urogenital infection one year ago, was admitted to the inpatient clinic of Neurology at Pamukkale University, Denizli. He complained of acute onset pain, stiffness in the ankles, numbness and progressive weakness in the lower extremities, and walking instability for previous two weeks. At presentation, he was afebrile and normotensive. He was unable to stand alone. He had no malar rash, no oral ulcers, no weight loss, no addictions and no high-risk sexual intercourse. Muscle strength in the legs was 3/5 proximally and 2/5 distally. Symmetric, acute onset ascending paraparesis was observed in the distal limbs and deep tendon reflexes were absent. Bilateral axillary and inguinal 1-2 cm superficial lymphadenopathies were revealed. The possible causative bacterial infections including tuberculosis, brucellosis, syphilis and lyme disease were excluded via negative serological tests. Also viral serologic markers including Hepatitis A, B, C, Human Immunodeficiency Virus, Epstein- Barr Virus, Cytomegalovirus, Herpes Simplex Virus, Rubella and Toxoplasmosis were negative by Enzyme- Linked ImmunoSorbent Assay. Cerebrospinal fluid and nerve conduction studies showed albuminocytologic dissociation and findings of dysautonomic inflammatory demyelinating polyneuropathy, respectively. He was firstly treated with standard therapies including intravenous immunoglobulin (IVIG) and plasmapheresis sessions for the diagnosis of Guillain Barre Syndrome, but did not respond well to these therapies. Brain and whole spine magnetic resonance imaging were normal and inguinal excisional lymph node biopsy resulted in reactive lymphoid hyperplasia. Serum angiotensin converting enzyme (ACE) level was normal and no lymph node or parenchymal involvement was detected in lung tomography. After excluding sarcoidosis, infections, malignancies, and antiphospholipid antibody syndrome, he was diagnosed as SLE with the presence of symmetrical arthralgia and positive immunological markers including positive antinuclear antibody, antidsDNA antibody, anti-Smith antibody, and anti-SSA antibody with low complement levels, meeting the minimum 4 of 11 criteria created by the American College of Rheumatology. Laboratory tests revealed no hematological or renal involvement. Intravenous pulse methylprednisolone and cyclophosphamide were given. After intensive immunosuppressive treatments, he had tonic clonic seizures and severe headaches. Hypertension was observed (160/110). Concurrent infections and cerebrovascular insults were exluded. His brain magnetic resonance imaging (MRI) was consistent with hyperintense white matter vasogenic edema, predominantly the right side of the posterior brain on T2 weighted images (Figure 1). He was treated with antiepileptic and antiedema therapies. For adequate blood pressure control, parenteral antihypertensive agents were titrated exactly. Complete resolution of vasogenic edema was seen after all of the supportive treatments on fluid attenuated inversion recovery (FLAIR) MRI images (Figure 2). Thus the diagnosis of PRES was confirmed by reversible MRI images. Although the patient was admitted to the intensive care unit with mechanical ventilation and all supportive measurements, no clinical improvement was seen. Consequently we did not prevent death.
Figure 1. T2-MRI (Hyperintense white matter vasogenic
edema, predominantly the right side of the posterior
brain on T2 weighted magnetic resonance imaging (T2-
MRI))
Discussion
This is the first original educational case where GBS and PRES occurred together in a SLE patient. Only 15 cases with PRES and GBS have been reported in the recent literature, with the vast majority of patients being female and older than the age of 55 [5]. PRES can develop in association with autoimmune diseases like SLE, GBS, and polyarteritis nodosa. Although the association between PRES and GBS are poorly understood, underlying possible mechanisms leading to PRES in GBS patients may include dysautonomia, autoimmunity, IVIG therapy, and activation of the sympathetic nervous system. Dysautonomic cardiac and cerebrovascular complications of GBS include tachybradycardia, hypo-hypertension, and PRES, respectively [6]. The rare coexistence of PRES and GBS has also been reported after spinal surgery [7], in association with hyponatremia and IVIG therapy [8] and head injury [9].
75% of patients with SLE can present with many different neuropysichiatric manifestations from headache to stroke during the course of the disease [10]. In patients with Guillain-Barre Syndrome, autoantibodies target peripheral nervous system cells. As a result, damaged nerves can not transmit signals from the brain to the muscles. Many studies showed that antecedent viral or bacterial infectious agents like Campylobacter play a role in the etiology and may trigger autoimmune peripheral neuropathy. The recent cases of GBS and SLE in the literature were treated successfully with a combination of IVIG, corticosteroids, plasma exchange, and/or intravenous cyclophosphamide. The response rate of treatments was stated as 77.4% of patients with different types of peripheral nervous system involvement [11]. Although multiple clinical trials demonstrated the significant benefits of intravenous immunoglobulin and plasma exchange for treatment of GBS, our patient did not respond to initial treatment modalities and his clinical condition was aggravated [12]. During the intensive immunosuppressive treatment including CyC and pulse steroid, he developed posterior reversible encephalopathy syndrome (PRES). This was first described in 1996 by Hinchey. This neuroradiological disease is characterized by classical symptoms like headache, altered mental function, visual symptoms, vomiting, seizures, and with typical bilateral posterior subcortical brain edema on magnetic resonance imaging. PRES is completely reversible with supportive treatments and does not require immunosuppressive drugs. The rate in SLE patients was 18% in a case series of 120 patients with PRES and patients diagnosed with SLE and PRES were analyzed retrospectively. Concurrent hypertension, treatment with high dose steroids and CyC have also been reported as risk factors [13]. CyC is a mainstay drug for neurolupus and lupus nephritis, and may trigger PRES via direct endothelial cytotoxic effects at the blood brain barrier. In an other study, renal insufficiency and high SLE Disease Activity Index (SLEDAI) were also shown to be risk factors for development of PRES [14]. Cui Hw et al. stated that SLE patients with PRES had more early disease onset with predominantly seizures and higher mortality rates than controls. Severe hypertension causes disruption in autoregulation of brain blood flow. Also, interleukin-6 (IL-6) related inflammation and endothelial damage are thought to cause hyperperfusion induced vasogenic edema and brain injury in SLE, which show a high mortality rate [15]. Male gender, atypic presentation with GBS, early disease onset, and unresponsiveness to previous treatment modalities were poor prognostic factors for our patient.
Here with, we report the first rare coincidental case with PRES, GBS and SLE. Underlying possible conditions in this patient were not clear. The underlying autoimmune diseases including SLE and GBS, concurrent hypertension, the use of cyclophosphamide (CyC), IVIG and pulse steroids may be predisposing causes for development of PRES. We did not have a chance for further distinction due to the poor lethal course of disease. Currently, there are no specific, diagnostic radiological or laboratory biomarkers for neurological involvement in SLE. Awareness of clinicians and, early recognition of neuropsychiatric involvements of disease are important for timely appropriate treatment. Delayed treatment may cause permanent damage, poor prognosis, long term morbidity, and even consequently death. We hope that this case could raise awareness of clinicians on typical or atypical presentations of neuropsychiatric involvement in SLE.
References
- Kampylafka E, Alexopoulos H, Kosmidis M. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus: a 3‐year prospective study of 370 patients. PLoS ONE. 8,e55843 (2013).
- Hanly JG, Urowitz MB, Sanchez-Guerrero J. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 56,265-73 (2007).
- Nadri Q, Althaf MM. Guillian-Barre syndrome as the initial presentation of systemic lupus erythematosus-case report and review of literature. Ann Saudi Med. 35,263-5 (2015).
- Tatjana Zekic, Mirjana Stanic Benic, Ronald Antulov. The multifactorial origin of posterior reversible encephalopathy syndrome in cyclophosphamide-treated lupus patients. Rheumatology International. 37,2105-14 (2017).
- Chen A, Kim J, Henderson G. Posterior Reversible Encephalopathy Syndrome in Guillain-Barre Syndrome. J Clin Neurosci. 22,914-916 (2015).
- Lovie J, Igbokwe E, Hinchey J. Posterior Reversible Encephalopathy Syndrome associated with the Dysautonomia of Guillain-Barre Syndrome. Neurol Bull. 1,7-10 (2009).
- Sanpei Y, Hanazono A. Guillain Barre Syndrome and Posterior Reversible Encephalopathy Syndrome following Spinal Surgery. Case Rep Neurol. 11(3),284-9 (2019).
- Drye C, Bose S. Guillain-Barre syndrome with concurrent posterior reversible encephalopathy syndrome and hyponatremia: mere coincidence. BMJ Case Rep. 12(7),e229749 (2019).
- Yonekura S, Anno T, Kobayashi. Posterior Reversible Encephalopathy Syndrome and Guillain-Barre syndrome after Head Injury: Case Report. Neurol Med Chir (Tokyo). 58(10),453-8 (2018).
- Kakati S, Barman B, Ahmed SU. Neurological manifestations in systemic lupus erythematosus: a single centre study from North East India. J Clin Diagn Res. 11,OC05-OC09 (2017).
- Van Doorn P. Diagnosis, treatment and prognosis of Guillain-Barre syndrome (GBS). La Presse Medicale. 42,e193-e201 (2013).
- Toledano P, Orueta R, Rodriguez-Pint. Peripheral nervous system involvement in systemic lupus erythematosus: prevalence, clinical and immunological characteristics, treatment and outcome of a large cohort from a single centre. Autoimmun Rev. 16,750-5 (2017).
- Fugate JE, Claassen DO, Cloft HJ. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 85,427-32 (2010).
- Houssiau FA, Vasconcelos C, D’Cruz D. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 46,2121-31 (2002).
- Cui Hw, Lei RY, Zhang SG. Clinical features, outcomes and risk factors for posterior reversible encephalopathy syndrome in systemic lupus erythematosus: a case-control study. Lupus. 28,961-9 (2019).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref