Review Article - Imaging in Medicine (2012) Volume 4, Issue 6
Rotator cuff ultrasound-guided procedures: technical and outcome improvements
Emanuele Fabbro1, Giulio Ferrero1, Davide Orlandi1, Chiara Martini1, Francesca Nosenzo1, Giovanni Serafini2, Enzo Silvestri3 & Luca Maria Sconfienza*4,51Scuola di Specializzazione in Radiodiagnostica, Università degli Studi di Genova – Viale Benedetto XV 6, 16132 Genova, Italy
2Dipartimento di Diagnostica per Immagini, Ospedale Santa Corona, ASL 2 Savonese – Via XXV Aprile 38, 17037 Pietra Ligure, Italy
3Diagnostica per Immagini, Ospedale Evangelico Internazionale – Corso Solferino 29, 16121 Genova, Italy
4Servizio di Radiologia, IRCCS Policlinico San Donato & Dipartimento di Scienze Biomediche per la Salute, Università degli Studi di Milano, Piazza Malan 2 – 20097 San Donato Milanese, Milano, Italy
5Dipartimento di Scienze Biomediche per la Salute, Università degli Studi di Milano – Piazza Malan 2, 20097 San Donato Milanese, Italy
- Corresponding Author:
- Luca
Maria Sconfienza
Servizio di Radiologia
IRCCS Policlinico San Donato & Dipartimento di Scienze Biomediche per la Salute
Università degli Studi di Milano
Piazza Malan 2 – 20097 San Donato Milanese, Milano, Italy
Tel: +39 02 5277 4468
Fax: +39 02 5277 4925
E-mail: io@lucasconfienza.it
Abstract
Ultrasound has emerged as a low-cost, radiation-free and effective imaging technique to guide percutaneous procedures. The shoulder joint, thanks to its superficial anatomical position, represents a good target to perform such procedures under ultrasound guidance with an in-plane approach that allows a clear visualization of the needles during their whole path. However, the knowledge of some technical aspects and tips is essential to act in the most accurate way on target tissues that can be as small as a few millimeters. This article attempts to review the aspects of such procedures with a practical approach, emphasizing precautions and tricks based on day-by-day experience that may help to improve the outcome of percutaneous ultrasound-guided procedures around the shoulder.
Keywords
calcific enthesopathy ▪ calcific tendinopathy ▪ interventional ultrasound ▪ musculoskeletal percutaneous procedures ▪ rotator cuff ▪ shoulder ▪ tendonitis ▪ ultrasound guidance
Rotator cuff (RC) tendonitis is a common finding when investigating shoulder problems [1,2]. With this term, we usually refer to a general pathologic condition of the RC that includes degenerative tendinopathy, calcific tendinopathy or calcific enthesopathy [3–6]. Furthermore, periarticular conditions, such as bursitis and tenosynovitis of the long head of biceps tendons, can also often be diagnosed [1,7,8]. Ultrasound has emerged as a low-cost and effective imaging technique for guiding percutaneous procedures aimed at treating these conditions with good results. It is also free from ionizing radiation [9].
This article attempts to review the aspects of such procedures with a practical approach, emphasizing precautions and tricks for outcome improvement.
RC degenerative tendinopathy
Chronic or degenerative tendinopathy is microscopically characterized by degenerative changes of the collagen f ibers with accompanying fibrosis and absent or minimal inf lammation. The macroscopic appearance of such degenerated tendons is that of a poorly organized tissue with loss of the tightly bundled collagen [2,4,5]. This condition typically affects elderly patients and can also be asymptomatic. At ultrasound examination, the ultrastructural alterations of degenerated tendons can be demonstrated well, in particular, the loss of the typical tendinous fibrillar echotexture and thickening of the affected portion [10–12]. Three main theories on tendon degeneration and subsequent rupture have been proposed in the literature, the combination of which could, at least in part, explain the etiology of degenerative tendinous changes: the mechanical, vascular and neural theories [2]. In the mechanical theory, it is supposed that abnormal loading within the normal physiological stress range of a tendon causes fatigue and eventually leads to tendon failure, explaining how chronic repetitive microtears could accumulate over time [13]. The vascular theory suggests that some tendinous portions (e.g., the critical area of the supraspinatus, located 1–2 cm from its enthesis) could be relatively hypovascularized and more susceptible to a reduced vascular supply in certain conditions, such as during exercise [14]. Some biomechanical factors, such as the subacromial impingement of the supraspinatus tendon, could also accelerate both mechanical and vascular damage mechanisms [15]. A third, more recent, neural theory has observed and underlined the close association within tendons of nerve cell endings and mast cells that could be responsible for the release of proinflammatory mediators (substance P and calcitonin generelated peptide) [16]. However, this last theory needs more evidence to be fully accepted.
RC calcific tendinopathy
The term ‘calcific tendinitis’ refers to the intratendinous deposition of calcium, predominantly carbonate apatite, which can affect every tendon in the body and especially the RC. It is a commonly seen condition, occurring in up to 20% of painful shoulders and up to 7.5% of asymptomatic shoulders [17–20]. Typically associated with an intact RC [11], this condition is more frequent in women in their 40s and 50s and does not seem to be related to physical activity [18,19]. The supraspinatus tendon (80% of cases), followed by the infraspinatus (15% of cases) and subscapularis (5% of cases) tendons, is the most commonly affected site [11,20].
RC calcific tendinitis is recognized as a dynamic process evolving through four stages: precalcific, calcific, resorptive and postcalcific [17–20]. In the precalcific stage, which is usually asymptomatic, microtraumatic factors associated with a local decrease in blood supply can lead to intratendinous fibrocartilaginous metaplasia with resulting calcification [19]. The subsequent calcific phase is considered to be a resting period characterized by low-grade subacute pain that usually increases at night, variably associated with mechanical symptoms according to the size of the deposit [11]. Eventually, triggered by unknown factors, there is resorption of the deposit, accompanied by vascular invasion, the migration of phagocytic cells with dissolution of the calcific focus (resulting in a ‘toothpaste’ appearance of the calcific deposit) and edema from intratendinous pressure, with sharp acute pain that limits shoulder movement and is resistant to high doses of oral anti-inflammatory drugs [10,11]. Fever, reflecting rupture of the calcification into the adjacent structures, is occasionally reported [11]. This acute phase of calcific tendinitis of the RC is regarded as a selfhealing condition, with spontaneous resolution of sharp pain in 7–10 days, but moderate pain may last for months. After resorption, in the postcalcific or reparative phase, fibroblasts restore the normal tendinous collagen pattern [19]. The ultrasound appearance and classification of these typical calcific deposits can be simplified as follows: type I consists of a hyper-reflexive lesion with a well-circumscribed dorsal acoustic shadow; type II deposits are well circumscribed, homogeneous hyperechoic foci with a faint posterior shadow; and type III are amorphous, inhomogeneous hyperechoic foci without posterior acoustic shadow [21,22]. The consistency is solid for deposits of types I and II and semiliquid for type III calcifications [21,22].
RC enthesopathy
Calcific enthesopathy of the RC is a common ultrasound finding. In this condition, tiny hyperechoic calcifications are found in the insertional area of the RC tendons and are usually coupled with degenerative alterations of the preinsertional tendinous portion. Equally affecting males and females, the exact incidence of this condition cannot be estimated because of the broad range of degenerative or inflammatory conditions that may result in calcific enthesopathy [11,23]. In symptomatic cases, a well-circumscribed pain at the level of the greater trochanter (supraspinatus, infraspinatus, or teres minor insertional areas) or the lesser trochanter (subscapularis insertion) is reported by patients, which is worsened by applied pressure, either by the examiner’s finger or by the probe during the examination.
Subacromial-subdeltoid bursitis
The general term ‘bursitis’ indicates a nonspecific inflammatory condition of the synovial walls of the bursa, an anatomic entity designed to reduce the mechanical friction between sliding anatomical structures (e.g., tendons and cortical bone; tendons and muscles) [11]. Subacromial-subdeltoid (SASD) bursitis is the most common finding in ultrasound evaluation of a painful shoulder, while minor asymptomatic abnormalities of this structure can be observed in up to 78% of patients [11,24]. Primary bursitis commonly originates from rheumatoid arthritis, gout, tuberculosis, polymyalgia rheumatica and other pathologic conditions [11]. The bursa may become secondarily inf lamed in RC tendinopathy/tears, with or without joint effusion [11]. It can be also found in anterosuperior impingement due to overhead activities [25]. Patients with acute SASD bursitis usually refer a restriction of abduction movements without previous trauma. Pain often worsens during the night, but also when performing overhead activities, as it is typically reported on the lateral and anterior aspect of the shoulder [11]. In this case, ultrasound examination can demonstrate the presence of an anechoic effusion distending the hyperechoic bursal walls, together with the presence of vascular spots at the color-Doppler interrogation. Patients with chronic SASD bursitis often complain of a dull shoulder ache, with tenderness over the greater tuberosity and beneath the deltoid muscle, while ultrasound can demonstrate thickened bursal walls and the presence of effusion as a result of chronic inflammation [11].
Isolated septic bursitis is more likely to be observed in very young infants or elderly patients with chronic debilitating disorders, or may derive from accidental introduction of bacteria during nonsterile percutaneous procedures [11]. Bursal walls can be thickened and peribursal hypoechoic strands reflecting edema in the surrounding soft tissues may be associated findings [11].
Existing treatments for RC pathology
Numerous different types of treatments are used in the management of tendon disorders in the shoulder but, unfortunately, few have a strong evidence base [2,11].
In a condition of asymptomatic degenerative tendinopathy or calcif ic enthesopathy, physiotherapy must always be considered [26]. When symptomatic degenerative tendinous tears are present, a surgical treatment is suggested [2,26]. In symptomatic calcific enthesopathy, percutaneous dry-needling or surgical tendinous debridement are valuable options [18,27–29]. Regarding RC calcific tendonitis, treatment is not required in asymptomatic cases. In patients with mild symptoms, the disease can be managed conservatively with physical therapy and a short course of oral nonsteroidal antiinflammatory drugs [11]. Lithotripsy has been demonstrated to only be partially effective [30,31]. An alternative therapeutic approach is to extract the calcific material by using arthroscopy [31,32] or an imaging-guided procedure [21,22,25,27,33–37].
When an isolated bursitis is diagnosed, oral anti-inflammatory drugs and intrabursal steroid injections are usually indicated in the acute phase. In chronic unresponsive cases, surgical removal may be helpful [11].
What the radiologist can do
The interventional radiologist should be able to recognize the abovementioned conditions, which in most cases are susceptible to an effective percutaneous ultrasound-guided treatment.
■ Degenerative tendinopathy & SASD bursitis
Degenerative tendinopathy can be treated less effectively and action is usually limited on a chronically-inflamed SASD bursa, or in the subacromial articular space [38–40]. In the case of isolated bursitis, an intrabursal steroid injection can be performed under ultrasound guidance with high accuracy [38,40,41]. The authors use 40 mg of methylprednisolone acetate. In refractory, chronic cases, an injection of steroid plus low molecular weight hyaluronic acid could be considered to debride the thickened bursal walls and to mechanically promote correct gliding between the bursal surfaces [38]. The whole injection procedure can be performed in less than 1 min.
■ Ultrasound-guided treatment of calcific tendinitis
When calcific tendinopathy is diagnosed, an ultrasound-guided percutaneous treatment is always immediately indicated in the acute phase of the pathology, with ultrasound f indings of type II (well-circumscribed, homogeneous hyperechoic foci with a faint posterior shadow; solid consistency) or III (amorphous, inhomogeneous hyperechoic foci without posterior acoustic shadow; semi-liquid consistency) calcifications [11,18,21,22,27,42]. In case of mildly symptomatic type I (hyper-reflective appearance with a well-circumscribed dorsal acoustic shadow; solid consistency) calcifications, elective treatment should be considered. Such treatment is not indicated if the calcification is very small (<5 mm), if it has migrated into the bursal space, is eroding the humeral cortical bone or if it is widely fragmented [42].
A double-needle ultrasound-guided percutaneous procedure has recently been demonstrated to be effective and minimally invasive in the treatment of RC calcific tendinitis [18,29]. In this technique, the target calcification is sonographically visualized along its long axis, while a small amount of local anesthesia (up to 10 ml of lidocaine) is injected in the SASD bursa and around the calcification with an in-plane approach. Two 16G needles are consequently inserted into the calcification and a syringe filled with saline solution is connected to one of the needles, applying a gentle intermittent pressure in order to dissolve the core of the lesion and allow the chalky washing fluid to exit through the second needle until a complete internal emptying is visualized [18,29]. The procedure is then concluded with a SASD intrabursal steroid injection (40 mg methylprednisolone acetate). The whole procedure has an approximate duration of 15–20 min.
A one-needle approach has also been reported. This technique is very similar to that reported above, except for the fact that the calcification is punctured with only one needle. Washing is performed, pushing the syringe plunger to hydrate the deposit. Calcium ref luxes back together with saline solution or anesthetic within the same syringe as the plunger is retracted [36].
■ Calcific enthesopathy dry needling
The term ‘dry needling’ usually indicates a percutaneous procedure aimed at tissutale scarification (also known as ‘barbotage’) under imaging guidance [34,43]. It is simply made by means of repeated punctures of a target tissue in order to produce small intratendinous bleeding and tissutale release of platelet-derived growth factors to promote healing of the tendon. When dealing with calcific enthesopathy, the dry needling procedure also accelerates the resorption of calcific material. After ultrasound visualization of the insertional tiny calcifications (which are not routinely treated with a doubleneedle technique as calcific deposits are very small and cannot be adequately removed), a small amount of local anesthetic is injected along the needle path in the SASD bursa and in the affected portion of the tendon under an in plane approach. A series of repeated punctures is then performed to fragment the insertional calcifications and to promote the healing mechanisms described previously [43]. The procedure is completed with an intrabursal steroid injection (40 mg methylprednisolone acetate). The whole procedure has an approximate duration of 5 min.
How to improve the outcome of ultrasound-guided treatment of calcific tendinitis
Some tips and tricks need to be used to minimize peri- and post-procedural complications, such as infections and steroid-induced tendinous rupture, and to reduce the procedure time. The procedure may be performed by two operators (one holding the probe and the other performing the procedure itself) until a sufficient experience is reached. Radiologists with a lot of experience in percutaneous image-guided procedures can easily perform the described treatments alone.
■ Patient positioning
To prevent patient movement during the procedure and the consequences of possible vagal reactions, the patient should be placed in a semisupine position. The arm of the affected shoulder should be completely extended along the body and placed with a slight internal or external rotation according to calcification location.
■ Probe & skin disinfection
An effective disinfection of the skin can be reached by means of a two-step process: first, using a iodine-based solution (e.g., 7.5% polivinilpirrolidone) and then with a color-free solution (e.g., 0.25% benzalkonium chloride plus 70% ethylic alcohol) [44]. The reasons for such a procedure is the advantage of making the disinfected cutaneous area clearly visible with the first substance, and then to further disinfect with the colorless solution that also clears the iodine-based one in order to protect the plastic cover of the probe from staining. Sterile surgical drapes with adhesive borders can also be used. The ultrasound probe can be protected using a sterile cover. A cheaper alternative could be the application of a sterile gauze imbued with a 2% peracetic acid solution (or 0.25% benzalkonium chloride plus 70% ethylic alcohol) kept in place for at least 5 min, or using presoaked diapers [41]. An accurate and stable positioning of the probe can help to prevent contact with the needles and to further maintain the asepsis. A sterile lubricating gel is also needed to optimize the contact between the probe and the skin.
■ Local anesthesia
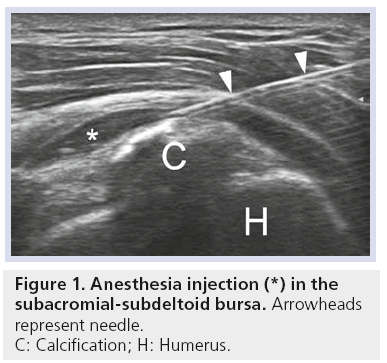
An effective local anesthesia can be achieved by injecting the drug under ultrasound guidance along the path of the needles, into the SASD bursa and in the pericalcific tendinous area (Figure 1). Different anesthetic solutions can be used: 2% mepivacaine chloridrate, 2% lidocaine chloridrate or 0.5% bupivacaine.
■ Correct needle positioning
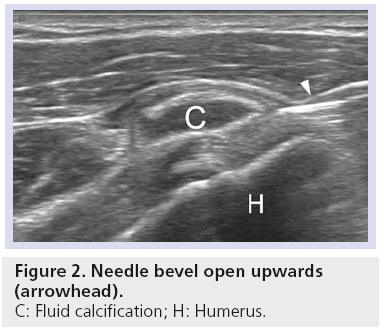
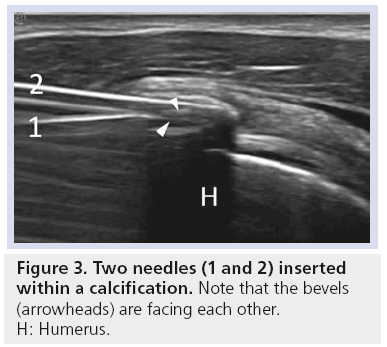
Since a suboptimal needle positioning could affect visualization for the whole procedure and reduce the efficacy of the treatment, accurate planning always needs to be considered. First, the needles must be inserted with an in-plane approach and parallel to the probe surface to have an optimal visualization under ultrasound guidance, with the bevel opened towards the probe (Figure 2) [28,37,44]. When performing a double-needle procedure, the deeper needle has to be inserted first, taking care not to go beyond the calcification in order to maintain the integrity of the calcific shell. The superficial needle is then inserted, as parallel as possible to the first, with bevel rotated downwards and towards the other needle (Figure 3).
■ Dry needling
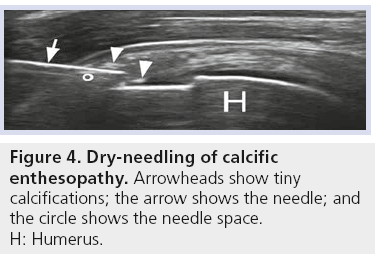
A series of approximately 20 repeated punctures is needed to fragment tiny insertional calcifications (which could not be treated with a double needle technique) in order to promote their resorption. In this case, the bevel of the needle should be maintained opened superficially or deeply (thus laying in the scanning plane), but not laterally because the longest part would not be visible (Figure 4).
■ Needle patency
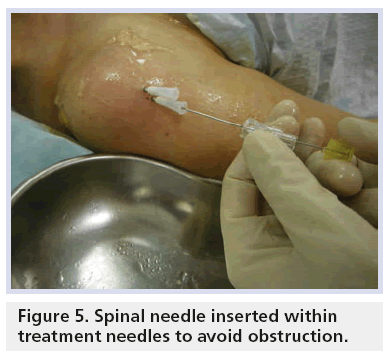
Once the needles are inserted in the calcification, its chalky material could obstruct their tips. The gentle use of an 18G spinal needle with the stylet slightly retracted into each needle could help in the removal of a sufficient amount of calcium from their tips and inside the calcification, and in creating an initial narrow space for the washing circuit of the saline solution (Figure 5) [42].
■ Saline solution
A recent study demonstrated a slight procedural time reduction when warm saline solution (40–42°C) is used instead of room temperature solution, as warm saline appeared to improve calcium deposit dissolution [45].
■ Optimizing the calcific debris removal
Gentle, intermittent pressures on the syringe plunger have to be performed, but excessive pressures that could break the outer shell of the calcification and dissipate the proinflammatory calcific material should be avoided [37,42]. It is essential that the solution injected in one needle exits from the other. If not, small adjustments of the needles are needed until a correct washing circuit is established.
■ Collecting the washing fluid
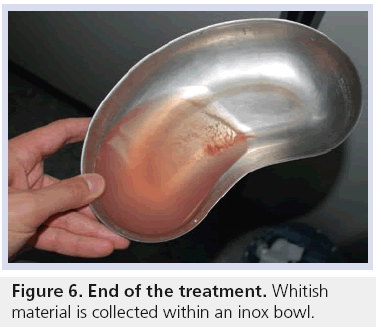
The use of an inox bowl to collect the washing solution allows the direct visualization of calcium crystals; thus, the operator can continue the washing procedure until the complete emptying of the calcification is confirmed by the spillage of a clean saline. Since a number of saline-filled syringes are usually needed, the periodic emptying of the bowl into a glass container can be helpful. This can also quantify the amount of calcium crystals collected during the whole procedure (Figure 6).
■ Reducing the risk of postprocedural bursitis
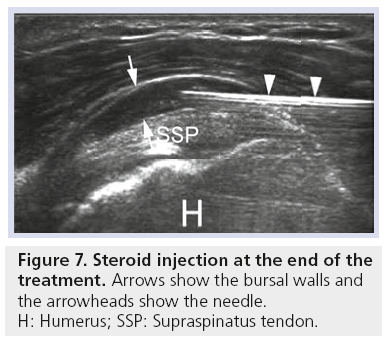
At the end of the procedure, ultrasound-guided intrabursal injection of slow-release steroids is indicated if no contraindications are present (e.g., diabetes). This seems to help in reducing the risk of postprocedural bursitis, which represents a short- to middle-time (3 months) complication in up to 14% of patients [37,45]. The bursal injection could be difficult in chronically inflamed bursae. In these cases, a gentle advancing of the needle into the bursa while injecting the steroid can help to debride thickened and collapsed bursal walls (Figure 7).
Discussion
Most pathologic conditions affecting tendons are usually referred to as tendinitis. However, we emphasize that this term is incorrect, as the underlying histology rarely includes inflammatory cells [6]. Thus, the term tendinosis may be more appropriate.
Several procedures have been proposed for the treatment of RC tendinous disorders.
In cases of RC calcific tendonitis, arthroscopic surgery is successful in up to 100% of cases [2,26,46,47], but it requires a hospital stay and rehabilitation; moreover, major complications, such as tendon rupture, have been encountered [47], albeit rarely. Extracorporeal shockwaves have been shown to be effective, facilitating short-term clinical improvement in 53–71% of patients [30,48] and long-term clinical improvement in 66–91% of patients [48–50]. However, extra-corporeal shockwaves require approximately three separate applications that are usually separated by 2–4 weeks and performed with dedicated equipment. In addition, this procedure is painful when performed during the acute pain attack. Percutaneous treatments of calcific tendonitis have been performed with fluoroscopic guidance since 1978 [34]. However, the disadvantages of this treatment are radiation exposure and potential difficulty in localizing the calcium deposit. Ultrasound guidance for calcific aspiration was first introduced in 1995 by Farin et al. [21]. The major difference of opinion among the various authors who have proposed the use of this approach is related to the use of one [27,35,36,51,52] versus two needles [18,21,22,53], although a recent work showed the absence of tendinous tears on a long-term surveillance, even in patients treated with a double-needle technique [18].
The dry needling technique performed in cases of calcific enthesopathy has its basis in the mechanical fragmentation of the small calcific insertional deposits added to the augmented tendinous vascularity induced by needle punctures inside the tendon that can speed their resorption; moreover, the release of growth factors and cytokines from the platelets in the affected area should stimulate tissue reparation.
When performing these procedures, a completely sterile setting is mandatory to avoid infective complications that could also threaten joint integrity. Moreover, a deep knowledge of technical aspects that could improve the visualization of the needles is also necessary, as well as the ability to choose the best percutaneous approach according to the size and location of the lesions.
Future perspective
Tendon physiopathology is quite well understood but the action of new substances on tendon damage are still to be investigated. Hyaluronic acid, an important component of the cartilage and cellular matrix, is currently used to contrast degenerative processes in the musculoskeletal system. Similarly, platelet-rich plasma has been demonstrated to play a role in treating degenerative tendon pathology with alternate results. Further studies should aim to assess whether these approaches may be used to improve RC pathology.
Conclusion
Ultrasound offers excellent image guidance when performing percutaneous musculoskeletal procedures. The shoulder joint, thanks to its superficial anatomical position, represents a good target on which to perform such procedures under ultrasound guidance with an in-plane approach that allows a clear visualization of the needles over their whole path. The continuous, real-time monitoring of the procedure offered by this radiation-free technique is widely accepted as a strength value, but the knowledge of some technical aspects and tips is essential for acting in the most accurate way on target tissues that can be as small as a few millimeters (e.g., the subacromion-subdeltoid bursa or tiny insertional calcifications). Based on day-by-day experience, there are some tips and tricks that may help to improve the outcome of percutaneous ultrasound- guided procedures around the shoulder.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann. Rheum. Dis. 54(12), 959–964 (1995).
- Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford). 45(5), 508–521 (2006).
- Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin. Sports Med. 22(4), 675–692 (2003).
- Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 27(6), 393–408 (1999).
- Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin. Orthop. Relat. Res. (415), 111–120 (2003).
- Khan KM, Cook JL, Kannus P, Maffulli N, Bonar SF. Time to abandon the ‘tendinitis’ myth. BMJ 324(7338), 626–627 (2002).
- Harrison AK, Flatow EL. Subacromial impingement syndrome. J. Am. Acad. Orthop. Surg. 19(11), 701–708 (2011).
- Nho SJ, Strauss EJ, Lenart BA et al. Long head of the biceps tendinopathy: diagnosis and management. J. Am. Acad. Orthop. Surg. 18(11), 645–656 (2010).
- Klauser AS, Tagliafico A, Allen GM et al. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European Society of Musculoskeletal Radiology. Eur. Radiol. 22(5), 1140–1148 (2012).
- Valle M, Zamorani MP. Muscle and tendon. In: Ultrasound of the Musculoskeletal System. Bianchi S, Martinoli C (Eds). Springer, Berlin, Germany, 45–91 (2007).
- Bianchi S, Martinoli C. Shoulder. In: Ultrasound of the Musculoskeletal System. Bianchi S, Martinoli C (Eds). Springer, Berlin, Germany, 190–331 (2007).
- Silvestri E, Orlandi D. The shoulder: focused ultrasound anatomy and examination technique. In: Normal Ultrasound Anatomy of the Musculoskeletal System: a Practical Guide. Silvestri E, Muda A, Sconfienza LM (Eds). Springer Verlag, Milan, Italy, 13–23 (2012).
- Mosler E, Folkhard W, Knörzer E, Nemetschek-Gansler H, Nemetschek T, Koch MH. Stress-induced molecular rearrangement in tendon collagen. J. Mol. Biol. 182(4), 589–596 (1985).
- Ling SC, Chen CF, Wan RX. A study on the vascular supply of the supraspinatus tendon. Surg. Radiol. Anat. 12(3), 161–165 (1990).
- Neer CS 2nd. Impingement lesions. Clin. Orthop. Relat. Res. (173), 70–77 (1983).
- Scott A, Bahr R. Neuropeptides in tendinopathy. Front. Biosci. 14, 2203–2211 (2009).
- Speed CA, Hazleman BL. Calcific tendinitis of the shoulder. N. Engl. J. Med. 340(20), 1582–1584 (1999).
- Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10‑year outcomes after two-needle US-guided percutaneous treatment – nonrandomized controlled trial. Radiology 252(1), 157–164 (2009).
- Uhthoff HK, Sarkar K. Calcifying tendinitis. Baillieres Clin. Rheumatol. 3(3), 567–581 (1989).
- Mole D, Gonzavez M, Roche O, Scarlat M. Introduction to calcifying tendinitis. In: The Cuff. Gazielly D, Gleyze P, Thomas T (Eds). Elsevier, Paris, France, 141–143 (1997).
- Farin PU, Jaroma H, Soimakallio S. Rotator cuff calcifications: treatment with US-guided technique. Radiology 195(3), 841–843 (1995).
- Farin PU, Räsänen H, Jaroma H, Harju A. Rotator cuff calcifications: treatment with ultrasound-guided percutaneous needle aspiration and lavage. Skeletal Radiol. 25(6), 551–554 (1996).
- Slobodin G, Rozenbaum M, Boulman N, Rosner I. Varied presentations of enthesopathy. Semin. Arthritis Rheum. 37(2), 119–126 (2007).
- van Holsbeeck M, Strouse PJ. Sonography of the shoulder: evaluation of the subacromialsubdeltoid bursa. AJR Am. J. Roentgenol. 160(3), 561–564 (1993).
- Farin PU, Jaroma H, Harju A, Soimakallio S. Shoulder impingement syndrome: sonographic evaluation. Radiology 176(3), 845–849 (1990).
- Lansdown DA, Feeley BT. Evaluation and treatment of rotator cuff tears. Phys. Sportsmed. 40(2), 73–86 (2012).
- Sconfienza LM, Serafini G, Sardanelli F. Treatment of calcific tendinitis of the rotator cuff by ultrasound-guided single-needle lavage technique. AJR Am. J. Roentgenol. 197(2), W366–W366 (2011).
- Serafini G, Sconfienza LM. Treatment of calcific tendonitis of the rotator cuff. In: Ultrasound-guided Musculoskeletal Procedures: the Upper Limb. Sconfienza LM, Serafini G, Silvestri E (Eds). Springer Verlag, Milan, Italy, 32–35 (2012).
- Cacchio A, Rompe JD, Serafini G, Sconfienza LM, Sardanelli F. US-guided percutaneous treatment of shoulder calcific tendonitis: some clarifications are needed. Radiology 254(3), 990; author reply 990–991 (2010).
- Hsu CJ, Wang DY, Tseng KF, Fong YC, Hsu HC, Jim YF. Extracorporeal shock wave therapy for calcifying tendinitis of the shoulder. J. Shoulder Elbow Surg. 17(1), 55–59 (2008).
- Hurt G, Baker CL Jr. Calcific tendinitis of the shoulder. Orthop. Clin. North Am. 34(4), 567–575 (2003).
- Jerosch J, Strauss JM, Schmiel S. Arthroscopic treatment of calcific tendinitis of the shoulder. J. Shoulder Elbow Surg. 7(1), 30–37 (1998).
- Pfister J, Gerber H. Chronic calcifying tendinitis of the shoulder-therapy by percutaneous needle aspiration and lavage: a prospective open study of 62 shoulders. Clin. Rheumatol. 16(3), 269–274 (1997).
- Comfort TH, Arafiles RP. Barbotage of the shoulder with image-intensified fluoroscopic control of needle placement for calcific tendinitis. Clin. Orthop. Relat. Res. 135, 171–178 (1978).
- Bradley M, Bhamra MS, Robson MJ. Ultrasound guided aspiration of symptomatic supraspinatus calcific deposits. Br. J. Radiol. 68(811), 716–719 (1995).
- Aina R, Cardinal E, Bureau NJ, Aubin B, Brassard P. Calcific shoulder tendinitis: treatment with modified US-guided fineneedle technique. Radiology 221(2), 455–461 (2001).
- Sconfienza LM, Viganò S, Martini C et al. Double-needle ultrasound-guided percutaneous treatment of rotator cuff calcific tendinitis: tips and tricks. Skeletal Radiol. doi:10.1007/s00256-012-1462-x (2012) (Epub ahead of print).
- Silvestri E. Subacromial-subdeltoid bursa injections. In: Ultrasound-guided Musculoskeletal Procedures: the Upper Limb. Sconfienza LM, Serafini G, Silvestri E (Eds). Springer Verlag, Milan, Italy, 25–28 (2012).
- Tagliafico A, Serafini G, Sconfienza LM et al. Ultrasound-guided viscosupplementation of subacromial space in elderly patients with cuff tear arthropathy using a high weight hyaluronic acid: prospective open-label nonrandomized trial. Eur. Radiol. 21(1), 182–187 (2011).
- Rutten MJCM, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 78(2), 254–257 (2007).
- Chen MJL, Lew HL, Hsu TC et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am. J. Phys. Med. Rehabil. 85(1), 31–35 (2006).
- Serafini G, Sconfienza LM. Treatment of calcific tendinitis of the rotator cuff. In: Ultrasound-guided Musculoskeletal Procedures: the Upper Limb. Silvestri E, Muda A, Sconfienza LM (Eds). Springer Verlag, Milan, Italy, 29–31 (2012).
- Lacelli F. Calcific enthesopathy dry-needling. In: Ultrasound-guided Musculoskeletal Procedures: the Upper Limb. Sconfienza LM, Serafini G, Silvestri E (Eds). Springer Verlag, Milan, Italy, 37–40 (2012).
- Conchiglia A, Gregori LM, Zugaro L, Masciocchi C. General aspects of US-guided musculoskeletal procedures. In: Ultrasoundguided Musculoskeletal Procedures: the Upper Limb. Sconfienza LM, Serafini G, Silvestri E (Eds). Springer Verlag, Milan, Italy, 1–9 (2012).
- Sconfienza LM, Bandirali M, Serafini G et al. Rotator cuff calcific tendinitis: does warm saline solution improve the short-term outcome of double-needle US-guided treatment? Radiology 262(2), 560–566 (2011).
- Wittenberg RH, Rubenthaler F, Wölk T, Ludwig J, Willburger RE, Steffen R. Surgical or conservative treatment for chronic rotator cuff calcifying tendinitis – a matched-pair analysis of 100 patients. Arch. Orthop. Trauma Surg. 121(1–2), 56–59 (2001).
- Weber SC, Abrams JS, Nottage WM. Complications associated with arthroscopic shoulder surgery. Arthroscopy 18(2 Suppl. 1), S88–S95 (2002).
- Daecke W, Kusnierczak D, Loew M. Longterm effects of extracorporeal shockwave therapy in chronic calcific tendinitis of the shoulder. J. Shoulder Elbow Surg. 11(5), 476–480 (2002).
- Wang CJ, Yang KD, Wang FS, Chen HH, Wang JW. Shock wave therapy for calcific tendinitis of the shoulder: a prospective clinical study with two-year follow-up. Am. J. Sports Med. 31(3), 425–430 (2003).
- Rompe JD, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin. Orthop. Relat. Res. (387), 72–82 (2001).
- Giacomoni P, Siliotto R. Echo-guided percutaneous treatment of chronic calcific tendinitis of the shoulder. Radiol. Med. 98(5), 386–390 (1999).
- del Cura JL, Torre I, Zabala R, Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. AJR Am. J. Roentgenol. 189(3), W128–W134 (2007).
- Galletti S, Magnani M, Rotini R et al. The echo-guided treatment of calcific tendinitis of the shoulder. Chir. Organi. Mov. 89(4), 319–323 (2004).
• Represents a practical guide for those who want to learn how to perform an adequate and comprehensive ultrasound examination of the joints of the upper and lower limbs.
• • Provides both a short- and long-term analysis of the outcomes achieved with the double-needle technique for ultrasound treatment in more than 200 patients affected by rotator cuff tendonitis.
• First paper presenting the double-needle ultrasound-guided technique.
• • Practical and illustrated guide about the most useful percutaneous ultrasound-guided musculoskeletal procedures on the upper limb.