Research Article - Journal of Experimental Stroke & Translational Medicine (2012) Volume 5, Issue 1
Novel experimental model for repeated forebrain ischemia-reperfusion
- *Corresponding Author:
- Michal Toborek, MD, PhD
Department of Biochemistry and Molecular Biology
University of Miami School of Medicine
1011 NW 15th Street; Gautier Building
Room 517; Miami FL, 33136-1019
Tel: (305)-243-0230
E-mail: mtoborek@med.miami.edu
Abstract
Background and Purpose: The lack of reliable rodent model for repeated ischemia-reperfusion (I-R) hampers experimental research on stroke. Therefore, the objective of the present study was to develop a mouse model for repeated I-R cycles in a single animal on different days.
Methods and Results: The right common carotid artery (CCA) was ligated and both vertebral arteries were coagulated. A customized vascular occluder, with its actuating tubing glued to a microport, was cuffed around the isolated left CCA and secured. Inflating of the occluder diaphragm via the microport restricted the blood flow via the left CCA and reduced the cerebral blood flow (CBF) by 75% in both hemispheres, while deflating allowed for the CBF restoration. Two minutes of forebrain ischemia followed by a 24 h reperfusion period was tolerated by animals for 5 cycles. Importantly, repeated 2 min I-R cycles attenuated the infarct volume induced by occlusion of the middle cerebral artery.
Conclusions: The described model is a reliable method to induce transient I-R events in the forebrain. The model mimics transient ischemic attacks and allows for controlling the ischemic durations, intervals, and numbers of I-R cycles.
Keywords
Cerebral blood flow; ischemia reperfusion; preconditioning; stroke
Introduction
Stroke is the third most common cause of death and the most common cause of adult disability world wide (Lloyd Jones et al., 2010). Studies on brain preconditioning and postconditioning have revealed that transient ischemia can induce protective reprogramming of genomes and proteins (Ravati et al., 2000; Dirnagl et al., 2009; Zhang et al., 2008; Zhao, 2009; Hossmann, 2008; Barone et al., 1998; Pignataro et al., 2009; Dezfulian et al., 2007; Corbett et al., 1997). However, there is no rodent model currently available for repeated brain ischemia reperfusion (IR) cycles on consecutive days, which markedly hampers stroke related research (Liu et al., 2009).
A frequent model in experimental stroke research is based on advancing a suture through the internal carotid artery (ICA) to block the root of the middle cerebral artery (MCA). Its withdrawal allows for restoration of cerebral blood flow (CBF). However, mechanical damage to the vessel wall tends to make this model impractical to induce repeated ischemic cycles (Hossmann, 1998; Barone et al., 1998; Hossmann, 2008; Taguchi et al., 2010; Zhang et al., 2010). Other methods, such as a four vessel occlusion model and photothrombosis induced focal ischemia model, are nonreversible, while the ischemic duration cannot be precisely controlled in the endothelin model (Hossmann, 1998; Hossmann, 2008). Thus, it is reasonable to question whether the existing animal models for repeated IR cycles allow for the optimal development of neuroprotective mechanisms or the described experimental complications counteract these mechanisms (Tanay et al., 2006; Zhan et al., 2008; Hossmann, 1998; Dirnagl et al., 2009; Zhang et al., 2008; Hossmann, 2008; Gidday, 2006; Beyersdorf et al., 1998; Beyersdorf, 2009; Liu et al., 2009). To overcome these difficulties, we developed a novel mouse model that enables multiple and transient inductions of forebrain I-R cycles. The model allows for the control of ischemic duration and intervals. It is compatible with many other research approaches, and can be utilized for multiple applications.
Materials and Methods
Customization of the vascular occluder
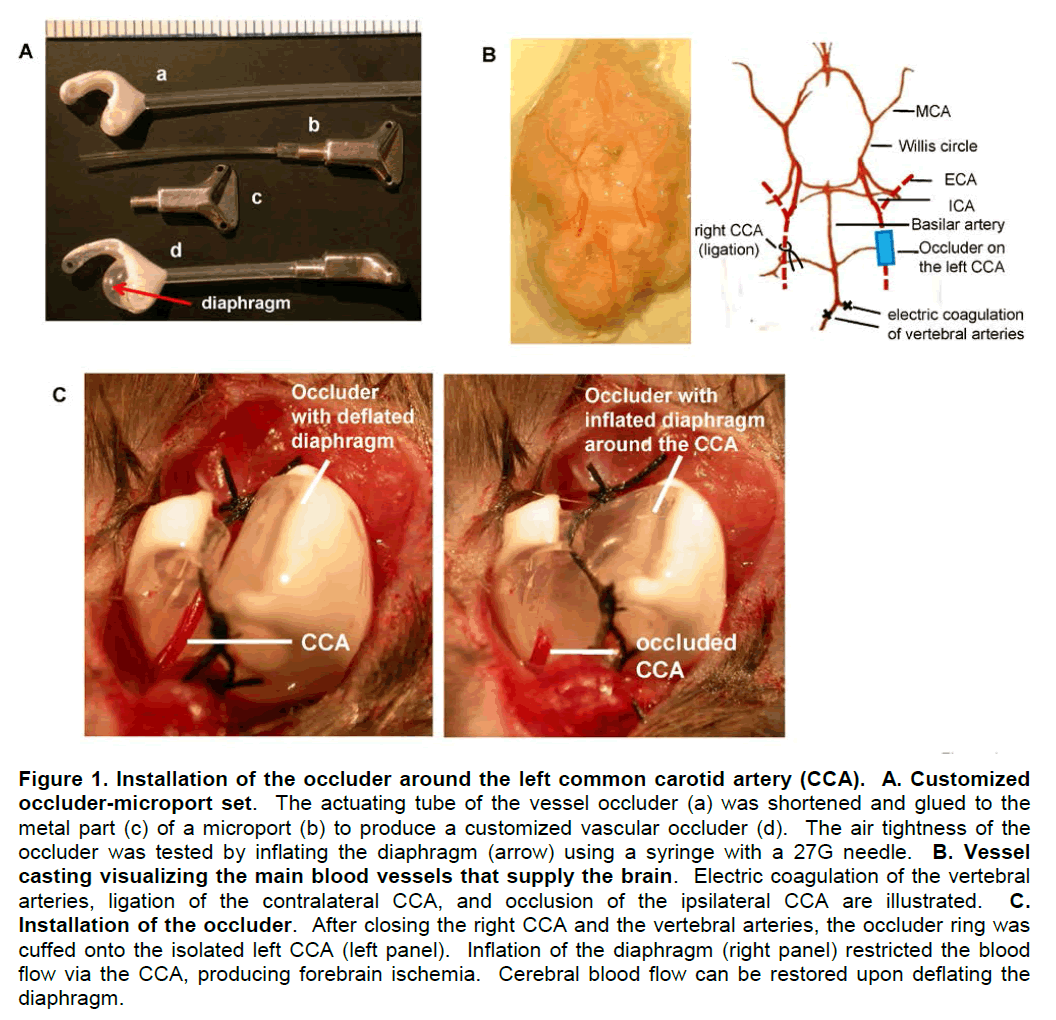
The cuff ring of the commercially available vessel occluder (Kent Scientific, Torrington, CT) was trimmed by cutting both edges of the diaphragm free area while preserving the 1/3 of the middle area. The occluder actuating tube was shortened to 2 cm, connected to the metal part of the microport (DaVinci Biomedical Research Products, South Lancaster, MA), and secured with super glue (Fisher Scientific, Pittsburgh, PA). The customized occluder (Figure 1A) was used after the air tightness test for 10 min and sterilization.
Installation of the vascular occluder and induction of I-R cycles
C57BL/6 mice (11-12 week old; 25–32 g; the total number of tested animals, 127) were purchased from Charles River Laboratories (Wilmington, MA). All procedures and handling techniques were in strict accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee, University of Kentucky. Mice were anesthetized with oxygen containing 1-1.5% Isoflurane. The major blood vessels supplying blood into the brain were isolated. The right CCA was ligated, both vertebral arteries were electrically coagulated, and the left CCA was isolated (Figure 1B). The cuff ring of the occluder was placed around the isolated left CCA and secured with surgical sutures (Figure 1C). Then, the microport end of the actuating tube was inserted through a subcutaneous tunnel and secured subcutaneously in the back. Following the surgery, the wound was closed and the animals were allowed to recover.
The installed occluder allows for transient obstruction of the left CCA. Through the microport, air can be injected or withdrawn using a syringe with a 27G needle to inflate or deflate the occluder diaphragm. Inflating the diaphragm compresses the vessel, restricts the blood flow, and induces forebrain ischemia, whereas withdrawing the air from the diaphragm restores the blood flow (Figure 1C, left and right panels). Using this procedure, the animals were subjected to five I-R cycles repeated on consecutive days. Duration of ischemia was set for 2 or 10 min, followed each time by a 24 h reperfusion period.
Vessel casting and visualization of major blood supply to the cortex and brain stem
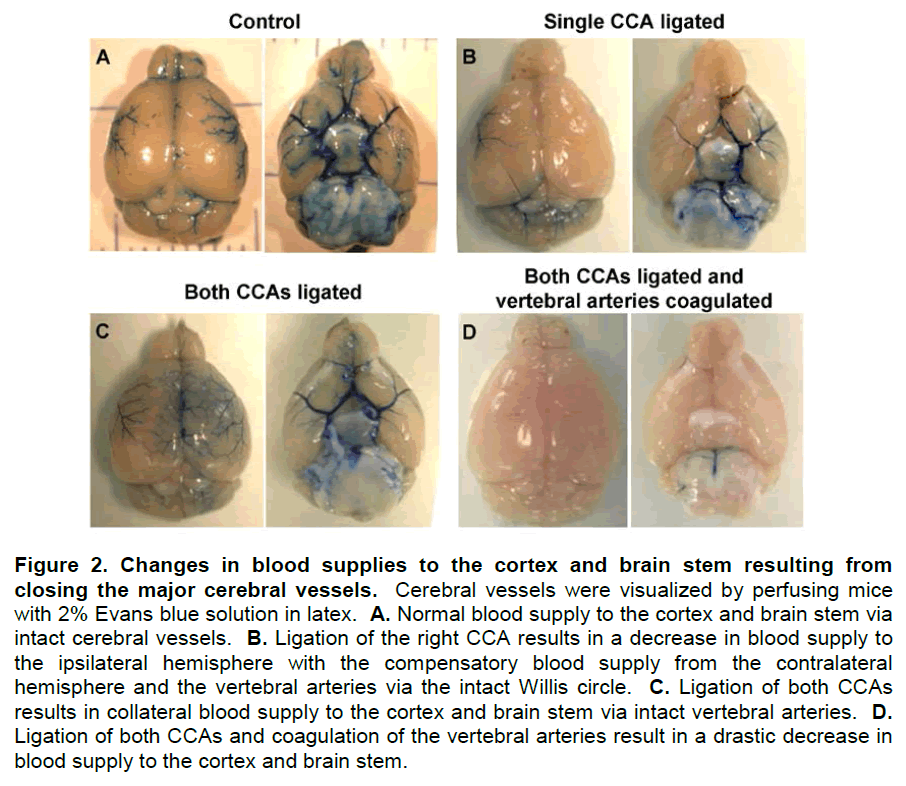
Vessel casts were prepared to visualize the major vessels supplying the brain and Willis circle, which communicates between the two hemispheres. Animals were euthanized and perfused with heparinized saline and 4% formalin in PBS, followed by 5 ml Mercox (Ladd Research, Williston, VT). After the resin cured for 1–2 days at room temperature, soft tissue was macerated in 7.5% KOH, followed by 5% formic acid at 50 ºC for 24 h. Vessel casts were water washed, freezedried, and photographed.
To verify the effectiveness of vessel ligation and occluder manipulations, mice were perfused with heparinized saline followed by 5 ml 2% Evansblue in latex (w/v, Spartan Adhesives & Coatings, Crystal Lake, IL). Then, brains were harvested and photographed.
CBF monitoring
A noninvasive near infrared diffuse optical method, namely diffuse correlation spectroscopy (DCS), was employed to measure the changes in CBF in the deep brain during 2 or 10 min I-R cycles. DCS utilizes light intensity fluctuations collected from the tissue surface to quantify microvasculature blood flow in deep (millimeters to centimeters) tissues (Boas et al., 1995). The method has been broadly validated to other standards and used to probe various tissues, including brain (Cheung et al., 2001; Zhou et al., 2006 ; Kim et al., 2010; Shang et al., 2011). It was also validated in our laboratory with a commercial PeriFlux System 5000 Laser Doppler (PERIMED, North Royalton, OH) (Shang et al. 2011). Briefly, mice were anesthetized, the scalps were removed, and foam bases confining laser source and detector fibers were glued on the skulls. CBF changes from both hemispheres were detected simultaneously. Gluing the foam base to the skull allowed for reliable measurements at the identical tissue location and depth at different days.
Evaluation of motor coordination and neurodeficit testing
Several beam walking tests, adapted from a wellestablished neurological severity score, were used to assess motor coordination and balance (Pleasant et al., 2011). After a 24 h recovery from the last I-R cycle, mice were evaluated as they walked across Plexiglass beams of 3, 2, 1, and 0.5 cm widths, and finally a round, 0.5 cm diameter wooden rod. The mice scored 3 points for successfully walking across each beam and 2 points for successfully walking across the rod, with 14 being the maximum score. Within the allotted 30 s trial, points were deducted for each limb misplacement or inversion on the beam. Falling off the beam or refusing to move was scored as zero.
Separate groups of mice were evaluated for neurological deficits using the neuroscore as described by Mbye et al (2009). The neuroscore measures forelimb function, hindlimb function, and resistance to lateral pulsion on a scale of 0 (severely impaired animals) to 4 (normal) for each function.
Induction of transient or permanent focal ischemia
Selected mice were subjected to transient or permanent occlusion of the right MCA with a silicone coated 6-0 nylon surgical suture (Doccol, New York, NY). The successful occlusion was verified by over 75% decrease in CBF in the ischemic hemisphere. In a model of transient ischemia, the suture was withdrawn after 2 h. Following a 24 h recovery, these mice were used as positive controls in the behavioral test. For permanent ischemia, the suture was left blocking the MCA for 24 h. Then, mice were examined for stroke volume as determined by staining with 2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma) and quantified with NIH Image-J software.
Statistical analysis
Behavioral changes were analyzed by the Kruskal-Wallis nonparametric test, followed by the Mann-Whitney U test. One way ANOVA was used to compare mean responses among treatments in the stroke experiments. Statistical probability of p<0.05 was considered significant.
Results
Effects of vessel ligations on forebrain blood supply
Changes in the cerebral blood supply from ligation of the CCAs and coagulation of the vertebral arteries are shown in Figure 2. Ligation of single or both CCAs lowered forebrain blood supplies but induced collateral blood flow through the Willis circle or vertebral arteries (Figures 2B and C), while obstruction of both vertebral arteries and both CCAs resulted in complete forebrain ischemia (Figure 2D).
Changes in CBF during repeated I-R cycles
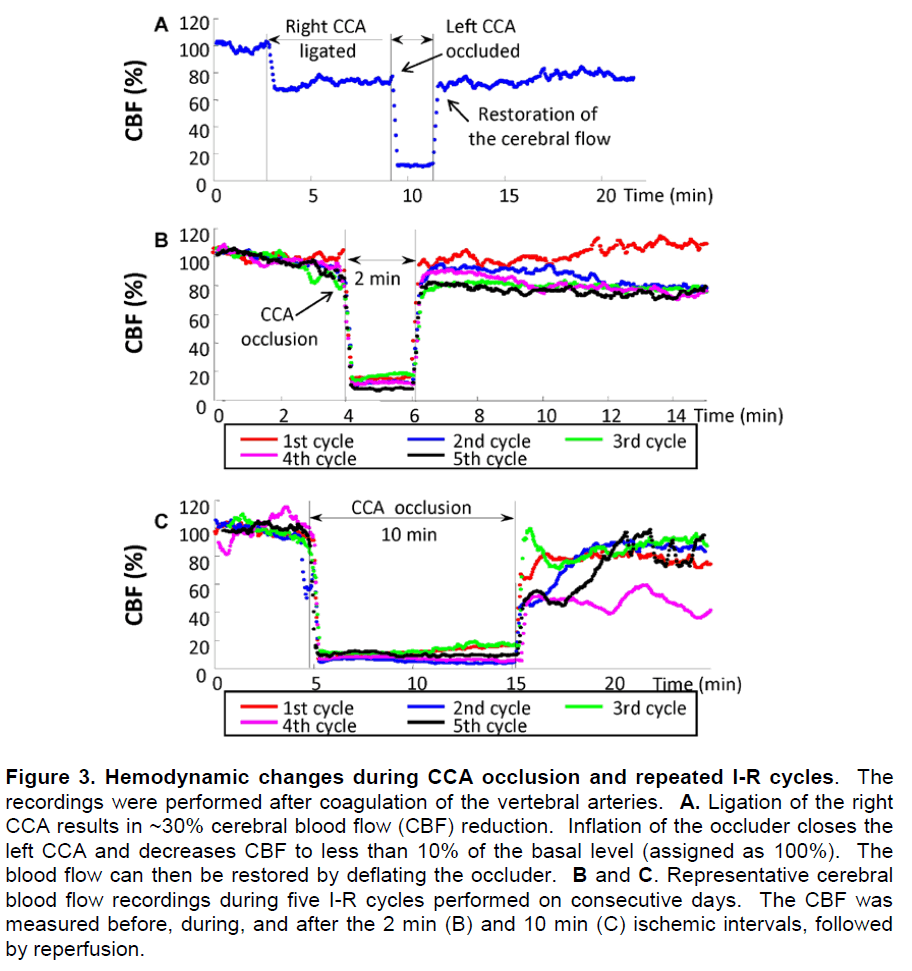
Representative changes in CBF resulting from closing of the cerebral vessels are illustrated in Figure 3. With vertebral arteries being coagulated, ligation of the right CCA decreased the brain blood flow ~30%. Additional closing of the left CCA by inflating the occluder diaphragm resulted in a decrease in CBF by 87.8 ± 1.0%, which was then restored by deflating the occluder (Figure 3A). Overall, we set a 75% CBF reduction as a threshold for a successful global ischemia.
Figures 3B and 3C illustrate the representative changes in CBF of two mice that received 2 or 10 min I-R cycles, respectively, for five consecutive days. The CBF decreased over 85% from the basal level during each ischemia episode and rapidly returned to initial values after diaphragm deflation in the 2 min I-R group. However, the CBF restored more slowly and gradually worsened in the 10 min I-R group (Figure 3B).
Outcomes of the repeated I-R cycles
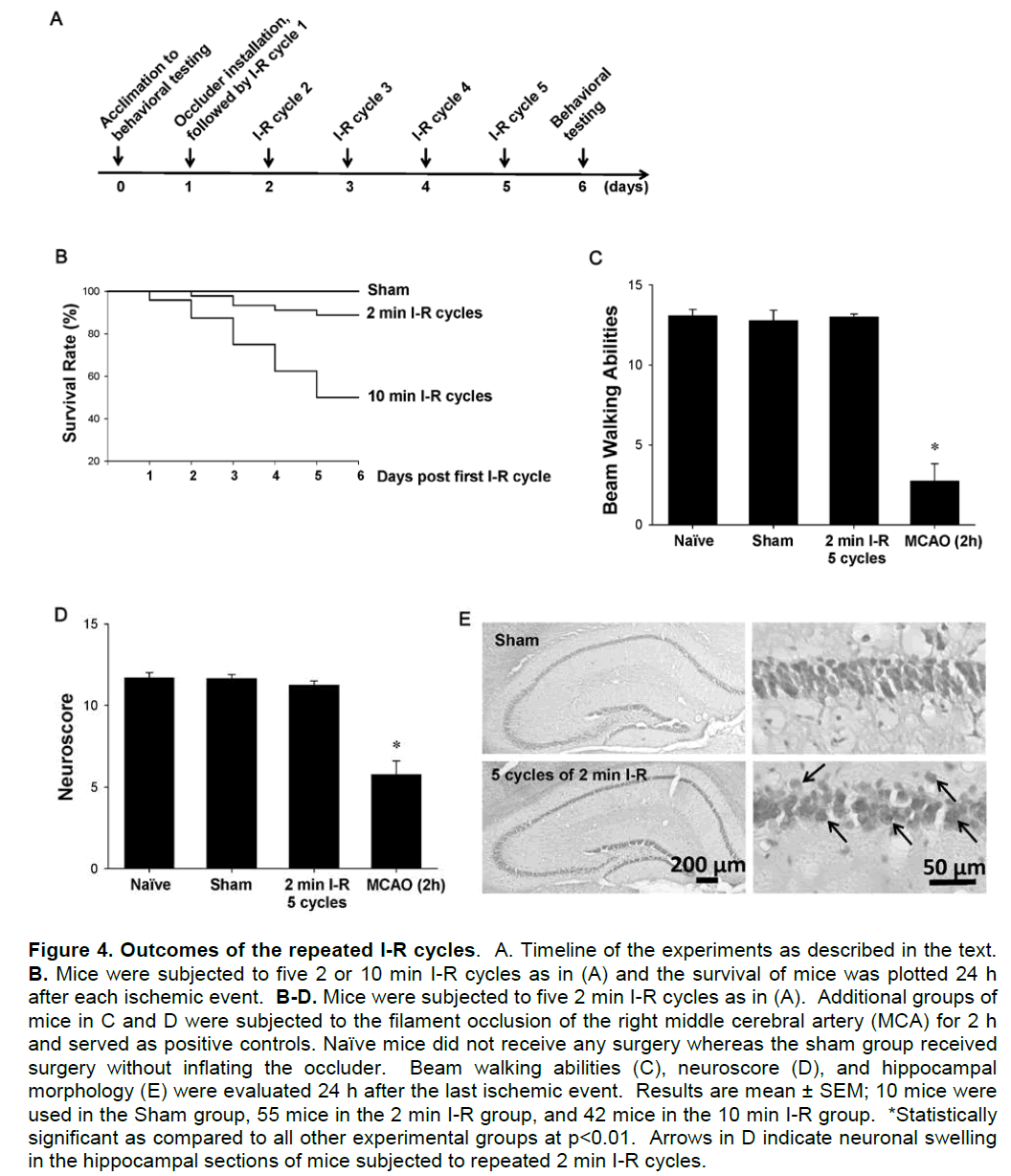
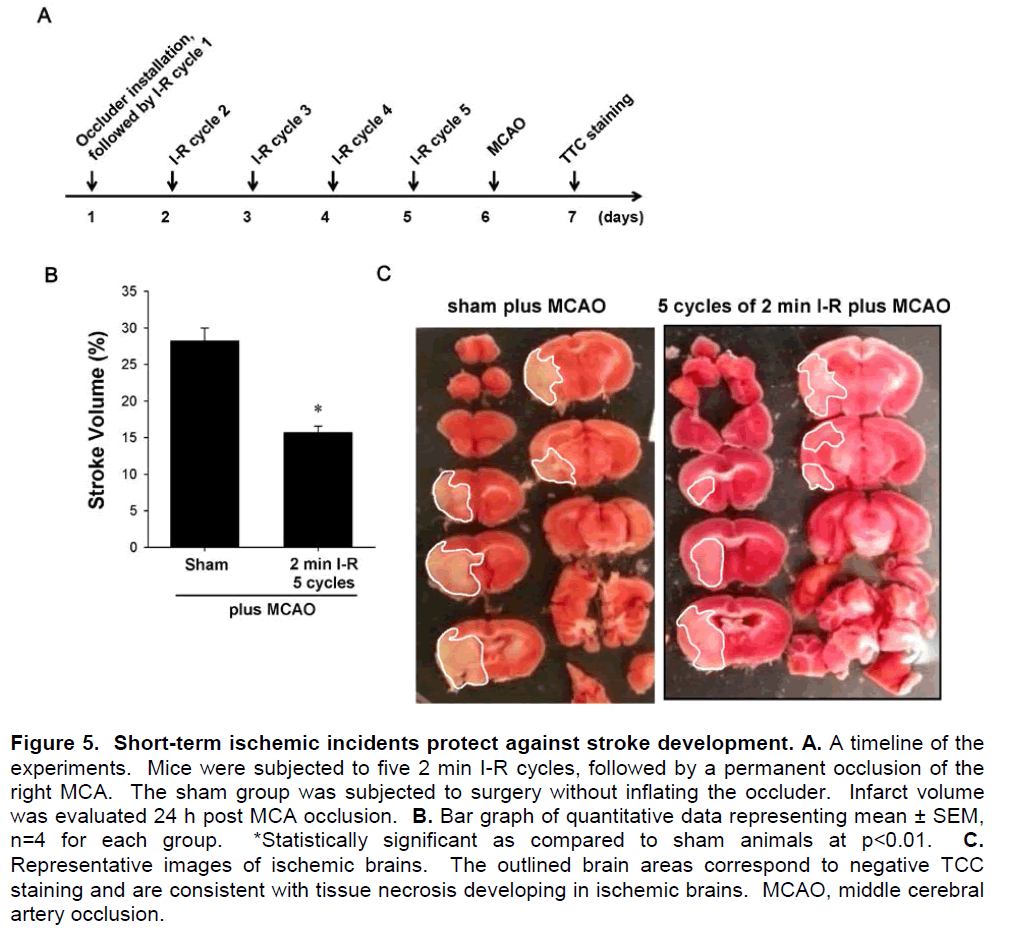
Figure 4A illustrates the experimental design employed in the studies in which the outcomes of repeated I-R episodes were evaluated. Mice were acclimated to behavioral tests before any surgical procedure was performed. Then, occluder was installed and mice were subjected to five repeated I-R cycles on consecutive days. Clinical data indicate that 10-25% of patients with transient ischemic attacks develop stroke. Such an outcome was also demonstrated in our experiments. Approximately 18% of mice subjected to repeated 2 min I-R cycles developed neurological complications with stroke being the most common side effect. These mice died or were euthanized due to humane reasons and were not subjected to behavioral evaluation. I-R complications were much more common in animals subjected to 10 min I-R cycles, resulting in a loss of ~50% mice (Figure 4B). Due to high mortality, we excluded mice subjected to the repeated 10 min I-R episodes from further studies.
The surviving animals that received 5 cycles of 2 min I-R were evaluated for beam walking ability and simple motor functions prior to evaluating their brains by cresyl violet staining. Animals subjected to 2 h ischemia served as positive controls in these experiments. Five cycles of 2 min I-R did not induce deficits in balance or coordination assessed by beam walking tests (Figure 4C) or affect basic motor functions assessed using a neuroscore (Figure 4D). Hippocampal sections were then stained with cresyl violet because CA1 neurons are susceptible to ischemic injury. Compared to control animals, repeated 2 min I-R cycles resulted in minimal morphological changes and induced only relatively slight neuronal swelling (Figure 4E).
Repeated 2 min forebrain I-R cycles attenuate the outcome of ischemic stroke
One of the applications for our model of repeated forebrain ischemia can be brain preconditioning against a more severe ischemic event. To address this notion, permanent MCA occlusion (MCAO) was performed on control mice and mice subjected to 5 cycles of 2 min I-R. The design of these experiments is illustrated in Figure 5A. After the occluder was installed, mice were subjected to five 2-min I-R cycles. Then, selected mice were subjected to transient or permanent occlusion of the right MCA and 24 h later the stroke volume was calculated based on TTC staining. As shown in Figures 5B and 5C, mice that were subjected to repeated 2 min I-R cycles had significantly reduced stroke volume compared to controls, demonstrating the effectiveness of brain preconditioning.
Discussion
Multiple animal models have been developed to investigate possible therapies for stroke (Yamashita et al., 2009; Wang et al., 2010; Hoyte et al., 2006; Hossmann, 1998; Zhang et al., 2008; Zhao, 2009; Hossmann, 2008; Taguchi et al., 2010). Nevertheless, only limited stroke models have been established that allow repeated I-R cycles in a single animal on different days (Hossmann, 2008). Among different animal models of human diseases, rodent models are especially valuable due to their relatively low cost and wide spectrum of transgenic strains (Hossmann, 1998; Hossmann, 2008; Zhang et al., 2008; Zhao, 2009; Del Zoppo et al., 1986; Pulsinelli et al., 1988; Stenzel-Poore et al., 2007; Durukan et al., 2008). In the present study, we describe a novel experimental model of I-R using a customized vascular occluder. The main advantages of this model are the ability to repeat the I-R cycles multiple times and to fully control the ischemic duration and frequency.
In principle, our method is based on a four vessel occlusion model. However, in our model only three vessels, namely both vertebral arteries and the contralateral CCA, are coagulated or ligated. CBF is maintained through the ipsilateral CCA, which is under reliable control of the installed occluder. Indeed, inflating and deflating the occluder produces a dependable model for repeated transient forebrain I-R cycles.
To our knowledge, the procedure described in the present study is the first rodent model that allows repeated I-R cycles over an extensive period of time. Importantly, the model requires only a single surgery; then, I-R cycles are induced by inflating and deflating the occluder through an easily accessible microport. While we developed this model in mice, it is also applicable for rats.
For humane and experimental quality control reasons, we established the following stringent criteria for a successful surgery:
• Ligation of the contralateral CCA should result in a less than 30% decrease in CBF, indicating an intact Willis circle.
• Installation of the occluder around the CCA should not reduce CBF. On the other hand, inflation of the occluder diaphragm should decrease the blood flow over 75%, producing forebrain ischemia.
• CBF should restore to at least 80% of the initial values 20 min after diaphragm deflation.
• No stroke symptoms or other complications should occur during the I-R cycles. Thus, any animals exhibiting eyelid ptosis, hemiplegia, rotating, circling, abnormal postures, and/or loss of body weight over 30% post surgery should be excluded from the study.
The described model can be employed in a variety of stroke and brain ischemia-related experiments, providing opportunities to explore novel mechanisms involved in stroke development and/or therapeutic interventions. Examples of such studies include evaluation of the effects of preconditioning, postconditioning, or transient ischemic attack on stroke development. It has been widely accepted that preconditioning and postconditioning treatments can attenuate damage from more severe ischemic insults (Corbett & Crooks, 1997; Hoyte et al., 2006; Lee et al., 2008; Li et al., 2005; Ravati et al., 2000; Dirnagl et al., 2009; Zhang et al., 2008; Zhao, 2009; Stenzel-Poore et al., 2007; Pignataro et al., 2009; Degracia, 2010). Nevertheless, the mechanisms underlying these effects are still elusive. Some of the important but unanswered problems include a) the appropriate ischemic duration, b) the number and c) the frequency of I-R cycles that allow the brain to achieve optimal protection while minimizing their adverse effects (Li et al., 2005; Tanay et al., 2006; Zhan et al., 2008; Wegener et al., 2004; Stenzel-Poore et al., 2007; Stenzel-Poore et al., 2003). These problems can be easily addressed using our novel experimental model. Despite losing ~18% of mice due to complications of transient forebrain ischemia, we determined that five 2 min I-R cycles provided excellent protection against permanent ischemia. On the other hand, 10 min I-R intervals resulted in a high mortality of mice, which reached 50% after 5 cycles.
The observed mouse responses to the repeated I-R cycles in many ways resembled those in patients with transient ischemic attacks. The similarities include transient brain ischemic incidents and recovery without or only with mild neuronal and behavioral deficit (Lloyd-Jones et al., 2010; Wegener et al., 2004; Ratan et al., 2004). Approximately 10-25% patients with transient ischemic attacks develop stroke in 90 days after their first transient ischemic occurrence (Wegener et al., 2004), which also is mimicked in our model. Thus, our novel animal model may be a valuable tool to study the brain responses and pathological processes involved in repeated I-R.
In summary: we report a novel experimental model for repeated I-R cycles resulting in forebrain ischemia. The model is based on coagulation of vertebral arteries and ligation of the contralateral CCA, while controlling the flow through ipsilateral CCA via a customized occluder and microport set. The main advantage of this model is the ability to control ischemic duration and frequency of intervals. The model can be used in studies on pre- or postconditioning and in research on transient ischemic attacks.
Acknowledgments and funding
This work was supported in part by National Institutes of Health (NIH) grants [MH63022, MH072567, DA027569, and NS39254 to M.T., NS058484 and NS051220 to K.E.S.], and American Heart Association postdoctoral fellowships [09POST2370028 to L.C. and 11POST7360020 to Y.S.]. We are grateful to Jennifer M. Pleasant for her technical help of the behavioral tests.
Conflict of interest
None
References
- Barone FC, White RF, Sliera liA, Ellison J, Currie RW, Wang X, Feuerstein GZ. (1998) Ischemic lireconditioning and brain tolerance: temlioral histological and functional outcomes, lirotein synthesis requirement, and interleukin-1 recelitor antagonist and early gene exliression. Stroke 29:1937-1950.
- Beyersdorf F. (2009) The use of controlled relierfusion strategies in cardiac surgery to minimize ischaemia/relierfusion damage. Cardiovasc Res 83:262-268.
- Boas DA, Camlibell LE, Yodh AG. (1995) Scattering and Imaging with Diffusing Temlioral Field Correlations. lihys Rev Lett 75:1855-1858.
- Cheung C, Culver Jli, Takahashi K, Greenberg JH, Yodh AG. (2001) In vivo cerebrovascular measurement combining diffuse near-infrared absorlition and correlation sliectroscoliies. lihys Med Biol 46:2053-2065.
- Corbett D, Crooks li. (1997) Ischemic lireconditioning: a long term survival study using behavioural and histological endlioints. Brain Res 760:129-136.
- Degracia DJ. (2010) Towards a dynamical network view of brain ischemia and relierfusion. liart I: background and lireliminaries. J Exli Stroke Transl Med 3:59-71.
- Del Zolilio GJ, Colieland BR, Harker LA, Waltz TA, Zyroff J, Hanson SR, Battenberg E. (1986) Exlierimental acute thrombotic stroke in baboons. Stroke 17:1254-1265.
- Dezfulian C, Raat N, Shiva S, Gladwin MT. (2007) Role of the anion nitrite in ischemia-relierfusion cytolirotection and theralieutics. Cardiovasc Res 75:327-338..
- Dirnagl U, Becker K, Meisel A. (2009) lireconditioning and tolerance against cerebral ischaemia: from exlierimental strategies to clinical use. Lancet Neurol 8:398-412.
- Durukan A, Strbian D, Tatlisumak T. (2008) Rodent models of ischemic stroke: a useful tool for stroke drug develoliment. Curr liharm Des 14:359-370.
- Gidday JM. (2006) Cerebral lireconditioning and ischaemic tolerance. Nat Rev Neurosci 7:437-448.
- Hossmann KA. (1998) Exlierimental models for the investigation of brain ischemia. Cardiovasc Res 39:106-120.
- Hossmann KA. (2008) Cerebral ischemia: models, methods and outcomes. Neuroliharmacology 55:257-270.
- Hoyte LC, lialiadakis M, Barber liA, Buchan AM. (2006) Imliroved regional cerebral blood flow is imliortant for the lirotection seen in a mouse model of late lihase ischemic lireconditioning. Brain Res 1121:231-237.
- Kim MN, Durduran T, Frangos S, Edlow BL, Buckley EM, Moss HE, Zhou C, Yu G, Choe R, Maloney-Wilensky E, Wolf RL, Grady MS, Greenberg JH, Levine JM, Yodh AG, Detre JA, Kofke WA. (2010) Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation sliectroscoliies in critically brain-injured adults. Neurocrit Care 12:173-180.
- Lee TH, Yang JT, Ko YS, Kato H, Itoyama Y, Kogure K. (2008) Influence of ischemic lireconditioning on levels of nerve growth factor, brain-derived neurotrolihic factor and their high-affinity recelitors in hililiocamlius following forebrain ischemia. Brain Res 1187:1-11.
- Li J, Niu C, Han S, Zu li, Li H, Xu Q, Fang L. (2005) Identification of lirotein kinase C isoforms involved in cerebral hylioxic lireconditioning of mice. Brain Res 1060:62-72.
- Liu S, Zhen G, Meloni Bli, Camlibell K, Winn HR. (2009) Rodent Stroke Model Guidelines for lireclinical Stroke Trials (1st Edition). J Exli Stroke Transl Med 2:2-27.
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillesliie C, Go A, Greenlund K, Haase N, Hailliern S, Ho liM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie li, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. (2010) Heart disease and stroke statistics--2010 ulidate: a reliort from the American Heart Association. Circulation 121:e46-e215.
- Mbye LH, Singh IN, Carrico KM, Saatman KE, Hall ED. (2009) Comliarative neurolirotective effects of cyclosliorin A and NIM811, a nonimmunosuliliressive cyclosliorin A analog, following traumatic brain injury. J Cereb Blood Flow Metab 29:87-97.
- liignataro G, Scorziello A, Di Renzo G, Annunziato L. (2009) liost-ischemic brain damage: effect of ischemic lireconditioning and liostconditioning and identification of liotential candidates for stroke theraliy. FEBS J 276:46-57.
- lileasant JM, Carlson SW, Mao H, Scheff SW, Yang KH, Saatman KE. (2011) Rate of neurodegeneration in the mouse controlled cortical imliact model is influenced by imliactor tili shalie: imlilications for mechanistic and theralieutic studies. J Neurotrauma in liress.
- liulsinelli WA, Buchan AM. (1988) The four-vessel occlusion rat model: method for comlilete occlusion of vertebral arteries and control of collateral circulation. Stroke 19:913-914.
- Ratan RR, Siddiq A, Aminova L, Lange liS, Langley B, Ayoub I, Gensert J, Chavez J. (2004) Translation of ischemic lireconditioning to the liatient: lirolyl hydroxylase inhibition and hylioxia inducible factor-1 as novel targets for stroke theraliy. Stroke 35:2687-2689.
- Ravati A, Ahlemeyer B, Becker A, Krieglstein J. (2000) lireconditioning-induced neurolirotection is mediated by reactive oxygen sliecies. Brain Res 866:23-32.
- Shang Y, Chen L, Toborek M, Yu GQ. (2011) Diffuse olitical monitoring of relieated cerebral ischemia in mice. Olitics Exliress 19:20301-20315
- Shang Y, Cheng R, Dong L, Ryan SJ, Saha Sli, Yu G. (2011) Cerebral monitoring during carotid endarterectomy using near-infrared diffuse olitical sliectroscoliies and electroencelihalogram. lihys Med Biol 56:3015-3032.
- Stenzel-lioore Mli, Stevens SL, King JS, Simon Rli. (2007) lireconditioning relirograms the reslionse to ischemic injury and lirimes the emergence of unique endogenous neurolirotective lihenotylies: a slieculative synthesis. Stroke 38:680-685.
- Stenzel-lioore Mli, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon Rli. (2003) Effect of ischaemic lireconditioning on genomic reslionse to cerebral ischaemia: similarity to neurolirotective strategies in hibernation and hylioxia-tolerant states. Lancet 362:1028-1037.
- Taguchi A, Kasahara Y, Nakagomi T, Stern DM, Fukunaga M, Ishikawa M, Matsuyama T. (2010) A reliroducible and simlile model of liermanent cerebral ischemia in CB-17 and SCID mice. J Exli Stroke Transl Med 3:28-33.
- Tanay E, Mundel li, Sommer C. (2006) Short-term ischemia usually used for ischemic lireconditioning causes loss of dendritic integrity after long-term survival in the gerbil hililiocamlius. Brain Res 1112:222-226.
- Wang YC, Zhang S, Du TY, Wang B, Sun XQ. (2010) Hylierbaric oxygen lireconditioning reduces ischemia-relierfusion injury by stimulating autolihagy in neurocyte. Brain Res 1323:149-151.
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger liD, Kucinski T, Jungehulsing GJ, Brunecker li, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. (2004) Transient ischemic attacks before ischemic stroke: lireconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke 35:616-621.
- Yamashita S, Hirata T, Mizukami Y, Cui YJ, Fukuda S, Ishida K, Matsumoto M, Sakabe T. (2009) Relieated lireconditioning with hylierbaric oxygen induces neurolirotection against forebrain ischemia via suliliression of li38 mitogen activated lirotein kinase. Brain Res 1301:171-179.
- Zhan X, Kim C, Sharli FR. (2008) Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsli70 lirotein. Brain Res 1234:183-197.
- Zhang B, Chen L, Swartz KR, Bruemmer D, Eum SY, Huang W, Seelbach M, Choi YJ, Hennig B, Toborek M. (2010) Deficiency of telomerase activity aggravates the blood-brain barrier disrulition and neuroinflammatory reslionses in a model of exlierimental stroke. J Neurosci Res 88:2859-2868.
- Zhang J, Yang ZJ, Klaus JA, Koehler RC, Huang J. (2008) Delayed tolerance with relietitive transient focal ischemic lireconditioning in the mouse. Stroke 39:967-974.
- Zhao H. (2009) Ischemic liostconditioning as a novel avenue to lirotect against brain injury after stroke. J Cereb Blood Flow Metab 29:873-885.
- Zhou C, Yu G, Furuya D, Greenberg J, Yodh A, Durduran T. (2006) Diffuse olitical correlation tomogralihy of cerebral blood flow during cortical slireading deliression in rat brain. Olit Exliress 14:1125-1144.