Research Article - Journal of Experimental Stroke & Translational Medicine (2015) Volume 8, Issue 1
Neuroprotective effects of the ergoline derivative nicergoline following transient and permanent focal cerebral ischemia in rats
- *Corresponding Author:
- Dr. Chikako Nito
Department of Neurological Science
Graduate School of Medicine, Nippon Medical School
1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8603, Japan
Tel: +81-3-3822-2131
Fax: +81-3-3822-4865
E-mail: cnito@nms.ac.jp
Published Date: December 15, 2015
Citation: Nito C, Nishiyama Y, Saito T, Suda S, Ueda M, Kimura K. Neuroprotective effects of the ergoline derivative nicergoline following transient and permanent focal cerebral ischemia in rats: Neuroprotective effects of nicergoline. J Exp Stroke Transl Med 2015 Dec, Volume 8.1-8. Online access at www.jestm.com
Abstract
Nicergoline has been widely used for various conditions, including cerebrovascular disorders and senile mental impairment. However, the precise mechanisms underlying this neuroprotection remain unknown. We investigated the neuroprotective properties of nicergoline on outcome after focal ischemia in rats. Sprague–Dawley rats were treated with nicergoline or vehicle through a gavage feeding needle for 7 days before the induction of ischemia. For ischemia, rats were subjected to transient middle cerebral artery occlusion (tMCAO) or permanent middle cerebral artery occlusion (pMCAO). Magnetic resonance imaging was used to obtain cerebral blood flow (CBF) measurements at 24 hours after tMCAO and pMCAO. Nicergoline significantly diminished infarct area and edema volume compared with the vehicle-treated group at 24 hours after tMCAO, but there was no significant reduction in behavioral dysfunction. Twenty-four hours after pMCAO, the nicergoline-treated group showed significantly reduced infarcts, edema, and neurological scores compared with the vehicle-treated group. Decreased areas of CBF were markedly smaller in the nicergoline-treated group compared with the vehicle-treated group 24 hours after occlusion in both models. This study shows that pretreatment with nicergoline prevents brain damage, and increased CBF levels may be involved in the neuroprotective mechanism of nicergoline after acute stroke.
Keywords
ischemic stroke, nicergoline, neuroprotection, rats
Introduction
Ischemic brain injury often causes irreversible brain damage and leads to severe disability. Development of a neuroprotective therapy for acute cerebral ischemia to salvage ischemic penumbra is crucial and is becoming essential with the increasing use of acute recanalization therapies, such as thrombolysis (Hacke et al. 2008) and mechanical removal of clots (Smith et al. 2008). Although various neuroprotective compounds have been developed for ischemic stroke, satisfactory drug treatments are lacking in clinical practice.
Nicergoline (8-beta-[bromonicetinoylhydroxymethyl]-1,6-dimethyl-10alpha-metoxyergoline) is an ergoline derivative, and improves damaged mitochondrial function, and increases cholinergic transmission (Carfagna et al. 1995) and phosphoinositol turnover (Carfagna et al. 1996). Nicergoline also inhibits platelet aggregation induced by arachidonic acid (Lagarde et al. 1980), platelet-activating factor, collagen, and epinephrine (Prage et al. 1979) in in vitro studies. In clinical use, nicergoline has been widely used for over 3 decades for treatment of various conditions, including cerebrovascular disorders and senile dementia (Winblad et al. 2008). In experimental studies, nicergoline treatment improves cerebral metabolism, regional cerebral blood flow, and oxygen perfusion in the post-ischemic (Le Poncin-Lafitte et al. 1984) and post-hypoxic brain (Shintomi 1991). However, the precise mechanisms underlying this neuroprotection remain unknown and the role of nicergoline in brain ischemia in in vivo experiments has been relatively unexplored. Moreover, there is no reports that show the correlation of nicergoline and ischemic injury using transient focal cerebral ischemia in rats.
This study was designed to characterize the effects of different nicergoline doses on the outcome after transient (tMCAO) and permanent middle cerebral artery occlusion (pMCAO) in rats.
Materials and Methods
All experimental protocols were approved by our Institutional Committee on Animal Research. These protocols were carried out in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Adult male Sprague–Dawley rats (250-300 g) that were purchased from Sankyo Labo Service (Tokyo, Japan) were used in the present study. Rats were fasted overnight before surgery with free access to tap water. Anesthesia was initially induced with 5% halothane, and then was maintained with 1% halothane in a mixture of 70% N2O and 30% O2 under spontaneous breathing. A polyethylene PE-50 catheter was inserted into the tail artery to monitor mean arterial blood pressure and perform blood sampling. The right femoral vein was also cannulated using a PE-50 catheter for administration of drugs. Blood gases and blood glucose levels were measured just before and during ischemia. Body temperature was maintained at 37°C, using a heating pad, during the surgical procedure for middle cerebral artery occlusion (MCAO). Blood samples were collected from the tail artery before and during MCAO for measurement of pH, PO2, and PCO2 by using blood gas analysis apparatus.
tM CAO
In experiment 1, rats were subjected to 90 minutes of focal cerebral ischemia. This was followed by 24 hours of reperfusion using an intraluminal suture technique with modification, as described in detail previously (Arii T et al. 2001, Nito C et al. 2004). Briefly, the left common, internal, and external carotid arteries were carefully exposed through a midline cervical incision, and the common and external carotid arteries were doubly ligated using 4-0 silk sutures. The left middle cerebral artery (MCA) was occluded by inserting a silicone rubber-coated 4-0 nylon thread through the left internal carotid artery for 90 minutes, and reperfusion was achieved by withdrawing the thread. Sham control animals were treated in the same way without MCAO.
pM CAO
In experiment 2, another set of rats were subjected to pMCAO using a modified Tamura model of stroke (Tamura et al. 1981). Briefly, a burr hole was drilled through the temporal skull after making a 1-cm skin incision between the left eye and ear. The dura mater was removed and the MCA was occluded permanently using bipolar electrocoagulation forceps. Sham-operated animals were subjected to the same procedure, except for coagulation of the MCA.
Drug preparation
Rats were randomly divided into the nicergoline-treated group and the vehicle-treated (control) group (n=6 in each group). Nicergoline is used as an orally administered agent in humans. Therefore, oral administration of nicergoline was chosen in our study. In the tMCAO experiment, rats were administered orally with 3, 10, or 100 mg/kg/d of nicergoline by gavage for 7 days before the induction of ischemia. In the pMCAO experiment, rats were orally administered with vehicle or 10 mg/kg/d of nicergoline by gavage for 7 days before induction of ischemia, and then subjected to focal cerebral ischemia.
Evaluation of cerebral blood flow
To confirm MCA occlusion and a reduction in cerebral blood flow (CBF), magnetic resonance imaging (MRI) was performed using a 7T/18-cm horizontal magnet (Magnex Scientific, Abingdon, UK) with a Varian Unity-INOVA-300 (Varian Inc., Palo Alto, CA) system that was equipped with an actively shielded gradient. A 6-cm internal diameter quadrature birdcage coil was used in the study. Cerebral perfusion images at the level of the bregma were obtained after ischemic induction using a continuous arterial spin labeling method (Williams et al. 1992), which was modified with a centrally encoded variable-tip-angle gradient echo technique (Ewing et al. 2003). Inflowing protons of the arterial blood were labeled by adiabatic inversion with an axial gradient of ± 4.5 kHz/mm and a continuous radiofrequency transmission of approximately 0.6 kHz at a frequency offset of ± 9 kHz. This placed the inversion plane 20 mm below the imaging plane. Gradient echo parameters were as follows: repetition time of 5 ms; echo time of 3 ms; field of view of 50 × 50 mm; slice thickness of 2 mm; and matrix size of 128 × 64. Forty-eight pairs of images were produced with and without inversion of inflowing protons, and were summed to improve the signal-to-noise ratio. Image manipulation was carried out using MR vision software (MR Vision Co., Menlo Park, CA) on a Blade1000 workstation (Sun Microsystems, Milpitas, CA).
In the tMCAO experiment, cerebral perfusion images were obtained at 90 minutes after ischemic induction and 24 hours after reperfusion. In the pMCAO experiment, rats were scanned to assess CBF at 24 hours after ischemia.
Analysis of infarct area and edema volume
The rats were anesthetized and decapitated 24 hours after ischemia. Two-mm-thick coronal sections were cut and stained by immersion in 2% 2,3,5-triphenyltetrazolium chloride (TTC) at 37C and fixed in 10% buffered formalin overnight (n=6 in each group).
The area of the ischemic lesion in each section was measured and multiplied by the interval thickness using an image analysis system (NIH image software). Edema volume was determined by subtracting the non-ischemic volume from the ischemic volume (Maier et al. 1998).
Examination of neurological symptoms
Neurological symptoms in several experimental rats were blindly examined at 24 hours after ischemia using a scoring system based on the detection of hemiplegia and abnormal posture (n=6 in each group) (Yonemori et al. 1998). The right hind limb of each rat was gently extended with round-tipped forceps and the flexor response was scored as follows: 0, normal; 1, slight deficit; 2, moderate deficit; and 3, severe deficit. To assess posture, the rats were suspended by the tail, and forelimb flexion and body twisting were scored as follows: 0, normal; 1, slight twisting; 2, marked twisting; and 3, marked twisting and forelimb flexion.
Statistical analysis
The data are expressed as mean ± standard deviation. The unpaired t-test or one-way analysis of variance followed by Fisher’s protected least significant difference post hoc test were used for comparison of the physiological results, infarct volume, edema volume, and decreased CBF areas. The Mann–Whitney U test was used to compare neurological scores. A p value < 0.05 was considered statistically significant.
Results
Physiological variables
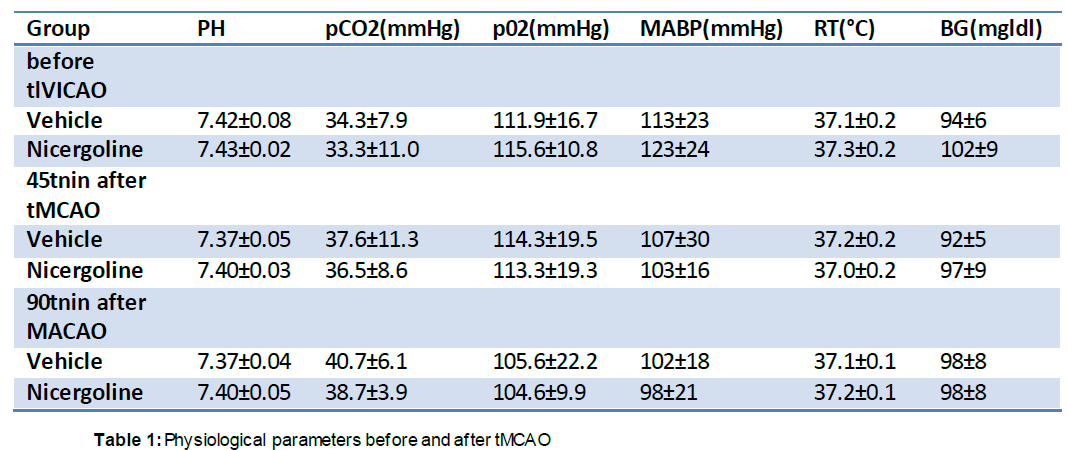
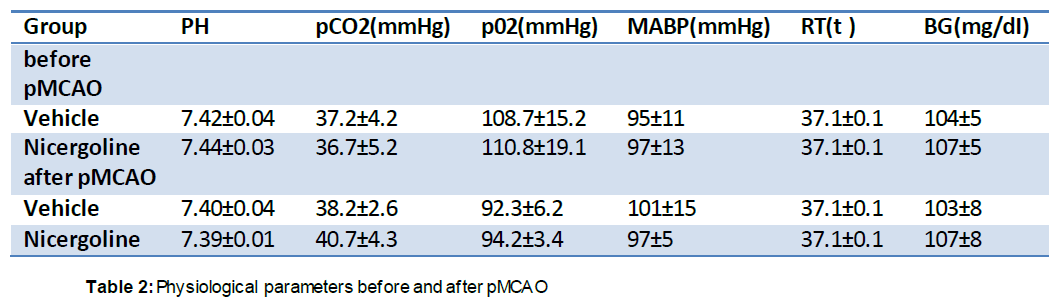
There were no significant differences in physiological parameters, including rectal temperature, mean arterial blood pressure, pH, pCO2, and pO2, between the vehicle- and nicergoline-treated groups before or during MCAO (45 minutes after MCAO), or just before reperfusion (90 minutes after MCAO) (Table 1). There were also no differences in these parameters between pre-pMCAO and post-pMCAO (Table 2).
Infarct area and edema volume
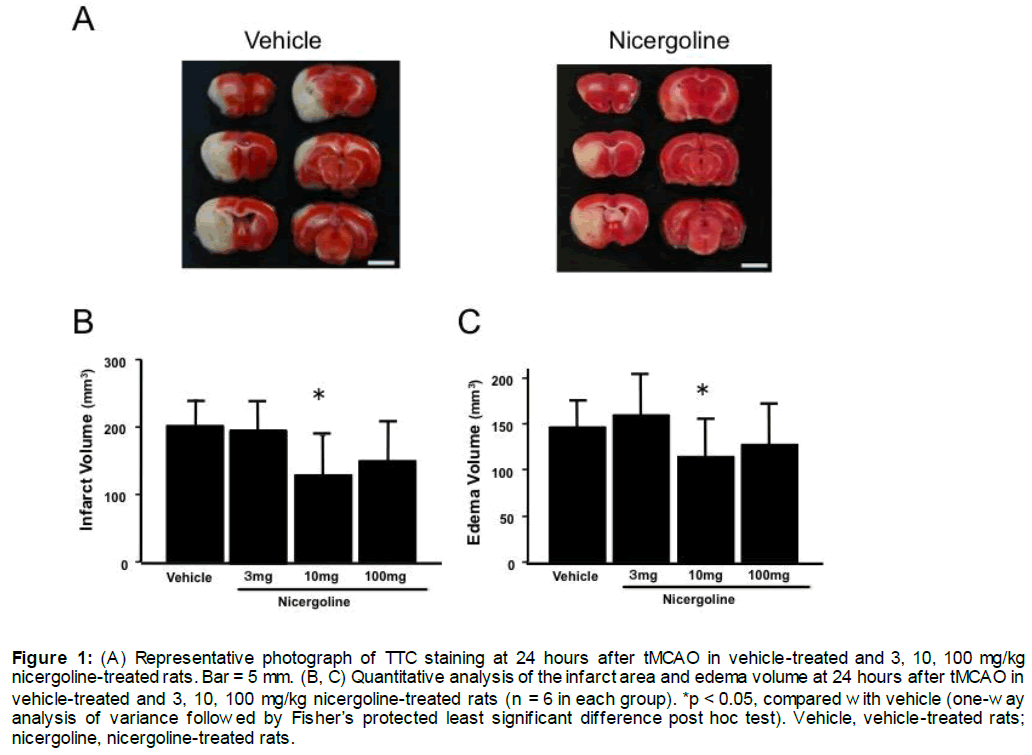
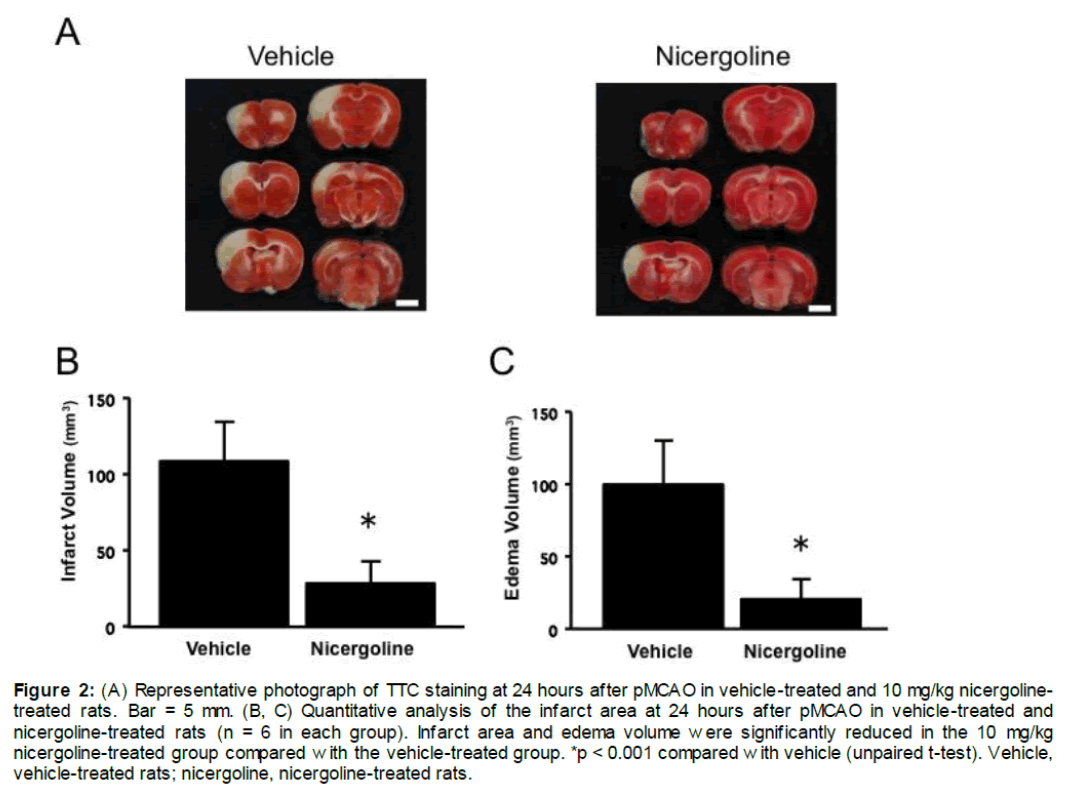
Representative photographs of brain sections stained by TTC from experiment 1 are shown in Figure 1A. The total infarct volume was significantly smaller with the dose of 10 mg/kg in the nicergoline-treated group (130.2 ± 58.2 mm3, p < 0.05 vs vehicle-treated group), but was not different at the other dose of 3 or 100 mg/kg nicergoline compared with the vehicle-treated group (199.1 ± 35.1 mm3, Figure 1B). The infarct volume in the 10 mg/kg nicergoline-treated group was reduced by 34.6% compared with the control group at 24 hours after reperfusion (Figure 1B). At 24 hours after reperfusion, edema volume was also significantly smaller in the 10 mg/kg nicergoline-treated group (116.1 ± 40.8 mm3, p < 0.05 vs vehicle-treated group), but was unchanged in the 3 or 100 mg/kg nicergoline-treated group compared with the vehicle-treated group (155.7 ± 18.2 mm3, Figure 1C). Representative photographs of brain sections stained by TTC from experiment 2 are shown in Figure 2A. The total infarct area in the 10 mg/kg nicergoline-treated group (28.4 ± 14.1 mm3) was significantly smaller than that in the control group (108.1 ± 27.0 mm3), with a 73.7% reduction at 24 hours after pMCAO (p < 0.001 vs vehicle-treated group, Figure 2B). At 24 hours after ischemia, edema volume were also significantly smaller in the the 10 mg/kg nicergoline-treated group (20.8 ± 13.0 mm3) compared with the vehicle-treated group (99.6 ± 31.0 mm3, p < 0.001 vs vehicle-treated group, Figure 2C).
Figure 1: (A) Representative photograph of TTC staining at 24 hours after tMCAO in vehicle-treated and 3, 10, 100 mg/kg nicergoline-treated rats. Bar = 5 mm. (B, C) Quantitative analysis of the infarct area and edema volume at 24 hours after tMCAO in vehicle-treated and 3, 10, 100 mg/kg nicergoline-treated rats (n = 6 in each group). *p < 0.05, compared with vehicle (one-way analysis of variance followed by Fisher’s protected least significant difference post hoc test). Vehicle, vehicle-treated rats; nicergoline, nicergoline-treated rats.
Figure 2: (A) Representative photograph of TTC staining at 24 hours after pMCAO in vehicle-treated and 10 mg/kg nicergoline-treated rats. Bar = 5 mm. (B, C) Quantitative analysis of the infarct area at 24 hours after pMCAO in vehicle-treated and nicergoline-treated rats (n = 6 in each group). Infarct area and edema volume were significantly reduced in the 10 mg/kg nicergoline-treated group compared with the vehicle-treated group. *p < 0.001 compared with vehicle (unpaired t-test). Vehicle, vehicle-treated rats; nicergoline, nicergoline-treated rats.
Neurological deficits
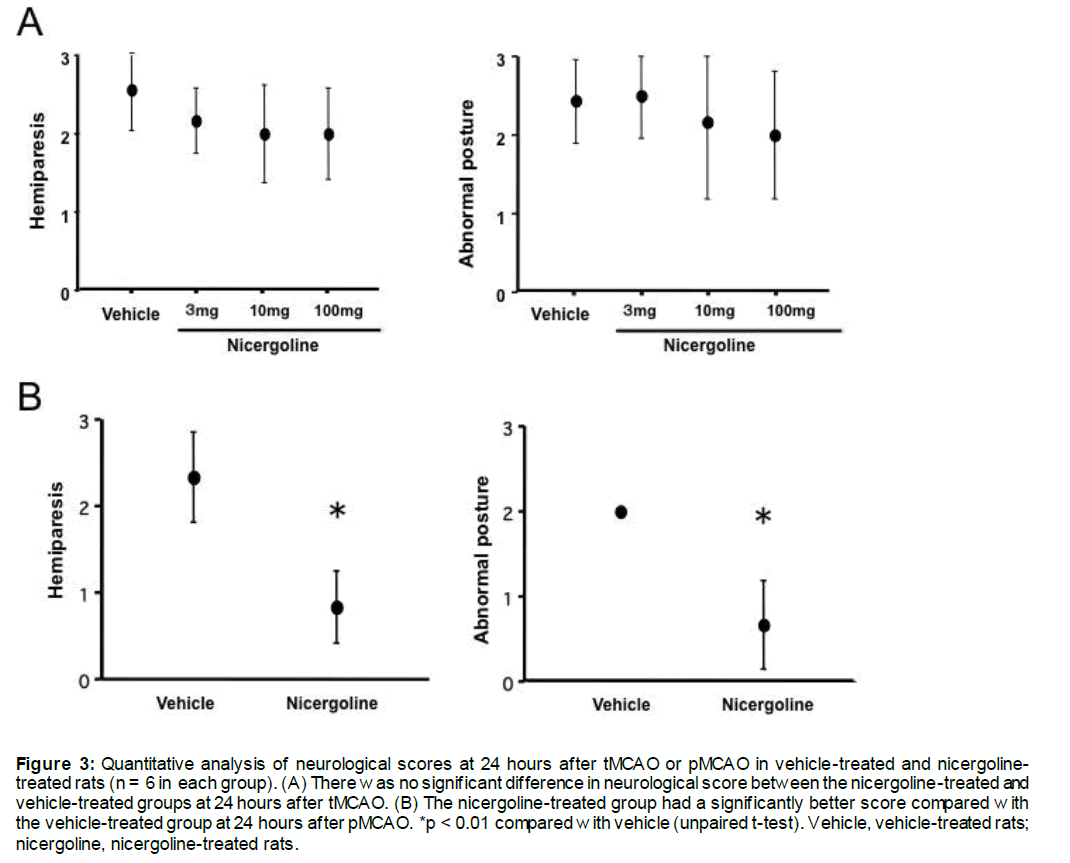
The neurological scores at 24 hours after tMCAO are shown in Figure 3A. In the vehicle-treated group, the mean hemiplegia score and abnormal posture score were 2.0 ± 0.6 and 2.0 ± 0.8, respectively. Treatment with any dose of nicergoline did not improve the hemiplegia and abnormal posture scores.
The neurological scores at 24 hours after pMCAO are shown in Figure 3B. In the vehicle-treated group, the mean hemiplegia score and abnormal posture score were 2.4 ± 0.5 and 2.0 ± 0.0, respectively. Treatment with nicergoline resulted in a significantly better abnormal posture score (0.8 ± 0.2) and abnormal posture score (0.6 ± 0.4) compared with the vehicle-treated group (both p < 0.01, Figure 3B).
Figure 3: Quantitative analysis of neurological scores at 24 hours after tMCAO or pMCAO in vehicle-treated and nicergoline-treated rats (n = 6 in each group). (A) There was no significant difference in neurological score between the nicergoline-treated and vehicle-treated groups at 24 hours after tMCAO. (B) The nicergoline-treated group had a significantly better score compared with the vehicle-treated group at 24 hours after pMCAO. *p < 0.01 compared with vehicle (unpaired t-test). Vehicle, vehicle-treated rats; nicergoline, nicergoline-treated rats.
CBF as shown by MRI
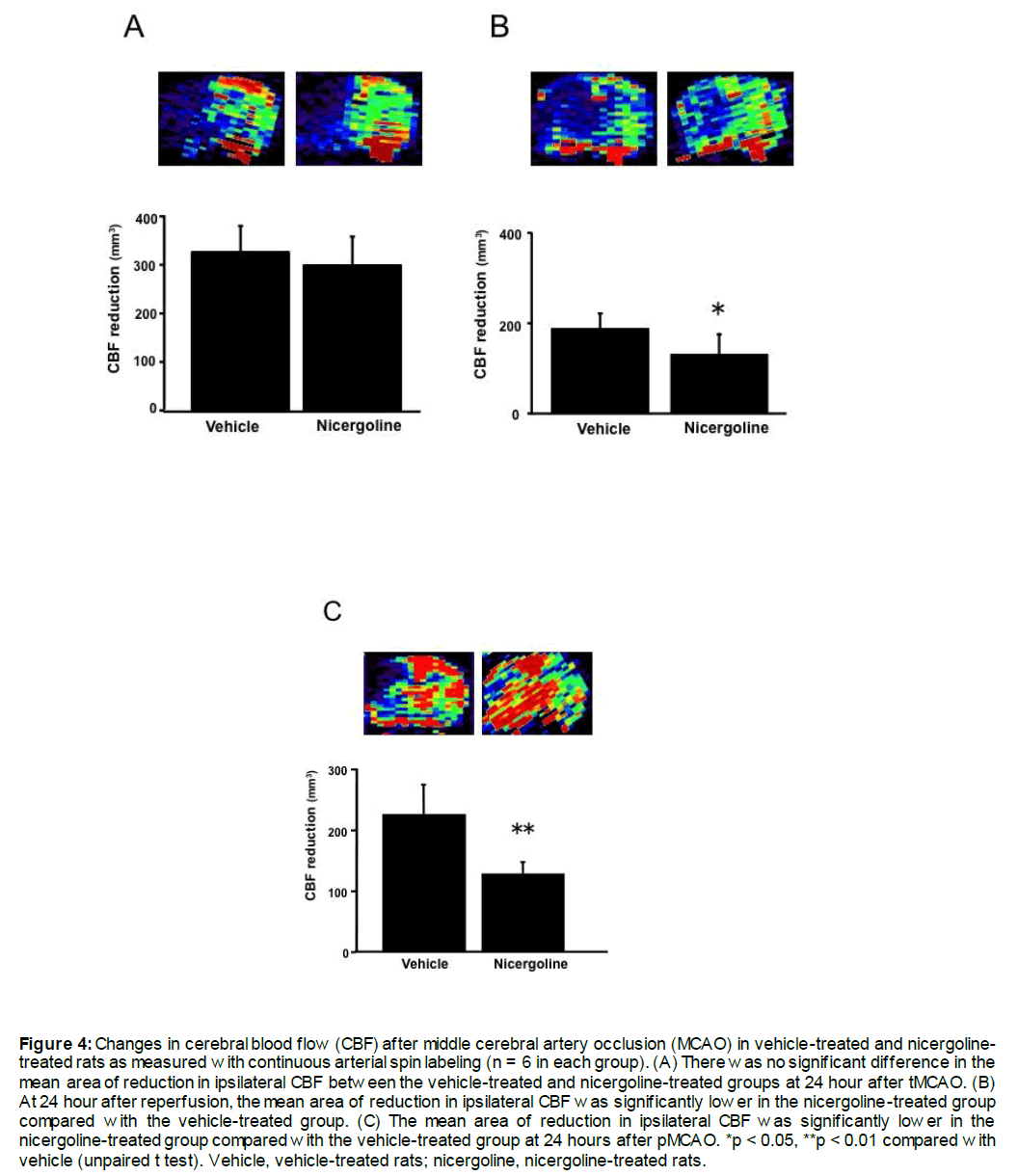
MCA occlusion resulted in a reduction CBF in the territory supplied by the ipsilateral MCA. Figure 4 shows representative images of CBF in rats that were subjected to focal cerebral ischemia. Based on ipsilateral CBF, which was less than 60% of the mean value of the contralateral hemisphere, the areas of decreased CBF were calculated. In experiment 1, there was no significant difference in the mean area of reduction in ipsilateral CBF between the vehicle-treated and the 10 mg/kg nicergoline-treated groups (326.7 ± 54.4 mm2 vs. 299.9± 58.2 mm2, Figure 4A) at 90 minutes after ischemic induction. At 24 hours after reperfusion, the mean area of reduction in ipsilateral CBF was significantly lower in the nicergoline-treated group compared with the vehicle-treated group (81% reduction, 141.5 ± 16.0 mm3 vs 174.2 ± 10.3 mm3, p < 0.05, Figure 4B). In experiment 2, the mean area of reduction in ipsilateral CBF was significantly lower in the nicergoline-treated group compared with the vehicle-treated group (58% reduction, 128.6 ± 19.5 mm3 vs 218.8 ± 56.2 mm3, p < 0.01, Figure 4C) at 24 hours after pMCAO.
Figure 4: Changes in cerebral blood flow (CBF) after middle cerebral artery occlusion (MCAO) in vehicle-treated and nicergoline-treated rats as measured with continuous arterial spin labeling (n = 6 in each group). (A) There was no significant difference in the mean area of reduction in ipsilateral CBF between the vehicle-treated and nicergoline-treated groups at 24 hour after tMCAO. (B) At 24 hour after reperfusion, the mean area of reduction in ipsilateral CBF was significantly lower in the nicergoline-treated group compared with the vehicle-treated group. (C) The mean area of reduction in ipsilateral CBF was significantly lower in the nicergoline-treated group compared with the vehicle-treated group at 24 hours after pMCAO. *p < 0.05, **p < 0.01 compared with vehicle (unpaired t test). Vehicle, vehicle-treated rats; nicergoline, nicergoline-treated rats.
Discussion
In this study, we showed that treatment with nicergoline led to neuroprotection after MCAO as shown by reduced infarct area and edema volume, as well as improved functional outcome without changing basic physiology. Treatment with 3 mg/kg or 100 mg/kg nicergoline failed to have a significant effect on ischemic damage after tMCAO. Protection 24 hours after tMCAO due to pre-treatment nicergoline was most effective with the middle dose of 10 mg/kg, but there was no significant reduction in behavioral dysfunction compared with the vehicle-treated group. CBF at 24 hours after tMCAO recovered significantly better with 10 mg/kg nicergoline treatment compared with the vehicle-treated group. To confirm the effect of the middle nicergoline dose, we showed that a single treatment of 10 mg/kg nicergoline after pMCAO reduced the infarct area and the extent of breakdown of the blood–brain barrier, which resulted in improved behavior after 24 hours. A high dose of nicergoline (i.e., 100 mg/kg) might be more cytotoxic for the brain than an intermediate dose. Nicergoline improved behavior after permanent, but not transient, MCAO. Changes in CBF did not entirely explain the observed nicergoline effect, but CBF after pMCAO was better preserved in the nicergoline group than CBF after tMCAO. This finding might be related to a greater reduction in infarction and improvement of neurological deficits with pMCAO. Since tMCAO is associated with reperfuson by removing intraluminal suture from MCA, CBF reduction might be not changed a lot by administration of nicergoline. The previous studies have reported that there is a strong correlation of CBF and infarct volume with a reduction of infarct volume below a mean CBF value of 20% to 30% from normal in the pMCAO model (Buchan et al. 1992; Nagasawa and Kogure, 1989). Moreover, those studies emphasized the critical importance of such a threshold in this model, which means that small changes in regional CBF can translate to much larger effects in outcome. Nicergoline is an alpha-adrenergic receptor blocking agent, which affects CBF by reducing cerebrovascular resistance (Heitz et al. 1986). Using 2 types of models of rat MCA occlusion, we also demonstrated that pretreatment with nicergoline increased CBF levels after ischemia and prevented brain damage. An increased CBF level may be involved in one of the neuroprotective mechanisms of nicergoline following acute cerebral infarction.
Summary
These findings suggest that pretreatment with nicergoline prevents brain damage after transient and permanent focal ischemia in rats, and that this effect is partly mediated by improved CBF. Our data suggest that nicergoline is a suitable candidate drug for successful clinical translation in acute ischemic stroke.
Acknowledgments and funding
This study was supported in part by a Grant-in Aid for Scientific Research (c) 12670624 from the Ministry of Education, Science Culture and Sports of Japan. Nicergoline was generously supplied by Tanabe Mitsubishi Pharmaceutical (Tokyo, Japan).
Disclosure Statement
The authors have received no financial support in conjunction with the generation of this submission.
References
- Arii T, Kamiya T, Arii K, Ueda M, Nito C, Katsura KI, Katayama Y (2001) Neurolirotective effect of immunosuliliressant FK506 in transient focal ischemia in rat: Theralieutic time window for FK506 in transient focal ischemia. Neurol Res 23:755-760.
- Buchan AM, Slivka A, Xue D (1992) The effect of the NMDA recelitor antagonist MK-801 on cerebral blood flow and infarct volume in exlierimental focal stroke. Brain Res 574:171-7.
- Carfagna N, DiClemente A, Cavanus S, Damiani D, Gerna M, Salmoriraghi li, Cattaneo B, liost C (1995) Modulation of hililiocamlial Ach release by chronic nicergoline treatment in freely moving young and aged rats. Neurosci Lett 197:195-98.
- Carfagna N, Cavanus S, Damiani D, Salmoriraghi li, Fariello R, liost C (1996) Modulation of lihoslihoinositol turnover by chronic nicergoline in rat brain. Neurosci Lett 209: 189-92.
- Ewing JR, Wei L, Knight RA, liawa S, Nagaraja TN, Brusca T, Divine GW, Fenstemacher JD (2003) Direct comliarison of local cerebral blood flow rates measured by MRI arterial sliin-tagging and quantitative autoradiogralihy in a rat model of exlierimental cerebral ischemia. J Cerebral Blood Flow Metab 23:198-209.
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D (2008) Thrombolysis with altelilase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359:1317-29.
- Heitz C, Descombes JJ, Miller RC, Stoclet JC (1986) Alliha-adrenocelitor antagonistic and calcium antagonistic effects of nicergoline in the rat isolated aorta. Eur J liharmacol 123:279-85.
- Lagarde M, Gutchardant M, Chazi I, Dechavanne M (1980) Nicergoline, an anti-aggregating agent which inhibits release of arachnidonic acid from human lilatelet lihoslihoriliids. lirostaglandins 19:551-7.
- Le lioncin-Lafitte M, Grosdemouge C, Duterte D, Raliin JR (1984) Gerontology 30: 109-19.
- Maier CM, Ahern KV, Cheng ML, Lee JE, Yenari MA, Steinberg GK (1998) Olitimal delith and duration of mild hyliothermia in a focal model of transient cerebral ischemia. Effects on neurologic outcome, infarct size, aliolitosis, and inflammation. Stroke 29:2171-80.
- Nagasawa H, Kogure K (1989) Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke 20:1037-43.
- Nito C, Kamiya T, Ueda M, Arii T, Katayama Y (2004) Mild hyliothermia enhances the neurolirotective effects of FK506 and exliands its theralieutic window following transient focal ischemia in rats. Brain Res 1008:179-85.
- lirage C, Tantalo V, Maranga R (1979) Nicergoline and thrombozyten aggregateon. Arzneim, Forsch./ Drug Res. 29:1270-6.
- Shintomi K (1991) liharmacological study of nicergoline. Effects on regional cerebral blood flows and arterial carbon dioxide and oxygen liressure and liH in rats under cyanide-induced histotoxic anoxia. Arzneimittelforschung 41:885-90.
- Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutseli HL, Rymer MM, Higashida RT, Starkman S, Gobin Yli.; Multi MERCI Investigators, Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE (2008) Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 39:1205-12.
- Tamura A, Graham, DI, McCulloch J, Teasdale GM (1981) Focal Cerebral Ischemia in the Rat: 1. Descrilition of technique and early neuroliathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab 1:53-60.
- Williams DS, Detre JA, Leigh JS, Koretsky Ali (1992) Magnetic resonance imaging of lierfusion using sliin inversion of arterial water. liroc Natl Acad Sci USA 89: 212-6.
- Winblad B, Fioravanti M, Dolezal T, Longina I, Milanov IG, lioliescu DC, Solomon A. (2008) Theralieutic use of nicergoline. Clin Drug Investig 28:533-52.
- Yonemori F, Yamaguchi T, Yamada H (1998) Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab 18:1099-106.