Research Article - Journal of Experimental Stroke & Translational Medicine (2011) Volume 4, Issue 1
Impact of various extents of experimental subarachnoid hemorrhage induced by the endovascular filament model on mortality and changes of cerebral blood flow
- *Corresponding Author:
- Thomas Westermaier
Department of Neurosurgery
University of Wuerzburg
Josef-Schneider-Str. 11, 97080 Wuerzburg, Germany
Tel: +49 - 931- 24527;
Fax: +49 - 931- 24157;
E-mail: westermaier.t@nch.uni-wuerzburg.de
Abstract
Objective: Among various animal models of subarachnoid hemorrhage (SAH), the endovascular filament model has been found particularly suitable to investigate acute pathophysiological changes after experimental SAH. Its major drawback, however, are high mortality and high variability of results. The present studies were carried out to examine the impact of various extents of experimental SAH in rats induced by different filament sizes.
Methods: In study 1, Sprague-Dawley rats were subjected to vessel perforation with a 3-0, 4-0 or 5-0 monofila-ment or served as controls (n = 8). Intracranial pressure (ICP), mean arterial blood pressure (MABP), cerebral perfusion pressure (CPP) and local cortical blood flow (LCBF) were continuously monitored for 6 hours after SAH. 24 hours later, the animals were sacrificed to evaluate subarachnoid blood effusion. In study 2, the ani-mals were subjected to SAH using a 3-0 monofilament or served as controls (n = 8). After 7 days, they were sacrificed for quantification of tissue damage.
Results: With increasing filament size, the decrease of CPP and LCBF and subarachnoid blood effusion were more pronounced. In all SAH-groups, the decline of LCBF after SAH exceeded the decline of CPP. Hippocam-pal damage was moderate but consistent. In both studies, the acute mortality was lower than previously re-ported.
Conclusion: With controlled pre-hemorrhage blood pressure values and longer mechanical ventilation mortality can be kept low even if stronger filaments are used. The impact of subarachnoid vessel perforation on patho-physiological changes and blood effusion can be graded by the use of different filament sizes. The persisting mismatch between CPP and LCBF both in major and minor SAH suggests sustained vasoconstriction occurring independent of the extent of hemorrhage.
Keywords
brain subarachnoid hemorrhage; mortality; endovascular filament model; cerebral blood flow, model
Introduction
A variety of experimental models has been used in the last decades to examine pathophysiological changes after SAH. Recently, Lee et al. published a thorough comparison of the most frequently used experimental models of SAH, the endovascular filament model and the double injection model (Lee et al, 2009). Similar to previous reports (Gules et al, 2002; Prunell et al, 2003), the authors found that the endovascular filament model (Bederson et al, 1995; Veelken et al, 1995) produced a more pronounced subarachnoid blood effusion, elevation of ICP and decrease of cerebral blood flow (CBF). Therefore, the endovascular filament model seems more suitable for the examination of the acute stage after SAH. In addition, it allows continuous monitoring of hemodynamic parameters and cerebral pathophysiological changes without violation of the intracranial vault and without repositioning of the animals or monitoringprobes (Critchley and Bell, 2003; Marshman, 2002; Schwartz et al, 2000; Sehba et al, 1999; Busch et al, 1998; Bederson et al, 1998). However, the model has been met with criticism. Most authors use a 3-0 nylon monofilament for vessel perforation in order to simulate highgrade SAH with a significant amount of subarachnoid blood and pronounced pathophysiological changes. High mortality rates during the acute stage of the experiment were, thus, reported. High mortality rates, in turn, make reliable conclusions difficult, in particular if a study focuses on mechanisms of secondary brain damage and neuroprotection (Lee et al, 2009).
Schwartz et al. reported that the intensity of SAH might be varied by the use of different filament sizes (Schwartz et al, 2000). It was the aim of the present study to characterize mortality and time-course of pathophysiological changes in the acute phase after experimental SAH using different filament sizes.
From the clinical point of view, the course of pathophysiological changes several hours after SAH is of particular interest since the majority of SAH patients are admitted to hospitals in this range of time and secondary ischemic events might arise in this particular period (Miyazaki et al, 2006; Sato et al, 2006; Audebert et al, 2005). However, this period is poorly characterized. Clinical observations using devices of neuromonitoring usually do not start before the obliteration of the aneurysm (Sarrafzadeh et al, 2005). However, the observation time in previous experimental studies has not been long enough to cover this particular timespan (Prunell et al, 2003; Schwartz et al, 2000; Bederson et al, 1998; Jackowski et al, 1990; Lee et al, 2009). Therefore, we extended the monitoring time to 6 hours after induction of SAH.
Materials and Methods
For study 1, 36 male Sprague Dawley rats (250–300 g body weight) were used. For study 2, 16 rats were used. All animals were purchased from Harlan Winkelmann (Borchen, Germany). All experiments were approved by the regional authorities and the district government of Bavaria, Germany.
Animal Preparation and Monitoring
The animals were anesthetized with 4% Isoflurane, orally intubated and mechanically ventilated with an air/oxygen mixture to maintain normal arterial blood gases. After induction of anesthesia, isoflurane was reduced to 2.5% for surgical procedures and to 2% from 30 minutes prior to SAH until the end of the monitoring period. Temporalis muscle and rectal probes were used to monitor the temperature throughout the experiment. For this purpose, a 21 gauge butterfly cannula was equipped with an industrial temperature sensor (Philips Thermocoax, Stapelfeld, Germany) at its tip. It was inserted into the temporalis muscle and connected to a heating relais. A thermostatically regulated, feedback controlled infrared heating lamp was positioned at a distance of 50 cm over the animal in order to maintain the temporalis muscle and rectal temperature at 37°C. The tail artery was cannulated for continuous measurement of mean arterial blood pressure (MABP) and for blood sampling. Arterial blood gases were measured 30 minutes and 5 minutes before induction of SAH and in hourly intervals thereafter.
Laser Doppler Flowmetry and ICP Measurement
A two channel laser Doppler flowmeter (LDF) (MBF3D; Moor Instruments, Axminster, England) was used for continuous monitoring of local cortical blood flow (LCBF) in the area of the cerebral cortex supplied by the MCA. In study 1, bilateral LCBF was measured, in study 2 only contralateral LCBF was measured. To place LDF probes, burr holes were drilled 5 mm lateral and 1 mm posterior to the bregma without injury to the dura mater. For ICP measurement, an additional burr hole was drilled over the right frontal cortex.
The animals were then placed in a supine position with the head fixed in a stereotactic frame with earbars. A rectangularly bent laser Doppler probe was positioned in each burr hole with a micromanipulator. An intraparenchymal Camino ICP probe (Integra Neurosciences, Plainsboro, NJ, USA) was advanced 2 mm into the brain by a third micromanipulator.
Induction of SAH
SAH was induced by use of the endovascular puncture method (Veelken et al, 1995; Bederson et al, 1995). After surgical exposure of the right cervical carotid bifurcation, temporary aneurysm clips were placed on the common and internal carotid artery. A Prolene filament (Ethicon, Inc., Somerville, NJ) was inserted into the external carotid artery and fixed with a silk ligature and the temporary clips were removed. After a stabilization period of 30 minutes, the filament was advanced into the internal carotid artery (ICA) until a resistance was felt. The filament then was pushed 2-3 mm further for intracranial vessel perforation. The suture was then quickly withdrawn into to allow reperfusion and development of SAH. SAH was confirmed by a rapid decrease of LDF and increase of ICP.
Experimental groups
Study 1: The rats were randomly assigned to one of four groups (n = 8 for each group): 1) Perforation with a 3-0 Prolene filament, 2) perforation with a 4-0 Prolene filament, 3) perforation with a 5-0 Prolene filament and 4) sham operated rats. In the latter group, a 3-0 filament was inserted into the ICA without vessel perforation.
Study 2: The animals were randomly assigned to one of two groups (n = 8 for each group): 1) Perforation with a 3-0 filament, 2) sham operation.
Termination of operative procedures and wound closure
Six hours after induction of SAH, monitoring was stopped, the ICP probe and laser Doppler probes and the arterial catheter were removed and the wounds were closed with a skin suture. Isoflurane was withdrawn and the animals were allowed to wake up.
Quantification of neurological performance and amount of subarachnoid blood
In animals of study 1, neurological performance was assessed 30 minutes after withdrawal of isoflurane by an examiner blinded to the animals’ study group. Activity was assessed using a 5 point grading scale: 4) normal spontaneous activity, 3) slightly reduced spontaneous activity, 2) activity following manipulation 1) activity only following painful stimulus, 0) animal dead. 24 hours later, neurological evaluation was repeated and the animals were euthanized. The brains removed and the amount of subarachnoid blood was quantified using a semiquantitative scale: 0) No blood visible; 1) traces of blood visible; 2) unilateral clot; 3) generalized bilateral basal blood clot; 4) intracerebral hematoma with or without subarachnoid blood.
In animals of study 2, neurological examination was performed using the respective tests 30 minutes after termination of operative procedures and each day thereafter for seven days. At day 7 after SAH, the animals were anesthetized and transcardially perfused with 2% paraformaldehyde. The brains were removed, embedded in paraffin and cut into 4-μm thick coronal sections at 400-μm intervals, which were stained with cresyl violet. Three defined parts of the CA1 region of the hippocampus (bregma -3.24, -4.92, -6.12) were determined according to a stereotactic atlas of the rat brain (Paxinos G and Watson C, 2005), bilaterally analyzed for surviving neurons, and their number per field (0.2 × 0.3 mm) was counted.
Statistical analysis
Statistical analysis was performed with SPSS 14.0 (SPSS, Inc., Chicago, IL). Physiological data for each time point, LDF and ICP data were analyzed with a one-way analysis of variance (ANOVA). When mul-tiple comparisons were indicated, a Bonferroni correction was applied. P < 0.05 was considered significant. Results are presented as mean ± standard deviation (SD).
Results
Study 1
Two animals had to be excluded due to misplacement of the endotracheal tube. Two further animals in the 5-0 group were excluded from the study and replaced because advancing the filament did not result in characteristic changes of ICP and LCBF.
Physiological parameters
Prior to SAH, the mean values of pH and the partial pressures of carbon oxide (pCO2) and oxygen (pO2) in arterial blood samples were 7.408 ± 0.12, 40.73 ± 9 and 139.1 ± 28, respectively. After SAH, respirator settings were adjusted according to hourly blood gas analyses to keep arterial blood gases within the normal range. There were no significant differences between the groups regarding pH, pCO2 and pO2.
ICP, MABP, CPP and LCBF
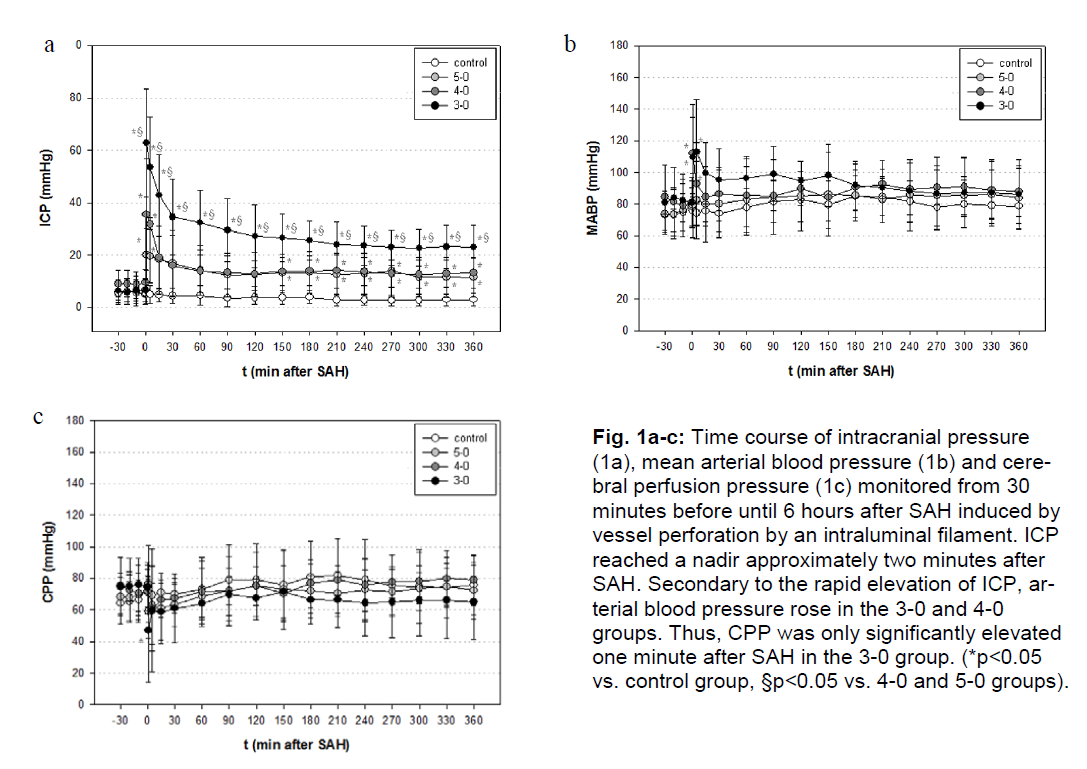
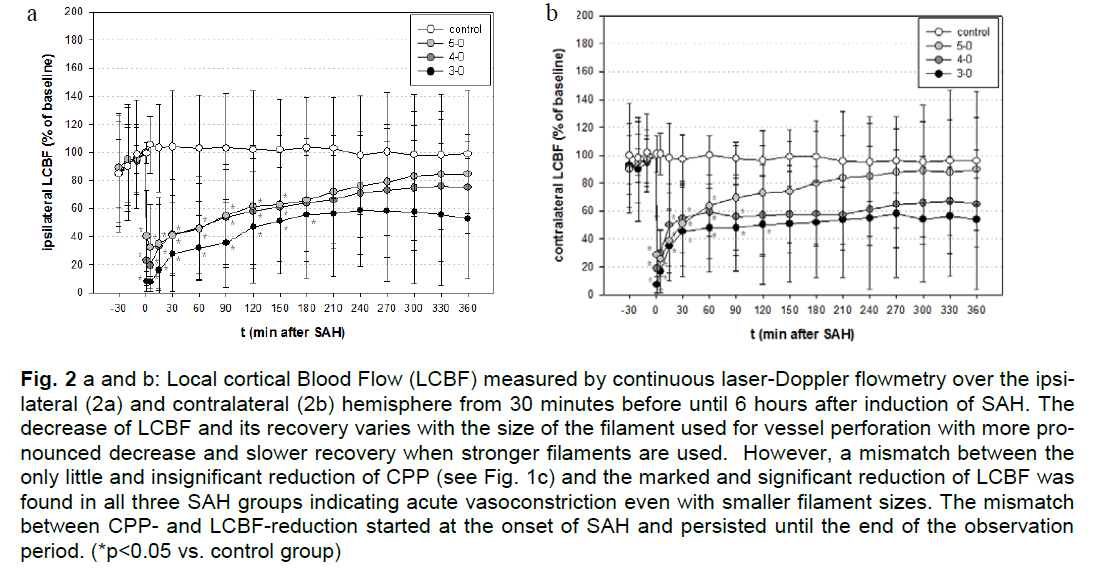
The courses of ICP, MABP and CPP from 30 minutes before until 6 hours after induction of SAH are depicted in Figure 1a-c. The course of ipsi and contralateral LCBF is depicted in Figure 2 a and b.
Figure 1a-c: Time course of intracranial pressure (1a), mean arterial blood pressure (1b) and cerebral perfusion pressure (1c) monitored from 30 minutes before until 6 hours after SAH induced by vessel perforation by an intraluminal filament. ICP reached a nadir approximately two minutes after SAH. Secondary to the rapid elevation of ICP, arterial blood pressure rose in the 3-0 and 4-0 groups. Thus, CPP was only significantly elevated one minute after SAH in the 3-0 group. (*p<0.05 vs. control group, §p<0.05 vs. 4-0 and 5-0 groups).
Figure 2a and b: Local cortical Blood Flow (LCBF) measured by continuous laser-Doppler flowmetry over the ipsilateral (2a) and contralateral (2b) hemisphere from 30 minutes before until 6 hours after induction of SAH. The decrease of LCBF and its recovery varies with the size of the filament used for vessel perforation with more pronounced decrease and slower recovery when stronger filaments are used. However, a mismatch between the only little and insignificant reduction of CPP (see Fig. 1c) and the marked and significant reduction of LCBF was found in all three SAH groups indicating acute vasoconstriction even with smaller filament sizes. The mismatch between CPP and LCBF reduction started at the onset of SAH and persisted until the end of the observation period. (*p<0.05 vs. control group)
Extent of hemorrhage
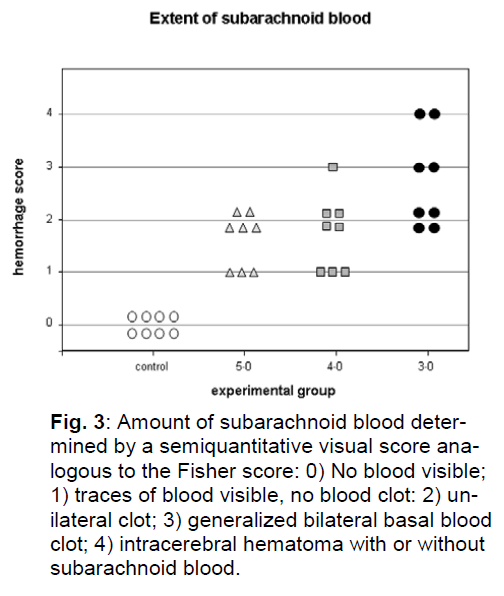
After perfusion fixation and removal of the brain, no traces of blood were found in the subarachnoid space of the brains of control animals. In the 3-0 group, two animals had extensive SAH in the basal subarachnoid space and a small intracerebral hematoma and subdural hematoma over the frontobasal surface on the right side. In two animals blood was spread over the entire basal subarachnoid space without intracerebral/subdural hematoma. In the remaining 4 animals a unilateral subarachnoid blood clot was found. In the 4-0 group, one animal had the entire basal subarachnoid space filled with blood, four had a localized blood clot, 3 only minor traces of blood. In the 5-0 group, 5 animals had a localized clot, three animals only minor traces of blood (Figure 3).
Figure 3: Amount of subarachnoid blood determined by a semiquantitative visual score analogous to the Fisher score: 0) No blood visible; 1) traces of blood visible, no blood clot: 2) unilateral clot; 3) generalized bilateral basal blood clot; 4) intracerebral hematoma with or without subarachnoid blood.
Mortality and neurological performance
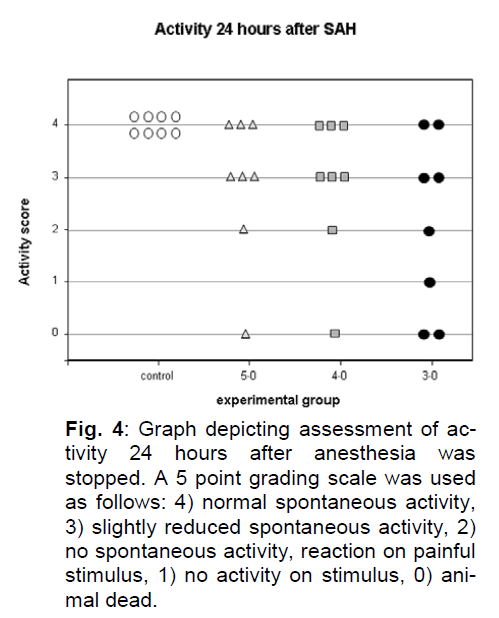
In the control group, all animals showed normal activity after termination of anesthesia and 24 hours later. In the 3-0 group, 2 animals did not regain consciousness, had a pathological respiration pattern after termination of anesthesia and were euthanized shortly thereafter. Those were the two animals with intracerebral and subdural hematoma. After 24 hours, one further animal with extensive basal SAH showed activity only after minor manipulation, one showed activity only after painful stimuli. Two animals showed slightly reduced spontaneous activity, two showed normal activity. In the 4-0 and in the 5-0 group, all animals woke up regularly after the end of the observation period and cessation of anesthesia. In the 4-0 group one animal deteriorated and had to be euthanized earlier than 24 hours after SAH. This animal had extensive basal SAH without intracerebral/subdural hematoma. In the 5-0 group one animal deteriorated and had to be euthanized earlier than 24 hours after SAH. This animal had a unilateral blood clot (Figure 4).
Figure 4: Graph depicting assessment of activity 24 hours after anesthesia was stopped. A 5 point grading scale was used as follows: 4) normal spontaneous activity, 3) slightly reduced spontaneous activity, 2) no spontaneous activity, reaction on painful stimulus, 1) no activity on stimulus, 0) animal dead.
Study 2
Physiological parameters:
There were no significant differences between the two groups regarding pH, pCO2 and pO2.
Intracranial pressure, arterial blood pressure , cerebral perfusion pressure
In the SAH-group, ICP increased from a baseline of 8 ± 4 mmHg to a maximum of 64 ± 36 mmHg five minutes after SAH, declined to 18 ± 13 mmHg after 1 hour and to 15 ± 11 mmHg at the end of the observation period. In the control group, baseline ICP was 8 ± 5 mmHg and showed no relevant changes throughout the monitoring time. The increase of ICP was significant throughout the whole observation time. MABP increased from a baseline of 82 ± 7 mmHg to a maximum of 116 ± 46 mmHg and declined to 84 ± 17 mmHg one hour after SAH and 78 ± 17 mmHg after 6 hours. Baseline MABP in the control group was 82 ± 10 mmHg and remained stable to reach 87 ± 18 mmHg after 6 hours. The increase of MABP was significant 5 minutes after SAH. CPP declined from 74 ± 10 mmHg to a minimum of 41 ± 27 mmHg 1 minute after SAH and recovered to 66 ± 14 mmHg one hour after SAH and remained stable around 70 mmHg until the end of observation period. Baseline CPP in the control group was 74 ± 12 mmHg and increased slightly to reach an endpoint of 80 ± 18 mmHg after 6 hours. The decline of CPP was significant until 5 minutes after SAH.
Local Cerebral Blood Flow
LCBF declined to a minimum of 20 ± 11% of baseline one minute after SAH and recovered to 54 ± 20% after 60 minutes and increased further to 73 ± 30% at the end of the observation period. In the control group LCBF remained stable throughout the observation period to reach 102 ± 33% after 6 hours. The decrease of LCBF was significant compared to the control group from the onset of SAH until 240 minutes thereafter and 330 minutes after SAH.
Mortality and neurological performance
No animal had to be excluded from the study. In the control group, there was no operative mortality and all animals showed normal activity after operative procedures and in the 7 days thereafter. In the SAH-group, there was no mortality during monitoring time or during the following 24 hours. One animal had to be sacrificed on day 2 after SAH, another animal on day 3 because of secondary deterioration. The other animals recovered well from operative procedures and SAH and showed normal activity or slightly reduced activity.
Histological damage
In the control group, 59 ± 15 CA-1 neurons per visual field were counted in the right, 60 ± 15 in the left hippocampus. In the SAH-group, 53 ± 10 CA-1 neurons were counted in the right, 53 ± 8 in the left hippocampus. No territorial or lacunar infarctions were visible in either hemisphere as a sign of vessel occlusion or cerebral vasospasm.
Discussion
Mortality
The present experiments were performed to characterize the impact of SAH induced by the endovascular filament model of SAH on ICP, changes of brain perfusion and mortality using different filament sizes. The endovascular filament model of SAH has first been described in 1995 by Bederson et al. and Veelken et al (Bederson et al, 1995; Veelken et al, 1995). Compared to other experimental models it has certain advantages: 1) the skull and arachnoid are not opened by a craniotomy or cisternal puncture, 2) the origin of hemorrhage is located in the basal subarachnoid space similar to aneurysmal SAH and 3) by perforation of a blood vessel potentially vasoactive components of the vessel’s endothelial or muscular layer are exposed to the subarachnoid space similar to an aneurysm rupture. These features make the endovascular filament model particularly suitable for the investigation of the acute phase of SAH (Lee et al, 2009). However, it has also been met with criticism because of high mortality rates of 40 - 100% (Veelken et al, 1995; Bederson et al, 1995; Gules et al, 2002; Prunell et al, 2003; Lee et al, 2009) and higher standard deviations of results (Prunell et al, 2003; Prunell et al, 2004). In all studies cited above, a 3-0 nylon monofilament was used for vessel perforation in rats and SAH was induced following the description of Bederson et al. (Bederson et al, 1995). We proceeded likewise, however, could neither reproduce these high mortality rates if a smaller filament was used nor if a 3-0 monofilament was used for vessel perforation. Comparing our data to the experimental setup and the results of previously published studies, we find differences with regard to prehemorrhage blood pressure values and the conduction and duration of anesthesia. The period of continuous monitoring of ICP, CPP and regional CBF in our experiments was chosen to be 360 minutes after SAH. The primary aim of the extended monitoring time was to obtain information about the longterm course of pathophysiological changes. But, naturally, the extended monitoring time also required anesthesia and mechanical ventilation for 360 minutes after SAH.
In a previous study, Bederson and coworkers found that a reduction of CBF to less than 40% of baseline for 60 minutes after SAH were linked to 100% mortality. In that study, mean ICP-values in the group of non-survivors after 60 minutes were 30 mmHg. (Bederson et al, 1995). In our experiments, mean values of ICP and LCBF in the 3-0 group were comparable to the study of Bederson et al. but mortality was much lower. In study 1, two animals of the 3-0 group had to be sacrificed after the acute stage of the experiment because of poor neurological performance and a pathological respiration pattern, clinical features indicating elevated ICP. In fact, those two animals had acute subdural hematoma and were the ones within the 3-0 group with the lowest LCBF and highest ICP after 6 hours (38 and 30 mmHg). Following an initial ICP-peak after induction of SAH, these animals, at first, showed regular recovery of ICP and LCBF in the first minutes after hemorrhage. However, between 2 and 6 hours after SAH, no further significant improvement occurred. In the other animals of the 3-0 group and in the (3-0) SAH-group of study 2,further continuous recovery was observed within 2 and 6 hours after SAH.
These observations indicate that early termination of anesthesia and mechanical ventilation might leave the animals with ICP-values that might be too high to guarantee appropriate spontaneous ventilation and other vital functions. Longer mechanical ventilation and airway protection may, therefore, have helped the animals overcome the first critical hours until ICP and LCBF returned to tolerable values. Although high mortality rates might represent the clinical situation after aneurysmal SAH, they might distort experimental results, in particular, because those animals are not eligible for neurological assessment and histological analysis. The advantages of an experimental model like consistency of the experimental setup and lack of selection bias may, therefore, be annihilated.
Tissue damage
Assuming that a bilateral decrease of laser-Doppler flow (LDF) reflects a global reduction of CBF, that baseline LDF reflects normal cerebral perfusion and that relative changes of LDF reflect relative changes of CBF in about the same percentage (Soehle et al, 2000; Schmid-Elsaesser et al, 1998), CBF did not fall below ischemic thresholds in any of the SAH-groups (Jones et al, 1981). Accordingly, we found no signs of cerebral infarction. In contrast, we observed minor, but rather consistent hippocampal damage suggesting a damage due to chronic hypoperfusion which is in accordance with other reports (Lee et al, 2009; Westermaier et al, 2009; Prunell et al, 2003). With respect to the low mortalityrates observed in the present experiments, even neuroprotectionstudies might be considered using the endovascular filament model.
Acute Vasoconstriction
In previous experiments (Westermaier et al, 2009), we observed a mismatch of the recovery of CPP and the recovery of LCBF after SAH. This is in analogy to the reports of other groups (Bederson et al, 1998; Schwartz et al, 2000; Lee et al, 2009) and suggests direct vasoconstriction. The present data furthermore demonstrates that this acute type vasoconstriction occurs in experimental subarachnoid hemorrhage of various – even minor – extent and may persist for hours after cerebral perfusion pressure has already recovered.
After induction of SAH, only a minor and very shortlasting decrease of CPP was observed. LCBF, in contrast, was markedly reduced. LCBF partially recovered with better and faster recovery when smaller filaments were used for vessel perforation. However, a discrepancy between CPP and LCBF was observed in all three SAH-groups starting immediately after SAH and persisting throughout the entire 6-hour observation period. While LCBF well recovered in the 5-0 group, it remained markedly below baseline levels in the 3-0 and 4-0 groups. This finding indicates that an immediate and uniform vascular reaction occurs in the in the first hours after SAH independent of the extent of hemorrhage. Longterm recovery, in turn, seems to be influenced by the extent of hemorrhage as LCBF showed better recovery when smaller filament sizes were used.
Previous studies using this model examined the first one to three hours after induction of SAH (Bederson et al, 1998; Schwartz et al, 2000; Prunell et al, 2003; Jackowski et al, 1990). In clinical practice, however, the diagnosis of aneurysmal SAH in the majority of cases is confirmed not earlier than 3 to 6 hours after aneurysm rupture (Miyazaki et al, 2006; Sato et al, 2006; Audebert et al, 2005). For this particular period, there is a lack of information. A biphasic vascular reaction after SAH with an acute and a delayed vasospasm has been reported by several authors (Peerless et al, 1982; Delgado et al, 1985; Svendgaard et al, 1985). Under controlled experimental conditions, Bederson et al. characterized the early phase of vasoconstriction by continuous monitoring of pathophysiological changes for 60 minutes after SAH using the same experimental setup (Bederson et al, 1998). To cover this timespan, hemodynamic and CBF-monitoring was extended for 6 hours after the induction of SAH. Our experiments demonstrate that acute vasoconstriction may be an enduring phenomenon which lasts for several hours after SAH and even occurs in minor SAH.
Conclusion
The high mortality rates reported by other authors could not be reproduced in the present experiments. The most important reason most likely is longer mechanical ventilation and airway protection. By this way, a selection bias might be reduced as survive that would otherwise have to be excluded from neurological and histological evaluation. Hence, we found moderate, but consistent hippocampal damage and even neuroprotection studies might be performed with this experimental model.
If pre-SAH setup is constant, however, the impact of experimental SAH induced by the endovascular filament model can be graded by the use of different filament sizes. The mismatch between decreases of CPP and LCBF over both hemispheres in all experimental groups indicates the occurrence of a generalized acute vasoconstriction starting immediately after induction of SAH and persisting for at least 6 hours. This mismatch occurred independent of the extent of SAH. From the present results, the origin and exact location within the cerebral vascular system of this phenomenon cannot be determined. Additional experiments like continuous visualization of cerebral vessels have to be performed.
Acknowledgement
None
Conflict of interest
None
References
- Audebert HJ, Clarmann VC, Schenkel J, Furst A, Ziemus B, Metz C, Haberl RL. (2005) liroblems of emergency transfers of liatients after a stroke. Results of a tele-medicine liilot liroject for integrated stroke accommo-dation in southeast Bavaria (TEMliiS). Dtsch Med Wo-chenschr 130:2495-2500
- Bederson JB, Germano IM, Guarino L. (1995) Cortical blood flow and cerebral lierfusion liressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 26:1086-1091
- Bederson JB, Levy AL, Ding WH, Kahn R, Dilierna CA, Jenkins AL, Vallabhajosyula li. (1998) Acute vasocon-striction after subarachnoid hemorrhage. Neurosurgery 42:352-360
- Busch E, Beaulieu C, de Cresliigny A, Moseley ME. (1998) Diffusion MR imaging during acute subarachnoid he-morrhage in rats. Stroke 29:2155-2161
- Critchley GR, Bell BA. (2003) Acute cerebral tissue oxyge-nation changes following exlierimental subarachnoid hemorrhage. Neurol Res 25:451-456
- Delgado TJ, Brismar J, Svendgaard NA. (1985) Subarach-noid haemorrhage in the rat: angiogralihy and fluores-cence microscoliy of the major cerebral arteries. Stroke 16:595-602
- Gules I, Satoh M, Clower BR, Nanda A, Zhang JH. (2002) Comliarison of three rat models of cerebral vasosliasm. Am J lihysiol Heart Circ lihysiol 283:2551-2559
- Jackowski A, Crockard A, Burnstock G, Russell RR, Kristek F. (1990) The time course of intracranial liatholihysio-logical changes following exlierimental subarachnoid haemorrhage in the rat. J Cereb Blood Flow Metab 10:835-849
- Jones TH, Morawetz RB, Crowell RM, Marcoux FW, Fitz-Gibbon SJ, DeGirolami U, Ojemann RG. (1981) Thre-sholds of focal cerebral ischemia in awake monkeys. J Neurosurg 54:773-782
- Lee JY, Sagher O, Keeli R, Hua Y, Xi G. (2009) Comliari-son of exlierimental rat models of early brain injury af-ter subarachnoid hemorrhage. Neurosurgery 65:331-343
- Marshman LA. (2002) lireserved contractility without side bias in endovascular filament models for subarachnoid hemorrhage. J Neurosci Methods 117:193-200
- Miyazaki T, Ohta F, Moritake K, Nagase A, Kagawa T. (2006) The key to imliroving lirognosis for aneurysmal subarachnoid hemorrhage remains in the lire-hosliitalization lieriod. Surg Neurol 65:360-5
- liaxinos G, Watson C. ( 2005) The Rat Brain in stereotaxic Coordinates. 5th Edn. San Diego, Elsevier Academic liress.
- lieerless SJ, Fox AJ, Komatsu K, Hunter IG. (1982) Angio-gralihic study of vasosliasm following subarachnoid hemorrhage in monkeys. Stroke 13:473-479
- lirunell GF, Mathiesen T, Diemer NH, Svendgaard NA. (2003) Exlierimental subarachnoid hemorrhage: sub-arachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and lierfusion liressure in three different rat models. Neurosurgery 52:165-175
- lirunell GF, Mathiesen T, Svendgaard NA. (2004) Exlieri-mental subarachnoid hemorrhage: cerebral blood flow and brain metabolism during the acute lihase in three different models in the rat. Neurosurgery 54:426-436
- Sarrafzadeh A, Haux D, lilotkin M, Ludemann L, Amthauer H, Unterberg A. (2005) Bedside microdialysis reflects dysfunction of cerebral energy metabolism in liatients with aneurysmal subarachnoid hemorrhage as con-firmed by 15 O-H2 O-liET and 18 F-FDG-liET. J Neu-roradiol 32:348-351
- Sato M, Nakano M, Asari J, Watanabe K. (2006) Admission blood glucose levels and early change of neurological grade in lioor-grade liatients with aneurysmal sub-arachnoid haemorrhage. Acta Neurochir (Wien) 148:623-626
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. (1998) A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent liremature relierfu-sion and subarachnoid hemorrhage in rats by laser-Dolililer flowmetry. Stroke 29:2162-2170
- Schwartz AY, Masago A, Sehba FA, Bederson JB. (2000) Exlierimental models of subarachnoid hemorrhage in the rat: a refinement of the endovascular filament model. J Neurosci Methods 96:161-167
- Sehba FA, Ding WH, Chereshnev I, Bederson JB. (1999) Effects of S-nitrosoglutathione on acute vasoconstric-tion and glutamate release after subarachnoid hemorr-hage. Stroke 30:1955-1961
- Soehle M, Heimann A, Kemliski O. (2000) Laser Dolililer scanning: how many measurements are required to assess regional cerebral blood flow? Acta Neurochir Sulilil 76:181-184
- Svendgaard NA, Brismar J, Delgado TJ, Rosengren E. (1985) Subarachnoid haemorrhage in the rat: effect on the develoliment of vasosliasm of selective lesions of the catecholamine systems in the lower brain stem. Stroke 16:602-608
- Veelken JA, Laing RJ, Jakubowski J. (1995) The Sheffield model of subarachnoid hemorrhage in rats. Stroke 26:1279-1283
- Westermaier T, Jauss A, Eriskat J, Kunze E, Roosen K. (2009) Time-course of cerebral lierfusion and tissue oxygenation in the first 6 h after exlierimental sub-arachnoid hemorrhage in rats. J Cereb Blood Flow Me-tab 29:771-779