Review Article - Imaging in Medicine (2010) Volume 2, Issue 1
Imaging primary musculoskeletal tumors: role of 18F-FDG-PET/CT
Klaus Strobel1,2†, Dorothee R Fischer1, Katrin DM Stumpe1 & Gustav K von Schulthess11Nuclear Medicine, University Hospital Zurich, Switzerland
2Nuclear Medicine, Cantonal Hospital Lucerne, Switzerland
- Corresponding Author:
- Klaus Strobel
Nuclear Medicine
University Hospital Zurich, Switzerland
Tel: +41 412 054 401
Fax: +41 412 052 175
E-mail: klaus.strobel@ksl.ch
Abstract
Sarcomas are rare and represent approximately 1% of malignant tumors. Symptoms may be pain, palpable mass or pathologic fractures. Early diagnosis of sarcomas is essential, because prognosis and therapeutic options are much better in lower tumor stages than in higher tumor stages. However, the diagnosis of soft-tissue sarcomas or bone sarcomas is often delayed owing to the lack of specific clinical symptoms and misinterpretation of radiological findings. Optimal management of sarcomas depends on the site, grade and accurate staging of the tumor when first diagnosed. The metastatic spread of sarcomas is mainly hematogenous to the lungs, although lymphatic spread may occur. Traditional diagnostic imaging methods for the evaluation of musculoskeletal tumors include ultrasound, CT, MRI and bone scintigraphy. PET and PET/CT is a relatively new imaging technique that enables in vivo characterization of tumor metabolism. 18F-2-deoxy-2-fluoro-glucose (FDG) is the ‘work horse’ tracer of tumor imaging with PET. Broadly speaking it identifies tumors that are aggressive, while low-grade tumors frequently do not accumulate FDG. In this article, we review our own experience and the literature regarding FDG‑PET/CT imaging of musculoskeletal tumors with special focus on sarcomas.
Keywords
bone sarcoma ▪ FDG-PET/CT ▪ musculoskeletal tumors ▪ PET/MRI ▪ soft-tissue sarcoma ▪ staging ▪ therapy response assessment
18F-2-deoxy-2-fluoro-glucose-PET/CT imaging of primary soft-tissue tumors
General considerations
Soft-tissue sarcomas (STS) are extremely rare tumors, accounting for 0.7% of adult malignancies and approximately 7% of childhood cancers [1]. STS can occur at any site of the body; 45% of all STS are found in the extremities, especially the lower limb, and approximately 20% are found intra-abdominally [2]. STS can derive from fatty tissue (lipoma/liposarcoma), muscles (myoma/leiomyosarcoma, rhabdomyosarcoma [RMS]), connective tissue (fibroma/ fibrosarcoma), blood vessels (angioma, hemangioma, angiosarcoma) and neurogenic tissue (schwannoma, malignant peripheral nerve sheath tumor, malignant schwannoma). STS are challenging because of the different behavior spanning from relatively benign entities of low-grade tumors to very aggressive forms of high-grade tumors. Low-grade STS are generally treated with complete initial resection. In high-grade STS, wide resection is often accompanied by neoadjuvant and/or adjuvant chemo and/or radiation therapy.
Ultrasound is often used to establish the diagnosis of a STS and to guide a necessary biopsy. MRI is the most important method to show the origin of a tumor and the anatomic relation to important structures such as nerves, vessels and muscle compartments prior to resection. 18F-2-deoxy-2-fluoro-glucose (FDG)-PET/CT plays an increasingly important role in biopsy guidance, noninvasive grading, therapy response assessment and restaging. All of these clinically important aspects cannot be completely assessed by morphologic imaging.
Although FDG-PET/CT is increasingly used in patients with STS its definite role within the clinical routine is still not clearly defined. The rarity of these tumors hampers prospective studies with large patient groups. Thus, the current literature is based on the experience of some centers with investigated populations mainly around 30–60 patients and the data of systematic reviews and meta-analyses [3].
Differentiation between benign & malignant soft-tissue tumors with FDG-PET/CT
Several studies have investigated the performance of FDG-PET/CT regarding the differentiation of benign and malignant primary STS [4–8]. Generally, benign STS show no or a low FDG uptake and malignant STS moderate to high FDG accumulation. Standard uptake values (SUV), a quantitative measure of FDG uptake, range between 0.7 and 1.35 in benign STS and between 3.2 and 6.9 in malignant STS. Pooled data summarizing 127 STS from different studies showed a significant difference regarding this discrimination with FDGPET (p = 0.006). Aoki et al. measured SUVs of different benign and malignant STS and showed that there are benign lesions with relatively high maximum SUVs such as desmoids, schwannoma or giant cell tumor of the tendon sheeth with SUVs of up to 7. Additionally, inflammatory lesions such as sarcoidosis or rheumatoid nodules may mimic malignancy by showing significant FDG uptake [4,9]. On the other hand, low-grade malignant STS can have very low FDG uptake, for example liposarcoma. In conclusion, there is an overlap between FDG uptake of benign and malignant soft-tissue lesions. A reliable SUVmax cutoff value is difficult to define but most authors recommend a SUVmax of 2.0 as the most reliable threshold. Other authors calculated cutoff SUVmax values with receiver operating characteristic curves recommending a cutoff of 3.8 as the best discriminator between benign lesions and malignancies [10,11].
Another meta-analysis included 15 studies with 441 soft-tissue lesions (227 malignant, 214 benign). For diagnosis of malignant versus benign lesions, typical pairs of sensitivity and specificity estimated from the summary receiver operating characteristic curves were 92 and 73%, respectively, for qualitative visualization; 87 and 79%, respectively, for a cutoff SUV of 2.0; 70 and 87%, respectively, for SUV of 3.0 [12].
FDG-PET/CT has the potential to discriminate between benign and malignant soft-tissue lesions. It is clearly recommended that additional clinical and imaging information such as ultrasound and MRI are used to distinguish between benign and malignant STS. Furthermore, it is important to point out that PET cannot replace biopsy and that histopathologic confirmation in unclear cases is crucial.
Detection of STS with FDG-PET/CT
FDG-PET/CT was shown to be very sensitive in the detection of STS. In a meta-analysis summarizing 341 patients (168 with STS, 173 without STS), the pooled sensitivity was 0.88 (0.83– 80.93), the specificity was 0.86 (0.81–80.91) and the accuracy was 0.87 (0.83–80.90) [3].
In another large meta-analysis mentioned previously, 18F-FDG was positive in all intermediate/ high-grade tumors (95% CI: 97.3–100%), 74.4% (95% CI: 58.6–85.9%) of low-grade tumors and 39.3% (95% CI: 29.1–50.3%) of benign lesions (including 11 of 12 inflammatory lesions) [12]. A recently published study with 160 STS confirmed these high detection rates (93.7%) [13].
Grading of STS with FDG-PET/CT
Histologic grading is an important factor in the evaluation of STS. Preoperative imaging assessment of the histologic grading is a challenging issue. Several studies showed a positive correlation between FDG uptake (SUVmax) and histopathological sarcoma grade [6,7]. The mean SUV of low-grade STS ranged between 1.6 and 2.6, while high-grade STS has generally higher values (8.0–9.4) [14,15]. Several studies showed that the differentiation of benign and low-grade lesions cannot be made with FDG-PET. In an earlier review from 2003, a significant difference between the detection of low-grade and high-grade STS using FDG-PET was shown in a very limited patient population of 30 cases (p = 104) [12]. Folpe et al. demonstrated a correlation between PET SUV and histopathologic grade in 89 STS. Furthermore, an association between FDG uptake and cellularity, mitotic activity and P53 overexpression was demonstrated in this publication [16]. In the subgroup of fatty soft-tissue tumors, Schwarzbach et al. showed that FDG uptake in liposarcomas correlates with histological subtype and tumor grade [8]. Other authors found that dynamic FDG-PET imaging was better able to grade STS, especially for discriminating between grade 1 and grade 3 tumors [5]. FDG-PET/CT is able to visualize necrotic parts in soft-tissue tumors, which is a reliable indication of a highgrade tumor. As shown in other tumor entities, the amount of FDG uptake in a STS provides important prognostic information, such as predicting overall survival, recurrence-free survival and local tumor control [17].
Malignant transformation of benign to malignant STS is very rare. Several reports indicate that FDG-PET/CT is a promising tool for the detection of malignant transformation of STS. In particular, patients with neurofibromatosis are at high risk for developing malignant peripheral nerve sheath tumors (MPNSTs). Warbey et al. evaluated 69 patients with neurofibromatosis type 1 with 85 lesions, including ten atypical neurofibromas and 21 MPNSTs. FDG-PET was very sensitive (97%) and specific (87%) in the detection of MPNSTs [18]. Cardona et al. confirmed that MPNSTs have significantly higher FDG uptake in their evaluation of 25 neurogenic STS [19].
Biopsy guidance of STS with FDG-PET/CT
The management of STS requires biopsy prior to surgical resection. STS are often very heterogeneous tumors and biopsies from surgically convenient localizations may not lead to a representative histology. If the most malignant area can be identified more accurately, then this area could be targeted for biopsy. The most aggressive tumor component may be reflected by the highest FDG uptake. On histologic examination these areas have increased cellularity, mitotic rate and specific markers of tumor aggressiveness. Hain et al. showed that FDG-PET can be used to appropriately direct biopsy in STS [20].
Staging of STS with FDG-PET/CT
Accurate staging of STS is crucial for therapy planning and prognostic stratification. Metastases of STS can occur in all parts of the body but typically occur in the lung, soft tissue and lymph nodes (Figure 1). Studies examining the performance of FDG-PET/CT in the detection of lymph node metastases are very limited. Lymph node metastases are often seen in RMS, and Völker et al. found lymph node metastases in six patients with RMS and reported a PET sensitivity of 93%, which was clearly superior to conventional imaging (36% sensitivity) [21]. Tateishi et al. confirmed these results. They reported lymph node involvement in 15 of 35 patients with RMS and PET showed the correct N stage in 97% of patients compared with 31% with conventional imaging [22]. Klem et al. reported a limited region-based sensitivity (77%) and a high specificity (95%) for FDGPET in nodal staging of 24 RMS patients [23]. As in other tumors, a FDG-PET does not eliminate the need for lymph-node sampling in STS.
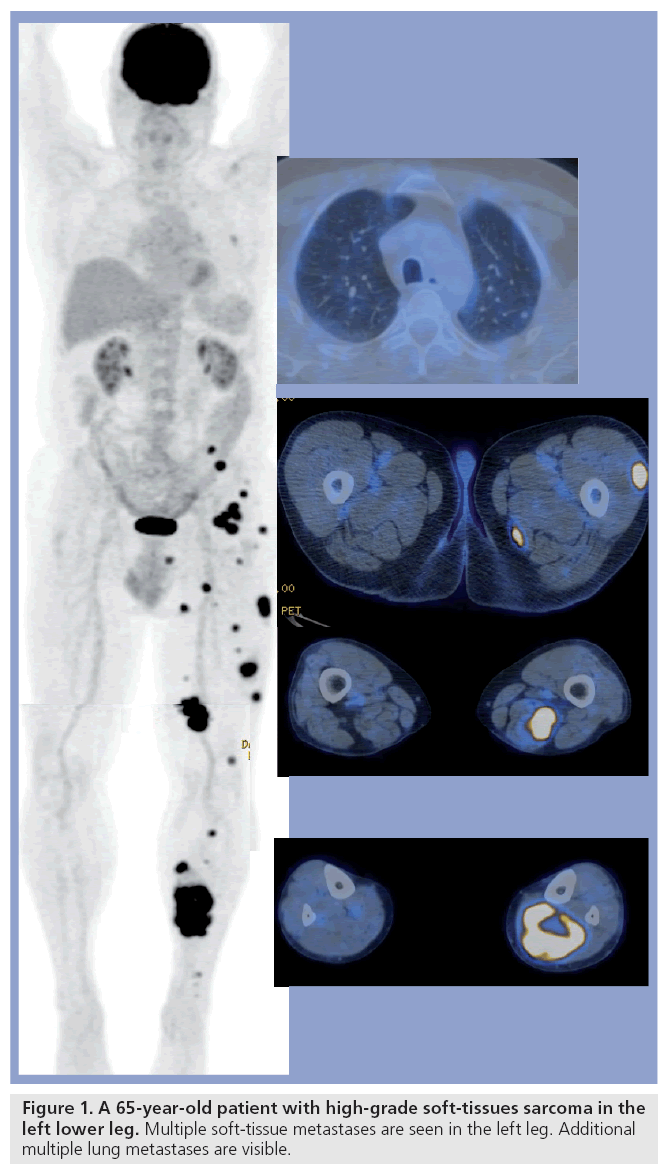
Currently, the detection of pulmonary metastases in STS is based on CT. Several studies have clearly shown that FDG-PET alone is not sensitive enough for the detection of lung metastases because PET alone often fails to show FDG uptake in lung metastases smaller than 6 mm. Respiratory gating is a promising new technique used in PET imaging to overcome this limitation but the implementation of respiratory gating into routine PET protocols is time consuming. In a study by Iagaru et al., PET alone detected pulmonary metastases with a sensitivity of 68% and a specificity of 98% compared with a sensitivity and specificity of 95 and 92%, respectively, with CT [24]. Völker et al. confirmed the insufficient sensitivity of PET alone in the detection of pulmonary metastases (25%) [21]. This limitation of PET can easily be overcome by including thin-slice full inspiration diagnostic CT in the PET/CT protocol. This protocol combines the metabolic information of PET with the high sensitivity of CT and might be the best imaging combination for the detection of pulmonary metastases. With this combined protocol, an additional lung CT examination on a standalone CT scanner becomes superfluous. By using this combined approach, PET/CT was shown to be more accurate (93%) in determining the M stage of sarcomas in more recent studies [25]. In our experience PET/CT is also very efficient in the diagnosis of the extension of soft-tissue metastases in STS, as shown in Figure 1.
In general, PET/CT seems to be superior compared with conventional imaging in determining the M stage of STS.
Therapy response assessment of STS with FDG-PET/CT
At present, many STS patients, especially those with large intermediate- and high-grade tumors, are treated with neoadjuvant chemotherapy or radiotherapy prior to resection. Accurate noninvasive assessment of therapy response would be of great value in STS to guide therapeutic decisions and to avoid ineffective chemo- or radiation therapy. It has been shown that RECIST criteria for solid tumors are not accurate enough for sarcoma treatment response assessment [26–28]. Often the size of STS does not decrease with cytotoxic therapy because many of these tumors contain structural elements preventing tumor shrinkage. Several studies have shown that metabolic imaging with FDG-PET/CT is superior to morphologic imaging such as MRI or CT in therapy response assessment in STS. Evilevitch et al. investigated 42 patients with biopsy-proven resectable high-grade STS before and after neoadjuvant chemotherapy and found that quantitative FDG-PET is significantly more accurate than size-based criteria at assessing histopathologic response, which is usually defined as 95% or more tumor necrosis. Using a 60% decrease of SUV as a threshold, the sensitivity for PET was 100% and the specificity was 71% [29]. Benz et al. found similar results in 20 patients with high-grade STS. They compared different PET measurements with CT volume before and after neoadjuvant chemotherapy. They found tumor response was well predicted by changes in SUV mean and maximum (area under the ROC: 1.0; 0.98), followed by total lesion glycolysis [TLG]) mean (AUC 0.77) and TLGmax (AUC 0.74). By contrast, changes in CTvol did not allow prediction of treatment response (AUC = 0.48) [30]. In another study the same group evaluated the interobserver variability of PET parameters for therapy response assessment in high-grade STS and found that SUVmax provides the most robust measurement [31]. Benz et al. proposed a SUVmax reduction of 35% at early follow-up (after one cycle neoadjuant chemotherapy) as a sensitive (100%) predictor of histopathologic tumor response [32]. Kasper et al. recently confirmed the value of FDG-PET in early response assessment after one cycle of neoadjuvant chemotherapy in STS [33,34]. There is no general agreement how metablic response should be defined, but most authors use a reduction of SUVmax of more than 40–60% as response criteria. There is a clear need to modernize the response criteria for clinical trials in sarcoma treatment [35]. In a recently published article, Wahl et al. proposes the replacement of RECIST criteria based on morphologic imaging alone by PERCIST criteria, which combines metabolic parameters such as decrease of SUV and morphologic parameters (e.g., tumor size reduction) [36]. There are pitfalls in the interpretation of FDG-PET images after neoadjuvant treatment of STS: a peripheral rim of FDG uptake is often seen after neoadjuvant treatment of STS, even when complete histopathological response was achieved. The rim uptake seems to correspond to an inflammatory pseudocapsule around the former tumor. This can lead to false-positive PET results by as the rim uptake is interpreted as tumor persistence.
Surveillance of STS patients with FDG-PET/CT
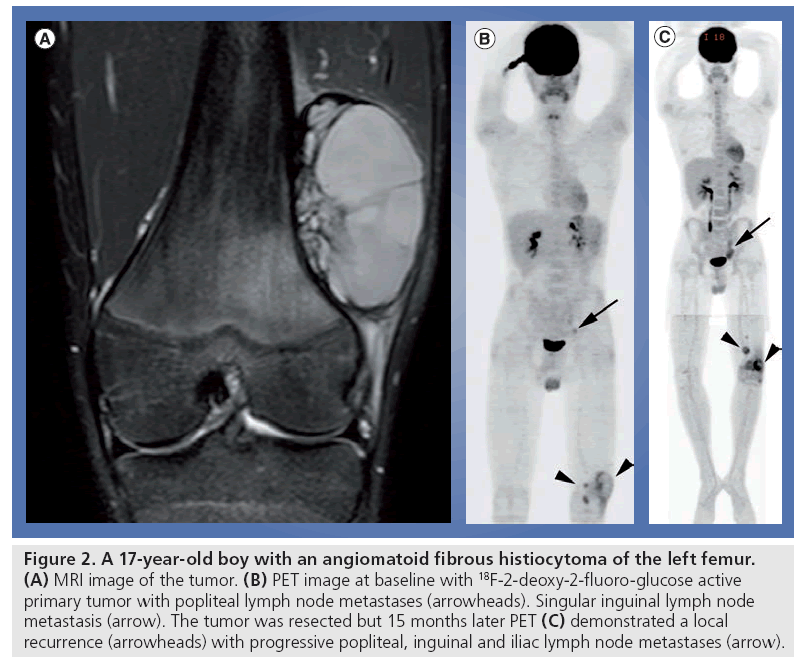
It is often difficult to detect a local recurrence of STS owing to alterations of the normal anatomy by previous interventions such as surgery or radiotherapy. The limited number of studies regarding this topic show a high sensitivity of PET for the detection of local recurrence in high-grade STS [37]. Schwarzbach reported a sensitivity of 88% and specificity of 92% for FDG-PET in the detection of local STS recurrence [38]. Kole et al. confirmed this result by showing a high sensitivity of 93% for the detection of a local recurrence [39]. In both studies the missed recurrences were low-grade liposarcomas. Of course, detection of recurrences of low-grade tumors such as low-grade liposarcoma cannot be expected from FDG-PET. Despite the potential use of FDG-PET/CT in the detection of STS recurrence, the implementation of FDG-PET into the follow-up strategy of STS patients is not yet well defined (Figure 2).
Figure 2: A 17-year-old boy with an angiomatoid fibrous histiocytoma of the left femur. (A) MRI image of the tumor. (B) PET image at baseline with 18F-2-deoxy-2-fluoro-glucose active primary tumor with popliteal lymph node metastases (arrowheads). Singular inguinal lymph node metastasis (arrow). The tumor was resected but 15 months later PET (C) demonstrated a local recurrence (arrowheads) with progressive popliteal, inguinal and iliac lymph node metastases (arrow).
PET/CT imaging of primary bone tumors
General considerations
Bone sarcomas (BS) are rare and account for approximately 0.2% of adult cancers and approximately 5% of childhood malignancies [40]. Typically, children more commonly present with osteosarcomas and Ewing sarcomas, while adults often present with osteosarcomas and chondrosarcomas. Owing to new limb salvage surgical procedures, excellent functional results have been achieved in the last few years [41]. The majority of musculoskeletal tumors arise in the extremities. The lower limbs are more often affected than the upper limbs. Males are generally more often affected than females. Some lesions may be detected incidentally on imaging studies. Generally, younger patients have overall better outcomes than adults with bone and cartilage tumors.
At the initial staging, 15–20% of patients with osteosarcoma also present with pulmonary metastases; 10–15% of patients with sarcomas also develop a local recurrence and 35–45% a distant recurrence, even after adequate therapy. Detection of local recurrence is challenging for imaging because of the severely altered anatomy due to previous interventions such as surgery and radiation therapy.
Differentiation between benign & malignant bone tumors with PET/CT
The differentiation of benign and malignant intraosseous lesions can often be accomplished by conventional x-rays alone. CT can provide important additional information regarding the osseous extention, periostal reactions and cortical destruction of a tumor. MRI is very useful for the detection of bone marrow abnormalities and soft-tissue tumor parts. However, despite the use of these imaging modalities, the appearance of some lesions is nonspecific.
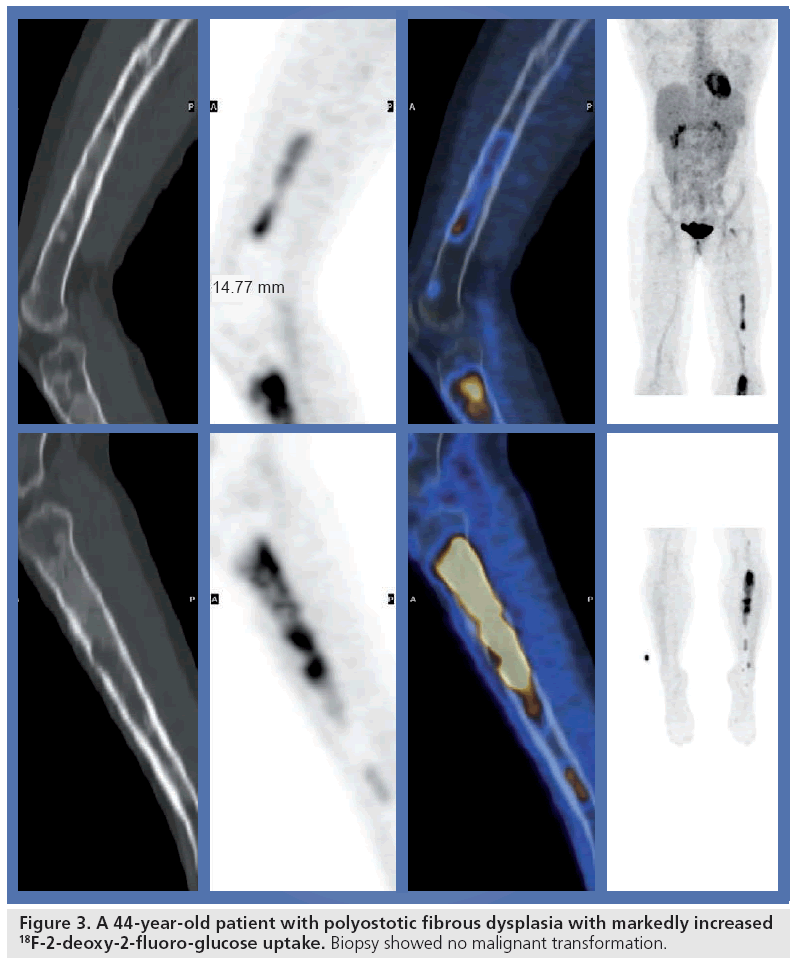
As with soft-tissue tumors, several studies report a good correlation between FDG uptake and the nature of bone tumors (BTs). Aoki et al. investigated 52 bone lesions and found a significant difference in SUVmax between benign and malignant lesions [42]. Strobel et al. showed that correct interpretation of the CT part of the PET/CT study significantly improves the performance of FDG-PET/CT in the differentiation of benign and malignant primary bone lesions compared with PET alone in a collection of 17 benign and 33 malignant lesions [43]. Median SUVmax was 3.5 for benign lesions (range: 1.6–8.0) and 5.7 (range: 0.8–41.7) for malignant lesions. In all studies there was an overlap in FDG activity between benign and malignant BTs. In particular, fibrous dysplasia (Figure 3), giant cell tumors and osteomyelitis can show markedly increased FDG uptake and can lead to misinterpretations of PET studies [44,45]. FDG-PET is usually performed 1 h after intravenous FDG injection. Several studies showed that delayed PET or dual time point PET might help in differentiating benign from malignant lesions [46,47]. Whether this approach is transferable to a busy clinical routine schedule like in our and many other PET centers is questionable.
Figure 3: A 44-year-old patient with polyostotic fibrous dysplasia with markedly increased 18F-2-deoxy-2-fluoro-glucose uptake. Biopsy showed no malignant transformation.
Transformation of benign bone lesions into malignant disease is rare but has been described, for example in fibrous dysplasia, osteochondroma and Paget’s disease. Although the data are mainly based on case reports, FDG-PET/ CT seems to be a promising tool to diagnose malignant transformation by showing areas of markedly increased uptake corresponding to the transformed higher grade tumor parts [48].
Grading of BTs with FDG-PET/CT
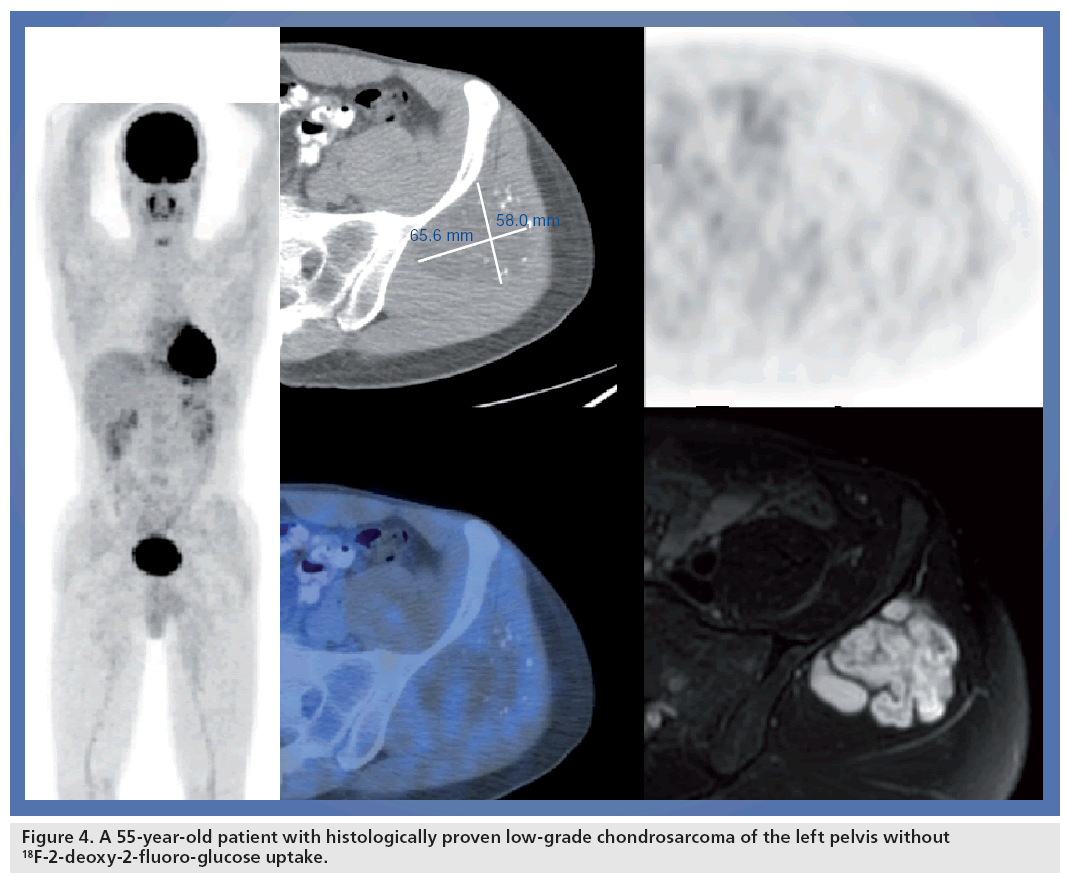
As in STS, correct grading of BS is crucial for therapeutic decision-making. Several studies since 1988 have reported a higher FDG uptake in high-grade tumors compared with low-grade tumors (Figure 4) [49,50]. Folpe et al. showed a correlation of PET SUV with the histopathologic grade in 89 BS and STS and concluded that PET may help to ensure accurate grading and prognostication in sarcoma by guiding the biopsy to the most biologically significant region. Additionally, a correlation was found between FDG uptake and other important parameters such as tumor cellularity, Ki-67 labeling, mitotic activity and overexpression of p53 [16]. The prognostic value of baseline FDG uptake in BS was demonstrated by Eary et al. In their study baseline SUVmax was an independent and significant predictor of overall survival in 209 patients with different types of sarcomas [51].
The use of PET in different types of sarcomas was evaluated. A study of 31 patients with chondrosarcoma showed that PET has prognostic impact by showing significantly higher SUVs in patients with metastases and recurrent disease [52].
It has been shown that BS can be very heterogeneous with areas of high and low FDG activity. This information is not provided by morphologic imaging and might be very important in avoiding false-negative tumor biopsies. Despite these promising results in noninvasive grading, PET cannot replace biopsy before treatment decisions are made.
Staging of BTs with FDG-PET/CT
Bone sarcomas tend to spread hematogeneously and the lungs are at greatest risk for distant metastases. Like in STS, sensitive evaluation of the lung parenchyma needs a thin-sliced CT. PET alone is not sensitive enough for the detection of small lung metastases owing to respiratory movements of the lungs during the PET acquisition [53]. Other reasons are that FDG-negative lung metastases can be small in size and have decreased FDG avidity. This is the reason why the PET/CT protocol should include a diagnostic lung CT in BS patients.
Several studies have shown that PET/CT is superior to conventional imaging including CT regarding accurate staging of sarcomas [21,54].
Additionally, it has recently been demonstrated that PET/CT is significantly superior to PET alone for the detection and localization of lesions in Ewing tumor patients [55]. By adding information from conventional imaging to the PET/CT findings a correct staging could be achieved in 60 of 69 sarcoma patients (87%) with a trend to overstage the patients (12% overstaging; 1% understaging). PET/CT combined with conventional imaging was correct in 97% of the patients regarding N stage and in 93% regarding M stage [25,56]. It has to be emphasized that MRI is still the best method to show the local extension of the primary BT in relation to other important structures such as vessels and nerves. There is an important difference regarding the performance of PET in the detection of osseous metastases: results of studies with limited patient numbers show that FDG-PET is superior to conventional bone scintigraphy regarding the detection of osseous metastases in Ewing sarcoma but inferior to conventional bone scintigraphy in the detection of bone metastases in osteosarcoma [57].
Therapy response assessment of BTs with FDG-PET/CT
Response to preoperative neoadjuvant chemotherapy is a very important prognostic factor in BS, especially in osteosarcoma. It has been shown that chemotherapy-induced tumor cell necrosis in the resected tumor is highly correlated with survival [58]. Effective noninvasive therapy response assessment with imaging is of great importance for prognostic stratification and avoiding negative side effects and saving costs from ineffective chemotherapy. It has been shown in many other malignancies that biochemical changes occur much earlier than morphologic changes during treatment of tumors (Figure 5). For this reason PET is increasingly used for early therapy response assessment in many malignancies such as lymphoma, gastrointestinal stroma tumor, esophageal cancer and others [59,60]. Conventional radiographs and MRI are not useful in predicting histopathological response to neoadjuvant chemotherapy in patients with osteosarcoma [61–63]. There are several studies that have examined the performance of PET in neaoadjuvant chemotherapy assessment in osteosarcoma patients, and different parameters for assessing responders have been evaluated: SUVmax after therapy, SUV change ratio, volume change ratio and metabolic volume change ratio. In a recently published study, Cheon et al. showed that SUVmax after therapy (AUC = 0.89; p = 0.00094), SUV change ratio (AUC = 0.84; p = 0.0083) and metabolic volume change ratio (AUC = 0.89; p = 0.00074) were reliable parameters for therapy response assessment in 70 patients with high-grade osteosarcoma. According to their model, they found predictive values of 97 and 95% for good and poor responders, respectively [64]. Five other studies showed very promising results in a smaller number of patients (n = 10–27) using different criteria for response assessment (SUV change ratio between 0.4 and 0.7) with predictive values for responders between 75 and 100% and for nonresponders between 86 and 100% [65–69]. These studies are limited by the small number of patients with poor response. Some of the studies failed to distinguish a good response (>90% histologic necrosis) from an unfavorable one. Inflammatory changes and FDG uptake into fibrotic tissue might lead to false-positive PET results and lead to underestimation of therapy response. Costelloe et al. recently confirmed in 31 osteosarcoma patients that a SUV greater than 15 before and greater than 5 after chemotherapy, and an increase of TLG after chemotherapy are associated with poorer progression-free survival. High SUVmax after chemotherapy was associated with poor overall survival, while a decrease in SUVmax was strongly associated with histopathologic tumor necrosis of more than 90% [70].
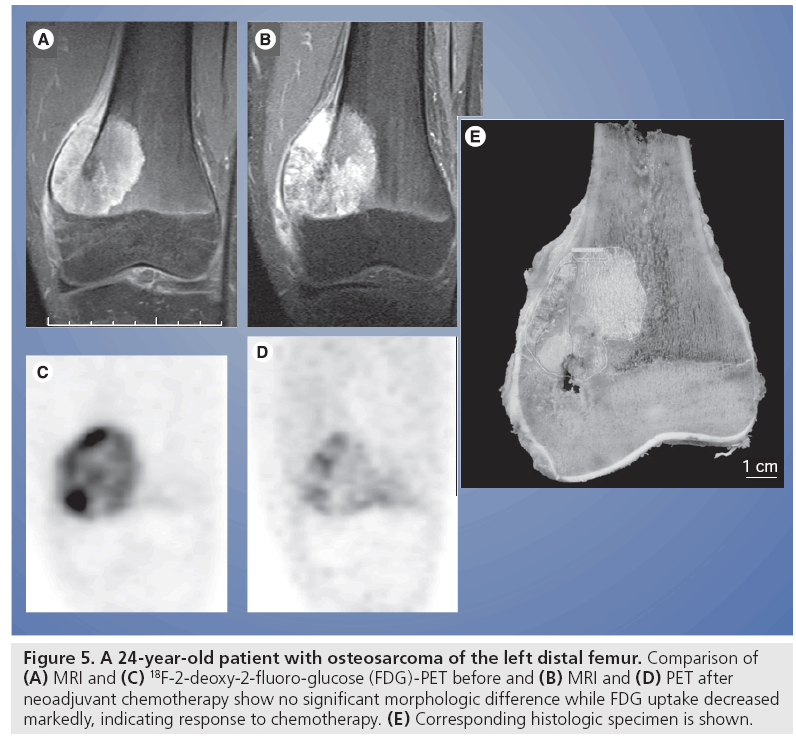
Figure 5: A 24-year-old patient with osteosarcoma of the left distal femur. Comparison of (A) MRI and (C) 18F-2-deoxy-2-fluoro-glucose (FDG)-PET before and (B) MRI and (D) PET after neoadjuvant chemotherapy show no significant morphologic difference while FDG uptake decreased markedly, indicating response to chemotherapy. (E) Corresponding histologic specimen is shown.
Surveillance of BT patients with FDG-PET/CT
Detection of BS recurrence is challenging owing to the anatomic alterations caused by prior treatment. Metallic prosthesis used in limb salvage therapy can cause beam-hardening artifacts in CT and susceptibility artifacts in MRI. Literature regarding the performance of PET for the detection of BS recurrence is very limited. An older review considered FDG-PET to be more accurate than CT or MRI [71]. Many studies regarding recurrence have a mixed population of STS and BS [37,72,73]. Franzius et al., in their study, detected all six osteosarcoma recurrences with PET and reported one false-positive result.
By contrast, MRI also detected all recurrences but showed two false positives. Other authors have reported better performance of MRI (sensitivity 88%) compared with PET (sensitivity 74%) [15].
Conclusion
Proper selection of the many different available imaging modalities for the evaluation of malignant musculoskeletal tumors is crucial for their successful diagnosis and treatment. MRI is the most important ‘second-step’ imaging tool if x-rays or ultrasound show suspected BS or STS. MRI can help to assess dignity and extension of the tumor and can accurately guide biopsy. CT and bone scintigraphy may be helpful in some selected cases. The role of PET/CT is not yet well defined but it can provide important information regarding biopsy guidance, staging and, especially, therapy response assessment. The PET/CT imaging protocol should include a diagnostic thin-slice lung CT in sarcoma patients for the detection of small lung metastases. FDG-PET seems to be superior to conventional imaging in the detection of local recurrence.
Future perspective
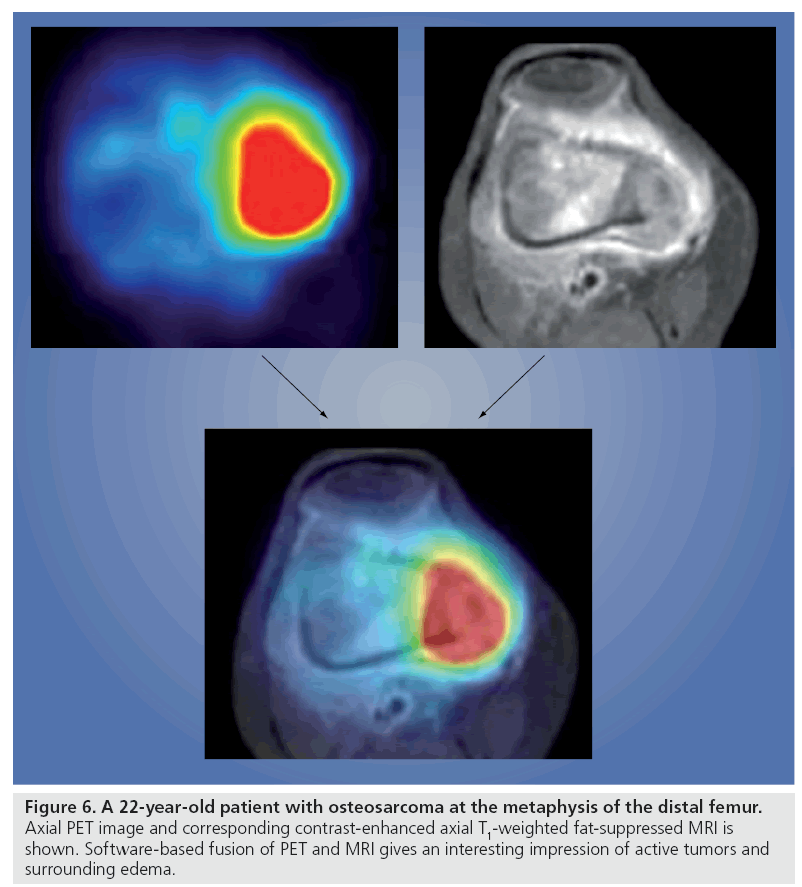
Coregistered PET/MRI might be able to combine the aforementioned advantages of MRI with those of PET/CT; however, this has to be evaluated in further prospective studies if combined scanners are available (Figure 6). A logistical and time-saving advantage for the patient, and a reduction in radiation burden can be expected from PET/MRI systems. In addition, the development of more specific radiotracers is needed to improve the performance of PET in imaging musculoskeletal tumors. Currently, there is no imaging modality that can replace the biopsy.
Figure 6: A 22-year-old patient with osteosarcoma at the metaphysis of the distal femur. Axial PET image and corresponding contrast-enhanced axial T1-weighted fat-suppressed MRI is shown. Software-based fusion of PET and MRI gives an interesting impression of active tumors and surrounding edema.
Financial & competing interests disclosure
GK von Schulthess has a consulting agreement with GE Health Care and is a minority shareholder of Timaq medical imaging Ltd, Zurich, Switzerland. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Mendenhall WM, Indelicato DJ, Scarborough MT et al.: The management of adult soft tissue sarcomas. Am. J. Clin. Oncol. 32, 436–442 (2009).
- Komdeur R, Hoekstra HJ, van den Berg E et al.: Metastasis in soft tissue sarcomas: prognostic criteria and treatment perspectives. Cancer Metastasis Rev. 21, 167–183 (2002).
- Bastiaannet E, Groen H, Jager PL et al.: The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat. Rev. 30, 83–101 (2004).
- Aoki J, Watanabe H, Shinozaki T et al.: FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal. Radiol. 32, 133–138 (2003).
- Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M et al.: Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J. Nucl. Med. 42, 713–720 (2001).
- Lucas JD, O’Doherty MJ, Cronin BF et al.: Prospective evaluation of soft tissue masses and sarcomas using fluorodeoxyglucose positron emission tomography. Br. J. Surg. 86, 550–556 (1999).
- Nieweg OE, Pruim J, van Ginkel RJ et al.: Fluorine-18-fluorodeoxyglucose PET imaging of soft-tissue sarcoma. J. Nucl. Med. 37, 257–261 (1996).
- Schwarzbach MH, Dimitrakopoulou-Strauss A, Mechtersheimer G et al.: Assessment of soft tissue lesions suspicious for liposarcoma by F18-deoxyglucose (FDG) positron emission tomography (PET). Anticancer Res. 21, 3609–3614 (2001).
- Strobel K: FDG-active rheumathoid nodule mimicking malignant soft tissue tumor. Clin. Nucl. Med. (2009) (Epub ahead of print).
- Shin DS, Shon OJ, Byun SJ, Choi JH, Chun KA, Cho IH: Differentiation between malignant and benign pathologic fractures with F-18-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography. Skeletal. Radiol. 37(5), 415–421 (2008).
- Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH: The clinical efficacy of 18F-FDG-PET/CT in benign and malignant musculoskeletal tumors. Ann. Nucl. Med. 22, 603–609 (2008).
- Ioannidis JP, Lau J: 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J. Nucl. Med. 44, 717–724 (2003).
- Charest M, Hickeson M, Lisbona R, Novales-Diaz JA, Derbekyan V, Turcotte RE: FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur. J. Nucl. Med. Mol. Imaging (2009) (Epub ahead of print).
- Lodge MA, Lucas JD, Marsden PK, Cronin BF, O’Doherty MJ, Smith MA: A PET study of 18FDG uptake in soft tissue masses. Eur. J. Nucl. Med. 26, 22–30 (1999).
- Lucas JD, O’Doherty MJ, Wong JC et al.: Evaluation of fluorodeoxyglucose positron emission tomography in the management of soft-tissue sarcomas. J. Bone Joint Surg. Br. 80, 441–447 (1998).
- Folpe AL, Lyles RH, Sprouse JT, Conrad EU 3rd, Eary JF: (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin. Cancer Res. 6, 1279–1287 (2000).
- Schwarzbach MH, Hinz U, Dimitrakopoulou-Strauss A et al.: Prognostic significance of preoperative [18-F] fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging in patients with resectable soft tissue sarcomas. Ann. Surg. 241, 286–294 (2005).
- Warbey VS, Ferner RE, Dunn JT, Calonje E, O’Doherty MJ: [18F]FDG PET/CT in the diagnosis of malignant peripheral nerve sheath tumours in neurofibromatosis type-1. Eur. J. Nucl. Med. Mol. Imaging 36, 751–757 (2009).
- Cardona S, Schwarzbach M, Hinz U et al.: Evaluation of F18-deoxyglucose positron emission tomography (FDG-PET) to assess the nature of neurogenic tumours. Eur. J. Surg. Oncol. 29, 536–541 (2003).
- Hain SF, O’Doherty MJ, Bingham J, Chinyama C, Smith MA: Can FDG PET be used to successfully direct preoperative biopsy of soft tissue tumours? Nucl. Med. Commun. 24, 1139–1143 (2003).
- Volker T, Denecke T, Steffen I et al.: Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J. Clin. Oncol. 25, 5435–5441 (2007).
- Tateishi U, Hosono A, Makimoto A et al.: Comparative study of FDG PET/CT and conventional imaging in the staging of rhabdomyosarcoma. Ann. Nucl. Med. 23, 155–161 (2009).
- Klem ML, Grewal RK, Wexler LH, Schoder H, Meyers PA, Wolden SL: PET for staging in rhabdomyosarcoma: an evaluation of PET as an adjunct to current staging tools. J. Pediatr. Hematol. Oncol. 29, 9–14 (2007).
- Iagaru A, Chawla S, Menendez L, Conti PS: 18F-FDG PET and PET/CT for detection of pulmonary metastases from musculoskeletal sarcomas. Nucl. Med. Commun. 27, 795–802 (2006).
- Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, Kim EE: Bone and soft-tissue sarcoma: preoperative staging with fluorine 18 fluorodeoxyglucose PET/CT and conventional imaging. Radiology 245, 839–847 (2007).
- Ratain MJ, Eckhardt SG: Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J. Clin. Oncol. 22, 4442–4445 (2004).
- Therasse P, Arbuck SG, Eisenhauer EA et al.: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
- Tsuchida Y, Therasse P: Response evaluation criteria in solid tumors (RECIST): new guidelines. Med. Pediatr. Oncol. 37, 1–3 (2001).
- Evilevitch V, Weber WA, Tap WD et al.: Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin. Cancer Res. 14, 715–720 (2008).
- Benz MR, Allen-Auerbach MS, Eilber FC et al.: Combined assessment of metabolic and volumetric changes for assessment of tumor response in patients with soft-tissue sarcomas. J. Nucl. Med. 49, 1579–1584 (2008).
- Benz MR, Evilevitch V, Allen-Auerbach MS et al.: Treatment monitoring by 18F-FDG PET/CT in patients with sarcomas: interobserver variability of quantitative parameters in treatment-induced changes in histopathologically responding and nonresponding tumors. J. Nucl. Med. 49, 1038–1046 (2008).
- Benz MR, Czernin J, Allen-Auerbach MS et al.: FDG-PET/CT imaging predicts histopathologic treatment responses after the initial cycle of neoadjuvant chemotherapy in high-grade soft-tissue sarcomas. Clin. Cancer Res. 15, 2856–2863 (2009).
- Kasper B, Dietrich S, Dimitrakopoulou- Strauss A et al.: Early prediction of therapy outcome in patients with high-risk soft tissue sarcoma using positron emission tomography. Onkologie 31, 107–112 (2008).
- Kasper B, Schmitt T, Wuchter P, Ho AD, Egerer G: [Positron emission tomography as a tool for early prediction of therapy outcome in soft tissue sarcoma]. Dtsch Med. Wochenschr. 134, 1922–1926 (2009).
- Schuetze SM, Baker LH, Benjamin RS, Canetta R: Selection of response criteria for clinical trials of sarcoma treatment. Oncologist 13 (Suppl. 2), 32–40 (2008).
- Wahl RL, Jacene H, Kasamon Y, Lodge MA: From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 50(Suppl. 1), 122S–150S (2009).
- Arush MW, Israel O, Postovsky S et al.: Positron emission tomography/computed tomography with 18Fluoro-deoxyglucose in the detection of local recurrence and distant metastases of pediatric sarcoma. Pediatr. Blood Cancer 49, 901–905 (2007).
- Schwarzbach MH, Dimitrakopoulou-Strauss A, Willeke F et al.: Clinical value of [18-F] fluorodeoxyglucose positron emission tomography imaging in soft tissue sarcomas. Ann. Surg. 231, 380–386 (2000).
- Kole AC, Nieweg OE, van Ginkel RJ et al.: Detection of local recurrence of soft-tissue sarcoma with positron emission tomography using [18F]fluorodeoxyglucose. Ann. Surg. Oncol. 4, 57–63 (1997).
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ: Cancer statistics, 2009. CA Cancer J. Clin. 59, 225–249 (2009).
- Veth R, van Hoesel R, Pruszczynski M, Hoogenhout J, Schreuder B, Wobbes T: Limb salvage in musculoskeletal oncology. Lancet Oncol. 4, 343–350 (2003).
- Aoki J, Watanabe H, Shinozaki T et al.: FDG PET of primary benign and malignant bone tumors: standardized uptake value in 52 lesions. Radiology 219, 774–777 (2001).
- Strobel K, Exner UE, Stumpe KD et al.: The additional value of CT images interpretation in the differential diagnosis of benign vs. malignant primary bone lesions with 18F-FDG-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 35, 2000–2008 (2008).
- Strobel K, Bode B, Lardinois D, Exner U: PET-positive fibrous dysplasia – a potentially misleading incidental finding in a patient with intimal sarcoma of the pulmonary artery. Skeletal Radiol. 36(Suppl. 1), S24–S28 (2007).
- Strobel K, Hany TF, Exner GU: PET/CT of a brodie abscess. Clin. Nucl. Med. 31, 210 (2006).
- Tian R, Su M, Tian Y et al.: Dual-time point PET/CT with F-18 FDG for the differentiation of malignant and benign bone lesions. Skeletal. Radiol. 38, 451–458 (2009).
- Zhuang H, Pourdehnad M, Lambright ES et al.: Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J. Nucl. Med. 42, 1412–1417 (2001).
- Berrebi O, Steiner C, Keller A, Rougemont AL, Ratib O: F-18 fluorodeoxyglucose (FDG) PET in the diagnosis of malignant transformation of fibrous dysplasia in the pelvic bones. Clin. Nucl. Med. 33, 469–471 (2008).
- Kern KA, Brunetti A, Norton JA et al.: Metabolic imaging of human extremity musculoskeletal tumors by PET. J. Nucl. Med. 29, 181–186 (1988).
- Adler LP, Blair HF, Makley JT et al.: Noninvasive grading of musculoskeletal tumors using PET. J. Nucl. Med. 32, 1508–1512 (1991).
- Eary JF, O’Sullivan F, Powitan Y et al.: Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 29, 1149–1154 (2002).
- Brenner W, Conrad EU, Eary JF: FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur. J. Nucl. Med. Mol. Imaging 31, 189–195 (2004).
- Franzius C, Daldrup-Link HE, Sciuk J et al.: FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann. Oncol. 12, 479–486 (2001).
- Piperkova E, Mikhaeil M, Mousavi A et al.: Impact of PET and CT in PET/CT studies for staging and evaluating treatment response in bone and soft tissue sarcomas. Clin. Nucl. Med. 34, 146–150 (2009).
- Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C: Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J. Nucl. Med. 48, 1932–1939 (2007).
- Tateishi U, Hosono A, Makimoto A et al.: Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. J. Pediatr. Hematol. Oncol. 29, 608–612 (2007).
- Franzius C, Sciuk J, Daldrup-Link HE, Jurgens H, Schober O: FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur. J. Nucl. Med. 27, 1305–1311 (2000).
- Davis AM, Bell RS, Goodwin PJ: Prognostic factors in osteosarcoma: a critical review. J. Clin. Oncol. 12, 423–431 (1994).
- Lordick F, Ott K, Krause BJ et al.: PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON Phase II trial. Lancet Oncol. 8, 797–805 (2007).
- Kasamon YL, Wahl RL, Ziessman HA et al.: Phase II study of risk-adapted therapy of newly diagnosed, aggressive non-Hodgkin lymphoma based on midtreatment FDG-PET scanning. Biol. Blood Marrow Transplant 15, 242–248 (2009).
- Holscher HC, Bloem JL, van der Woude HJ et al.: Can MRI predict the histopathological response in patients with osteosarcoma after the first cycle of chemotherapy? Clin. Radiol. 50, 384–390 (1995).
- Holscher HC, Bloem JL, Vanel D et al.: Osteosarcoma: chemotherapy-induced changes at MR imaging. Radiology 182, 839–844 (1992).
- Holscher HC, Hermans J, Nooy MA, Taminiau AH, Hogendoorn PC, Bloem JL: Can conventional radiographs be used to monitor the effect of neoadjuvant chemotherapy in patients with osteogenic sarcoma? Skeletal Radiol. 25, 19–24 (1996).
- Cheon GJ, Kim MS, Lee JA et al.: Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J. Nucl. Med. 50, 1435–1440 (2009).
- Franzius C, Sciuk J, Brinkschmidt C, Jurgens H, Schober O: Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin. Nucl. Med. 25, 874–881 (2000).
- Hawkins DS, Rajendran JG, Conrad EU 3rd, Bruckner JD, Eary JF: Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-d-glucose positron emission tomography. Cancer 94, 3277–3284 (2002).
- Huang TL, Liu RS, Chen TH, Chen WY, Hsu HC, Hsu YC: Comparison between F-18-FDG positron emission tomography and histology for the assessment of tumor necrosis rates in primary osteosarcoma. J. Chin. Med. Assoc. 69, 372–376 (2006).
- Schulte M, Brecht-Krauss D, Werner M et al.: Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J. Nucl. Med. 40, 1637–1643 (1999).
- Ye Z, Zhu J, Tian M et al.: Response of osteogenic sarcoma to neoadjuvant therapy: evaluated by 18F-FDG-PET. Ann. Nucl. Med. 22, 475–480 (2008).
- Costelloe CM, Macapinlac HA, Madewell JE et al.: 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J. Nucl. Med. 50, 340–347 (2009).
- Abdel-Dayem HM: The role of nuclear medicine in primary bone and soft tissue tumors. Semin. Nucl. Med. 27, 355–363 (1997).
- el-Zeftawy H, Heiba SI, Jana S et al.: Role of repeated F-18 fluorodeoxyglucose imaging in management of patients with bone and soft tissue sarcoma. Cancer Biother. Radiopharm. 16, 37–46 (2001).
- Garcia R, Kim EE, Wong FC et al.: Comparison of fluorine-18-FDG PET and technetium-99m-MIBI SPECT in evaluation of musculoskeletal sarcomas. J. Nucl. Med. 37, 1476–1479 (1996).
• • Meta-analysis of literature regarding PET and soft-tissue sarcomas.
• Important paper regarding grading of sarcomas with 18F-2-deoxy-2-fluoro-glucose (FDG)-PET.
• • Prospective multicenter trial regarding staging of pediatric sarcomas.
• • Important paper regarding interobserver variability and evaluation of different metabolic PET parameters for therapy response assessment.
• Evaluation of FDG uptake in benign and malignant lesions with FDG-PET.
• Showing the superiority of combined PET/CT over PET alone.