Review Article - Interventional Cardiology (2012) Volume 4, Issue 2
Hypothermia and percutaneous coronary intervention during acute myocardial infarction
- Corresponding Author:
- Michael W Dae
University of California San Francisco
185 Berry Street, Suite 350, San Francisco, CA, USA
E-mail: michael.dae@ucsf.edu
Abstract
Keywords
cardioprotection, cell death, endovascular cooling, infarct size, mild hypothermia, myocardial infarction, reperfusion injury
Successful reperfusion is the most definitive michael.dae@ucsf.edu treatment to date for reducing injury to the heart following myocardial infarction (MI). Reperfusion must be instituted in a timely manner in order to salvage ischemic tissue. However, studies have demonstrated that in spite of full restoration of distal coronary perfusion in over 90% of patients treated with percutaneous coronary intervention (PCI), a substantial number of patients fail to re-establish microvascular flow at the tissue level (low or no reflow) [1–3]. Patients with reduced microvascular perfusion following successful PCI have larger infarcts, a higher incidence of malignant arrhythmia, more congestive heart failure and greater mortality [1–3]. Large infarcts still occur in many patients that receive state-of-the-art reperfusion therapy. There remains a clinical need for adjunctive therapies to reduce infarct size following PCI. Numerous studies have shown that mild hypothermia (32– 35°C) may be an effective adjunctive therapy that can be applied during reperfusion therapy for acute MI (AMI) [4,5]. This article will review some of the evidence for mild hypothermia cardioprotection in preclinical studies and relate the findings to recent clinical trials designed to show a reduction in infarct size.
Cell death in MI
Numerous studies have shown a pattern of evolution of injury and cell death during coronary occlusion where necrosis starts in the subendocardium and spreads towards the subepicardium – the wavefront phenomenon [6]. This time course varies according to species, with near maximal amounts of cell death resulting from a duration of ischemia ranging from 60–90 min in rabbits and pigs and up to 3–6 h in primates and humans [7,8]. Among the major determinants of infarct size are: size of the ischemic bed (the area at risk), presence or absence of collateral blood flow, the duration of ischemia and temperature. In patients with AMI, the size of the area at risk and the presence or absence of collateral flow are not modifiable at the time of presentation. This leaves reduction in the duration of ischemia with prompt reperfusion as the major strategy for intervention. The possibility that temperature manipulation is adjunctive to reperfusion holds great promise, as discussed below.
A cascade of events leads to cell death during ischemia and reperfusion. Tissue damage can result from necrosis, apoptosis and autophagy [9]. With the abrupt cessation of coronary flow, there is a gradual depletion of energy in the ischemic myocardium and cellular ATP levels decline at a sustained rate. Opening of the mitochondrial permeability transition pore results in catastrophic depletion of cellular ATP levels below the critical amounts needed for membrane pumps to maintain ionic gradients, the cell swells, membrane integrity is lost and leakage of cellular contents to the extracellular space ensues via necrosis [9]. Consistent with the initial onset of necrosis in the subendocardium, cellular ATP levels decline more rapidly in the subendocardium, prior to declines in the midmyocardial and subepicardial regions [10]. For a more detailed discussion of the mechanisms of cell death, the reader is referred to a recent review [9].
The rates of ATP depletion are much slower in human myocardium than that described in dogs [11]. In patients, ATP decreased to 43% of its initial value after 2 h of coronary occlusion. By contrast, in dogs, ATP dropped to 6% of its normal value after only 40 min of acute coronary occlusion. This difference may be explained in part by better collateral flow in humans with coronary artery disease, as opposed to healthy experimental animals. However, an innate cellular protection is suggested in the hearts of higher species such as primates, where the rate of cell death is slower, in spite of negligible collateral flow [7,12]. The time course of injury described experimentally in animal studies showing the wavefront phenomenon, has been demonstrated in humans. Pooled analysis of infarct size data determined by Tc‑99m sestamibi myocardial SPECT perfusion imaging in several recent clinical trials clearly shows that infarct size increases as the duration of ischemia increases [13].
As opposed to necrosis, apoptosis is an energy requiring process characterized by DNA fragmentation and cellular disintegration, with the breakdown of cells into multiple apoptotic bodies that retain membrane integrity [14]. Early events in these two modes of cell death are similar, and the signals bifurcate later, leading to either apoptosis or necrosis. The determinate of outcome is likely related to the levels of ATP depletion that occurs during ischemia. Although the relative contribution of necrosis to final infarct size is greater, apoptosis does contribute significantly [15]. Execution of both apoptotic and necrotic modes of cell death depends upon many signaling events, such as the generation of reactive oxygen species (ROS), activation of stress kinases, release of cytochrome c, induction of heat shock proteins, and ultimately, depolarization of mitochondria and opening of the mitochondrial permeability transition pore [16]. The mitochondrion is central to the fate of the ischemic myocyte. Recent studies suggest that alteration in mitochondrial dynamics or clearance of mitochondria by autophagy play a role in myocyte cell death [17]. Inflammatory processes occur after reperfusion and may also play a role in subsequent cell death in myocytes and in the microvasculature [18,19].
Mechanisms of injury occur both during the ischemic period and following reperfusion. The concept of ‘reperfusion injury’ has been debated for many years and few studies have convincingly confirmed its existence. However, there is general agreement that myocytes are conditioned by ischemia for a deleterious response to reperfusion [20]. The main support for reperfusion related injury comes from the institution of therapies during the reperfusion period that reduce infarct size. Of particular note are recent studies whereby controlled rates of reperfusion (postconditioning) resulted in decreased infarct size in a number of species, including man [21,22].
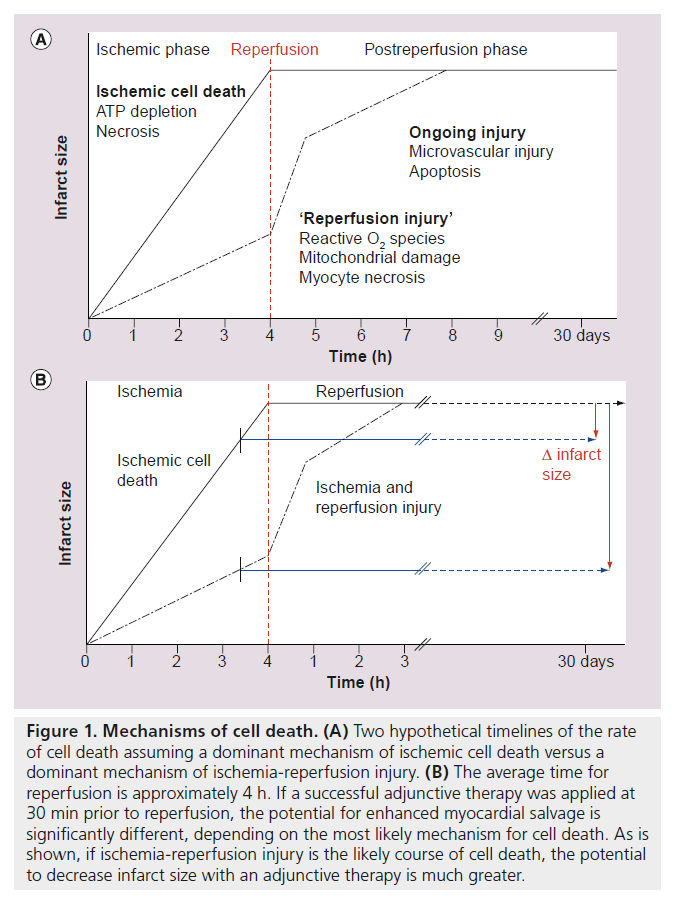
The microvasculature seems particularly prone to ongoing injury during reperfusion and evidence of expansion of the no-reflow zone during reperfusion has been reported [23]. A consequence of expanded microvascular injury after reperfusion, is continued ischemia and ongoing myocyte cell death. Figure 1 shows a schematic that describes two hypothetical rates of cell death during acute coronary occlusion: a more rapid rate for ischemic cell death and a slower rate for ischemia/reperfusion injury. As shown in Figure 1A, if ischemic cell death is the dominant or sole mechanism for eventual necrosis, there is little practical opportunity for an intervention that is timed just prior to reperfusion to have a significant impact on infarct size. As described by Stone et al., the average time from symptom onset to reperfusion by PCI is approximately 4 h [13]. Intervention timed just prior to PCI could potentially arrest cell death, but most of the damage will have been done. The hypothesis that ischemic cell death is the dominant or sole mechanism that determines final infarct size is not likely, however. As discussed below, evidence is accumulating indicating that therapies can protect a significant amount of myocardium when initiated prior to reperfusion. Thus, the pathway of ischemia plus reperfusion injury, as shown in Figure 1B, is a more likely scenario, where ample opportunity to reduce infarct size remains, if effective therapy can be administered prior to reperfusion.
Figure 1. Mechanisms of cell death. (A) Two hypothetical timelines of the rate of cell death assuming a dominant mechanism of ischemic cell death versus a dominant mechanism of ischemia-reperfusion injury. (B) The average time for reperfusion is approximately 4 h. If a successful adjunctive therapy was applied at 30 min prior to reperfusion, the potential for enhanced myocardial salvage is significantly different, depending on the most likely mechanism for cell death. As is shown, if ischemia-reperfusion injury is the likely course of cell death, the potential to decrease infarct size with an adjunctive therapy is much greater.
Mechanisms of protection with hypothermia
The following discussion pertains to the use of mild hypothermia, as opposed to 4°C (deep hypothermia) and cardioplegia. Hypothermia has been shown to have effects on many of the mechanisms related to cell death discussed above, including: ATP depletion, ROS generation, apoptosis and inf lammation. The mechanisms underlying cell protection with hypothermia are likely to be multifactorial. One consequence of hypothermia is a reduction in metabolic rate. Jones et al. showed in the globally ischemic dog heart that decrements in temperature from 40–28°C slowed the rate of cellular ATP depletion [24]. As noted above, ATP depletion during ischemia is fundamentally linked to cellular necrosis. The preservation of cellular ATP likely delays the initiation of irreversible biochemical cascades, thus preserving cell viability for longer periods of time. The clinical consequence may be an extension of the treatment window for salvaging myocardium. Hypothermia induces bradycardia, however, experimental studies where the heart rate was maintained at baseline levels still demonstrated cardioprotection with hypothermia. Thus, the effects are not likely related to changes in hemodynamics.
The level of mitochondrial calcium and ROS formation during continued ischemia determine the degree of ischemia/reperfusion injury. The role of ROS in effecting cardiac injury during reperfusion following ischemia is well established. Studies in intact myocardium show that during ischemia, hypothermia has a direct effect on mitochondria to preserve mitochondrial energy balance, decrease ROS formation and prevent Ca2+ overload [25].
Ning et al. showed that hypothermia protected mitochondria and enhanced recovery of function in isolated perfused rabbit hearts [26]. Heat shock protein, a cardioprotective agent, was also induced by hypothermia. Thus, the actions of hypothermia to preserve ATP are likely to be more than just the inhibition of metabolic rate and ATP consumption, but also related to direct protective effects on mitochondrial structure and function. Recent studies in a murine cardiomyocyte model of ischemiareperfusion inury showed that hypothermia cardioprotection is mediated by enhanced Akt phosphorylation and enhanced nitric oxide generation, resulting in attenuation of ROS generation and decreased cell death during reperfusion [27]. In mice deficient in Akt, hypothermia failed to confer cardioprotection [28]. Recent studies in isolated rabbit myocardium showed that hypothermia conferred cardioprotection via activation of ERK, a member of the RISK family of protective kinases [29].
Further mechanisms of protection by hypothermia relate to inhibition of apoptosis and inflammation. Ning et al. showed that a relatively mild degree of hypothermia modified postischemic gene expression for several proteins that contribute to the regulation of apoptosis in rabbit myocardium [30]. The expression of the proapoptotic protein, bak, was inhibited by hypothermia, while the the antiapoptotic protein, Bcl‑x, was accentuated. It is well established that hypothermia inhibits the migration and infiltration of inflammatory cells [31,32]. Inflammation and thrombosis are likely important and synergistic mechanisms leading to microcirculatory dysfunction in ischemic disorders. It has long been appreciated that hypothermia inhibits the coagulation cascade [33] and inhibits platelet activation and aggregation [34]. The mechanisms of hypothermia protection are summarized in the Box 1.
Box 1. The various mechanisms of protection with mild hypothermia are listed and grouped by likelihood of playing a role during the ischemic phase versus the reperfusion phase.
Effects of hypothermia on infarct size
▪ Hypothermia before ischemia
Mild hypothermia, or lowering of body temperature, is a strategy that is used successfully throughout nature to protect organisms during periods of metabolic deprivation. When exposed to hypoxia, rodents will actively seek lower ambient temperatures in order to reduce their core body temperature [35]. Cold seeking behavior and induced hypothermia is used as a means to enhance survival in many species [36]. The underlying principle is fundamental: metabolism is reduced at lower temperatures, thereby preserving precious energy, hopefully long enough for the return of more favorable conditions for survival.
Hypothermia has been used for myocardial protection during coronary artery bypass surgery for decades. A number of experimental studies have shown a reduction in myocardial infarct size when hypothermia is induced during the ischemic period either before or after coronary occlusion. Dunker et al. showed a linear relationship between core body temperature and infarct size in pigs made hypothermic prior to coronary occlusion [37]. Reductions in infarct size by mild hypothermia maintained throughout ischemia were reported by Chien et al. in rabbits [38], and Hamamoto et al. in sheep [39]. Thus the findings are not species specific. Of course, initiation of hypothermia prior to development of ischemia is not a practical option for therapy in patients with MI.
▪ Hypothermia during ischemia before reperfusion
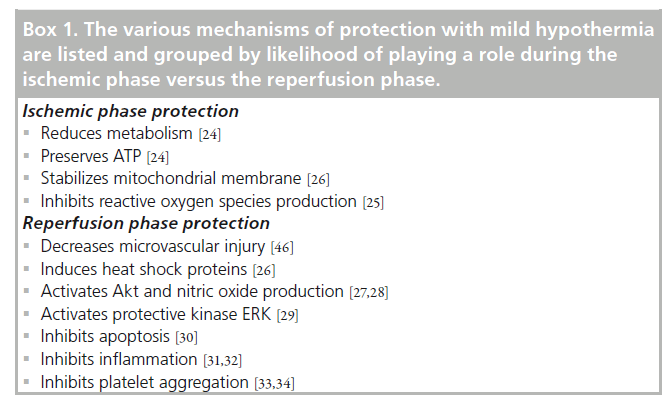
Numerous experimental studies have examined the effects of hypothermia administered after the onset of coronary artery occlusion on myocardial infarct size [40–42]. Several themes have emerged from preclinical studies concerning the depth and duration of hypothermia needed to reduce infarct size. In a study by Miki et al., rabbits underwent 30 min of coronary artery occlusion, and were divided into different groups (Figure 2) [40]. In the hypothermia animals, cooling from 38–35°C was instituted at the onset of occlusion (35/0), 10 min following occlusion (35/10), or 20 min following the onset of occlusion (35/20). Significant reductions in infarct size occurred with early onset, and 10‑min onset occlusion, but not in late onset cooling at 20 min of a 30‑min ischemic period. Thus, at 35°C, cooling over approximately two-thirds of the duration of ischemia was necessary for reduction of infarct size. When myocardial temperature was lowered to 32°C, starting 20 min after coronary occlusion, a significant reduction in infarct size occurred. At this lower temperature, a reduction in infarct size occurred with cooling during the latter one-third of the duration of the ischemic period. Hale et al. studied even later initiation of cooling in a rabbit model, and found that cooling to 32°C initiated at 25 min of a 30‑min occlusion period, did not significantly reduce infarct size [41]. Cellular models with isolated cardiomyocytes have provided further clues to the timing of hypothermia induced cardio-protection [43]. Hypothermia induced at the onset of reperfusion in this cell model reduced cell death. However, when hypothermia was delayed for 15 min after reperfusion, the protection was lost. The best protection occurred when hypothermia was induced prior to reperfusion, even at the expense of prolonging the duration of ischemia. These in vitro results are consistent with hypothermia protection against reperfusion injury. A noteworthy feature of these preclinical studies is that myocardial temperature was lowered to target levels prior to reperfusion. More recent animal studies have confirmed that hypothermia started during ischemia, but not during reperfusion, results in a decrease in infarct size [44]. Gotberg et al. showed that late onset cooling, 5 min prior to reperfusion in pigs, resulted in a modest 18% reduction in infarct size [45]. Hence, the ability for hypothermia to protect against reperfusion injury, per se, is still debated [46]. Overall, however, these studies suggest that: earlier onset of cooling is better; for delayed onset, a colder temperature is better; and there may be a minimum duration of hypothermia required prior to the onset of reperfusion to achieve a reduction in infarct size.
Figure 2. Infarct size normalized as a percentage of the risk zone is plotted for the groups experiencing 30 min of regional ischemia. Open and closed circles indicate individual experiments and group means, respectively. Note that the protection observed depends both on the temperature and the duration of cooling.
*p < 0.01 versus control.
Reproduced with permission from [40].
Prior studies have shown that injury to the microvasculature (no reflow) progresses during the reperfusion period [23]. Hale et al. recently studied the effects of late onset cooling followed by sustained hypothermia for 2 h after reperfusion on microvascular ‘no reflow’ in a rabbit model [47]. In hypothermic hearts, regional myocardial blood flow after 3 h of reperfusion was significantly higher; relative reflow to the previously ischemic region was significantly higher; and infarct size was reduced. The improvement in reflow was not due solely to a reduction in necrosis. Their data showed that for any given amount of necrosis, the extent of no reflow was less with hypothermia treatment. This was the first study to show a dissociation between a reduction in necrosis and improved reflow, and suggests that hypothermia during reperfusion decreases microvascular reperfusion injury. Reduced injury to the microvasculature may improve healing postinfarction, reduce remodeling of the heart after MI, as well as provide a conduit for delivery of regenerative substances such as stem cells. Other potential benefits of hypothermia include improvement in postischemic contractile dysfunction and hemodynamics [48,49].
Clinical trials of hypothermia during MI
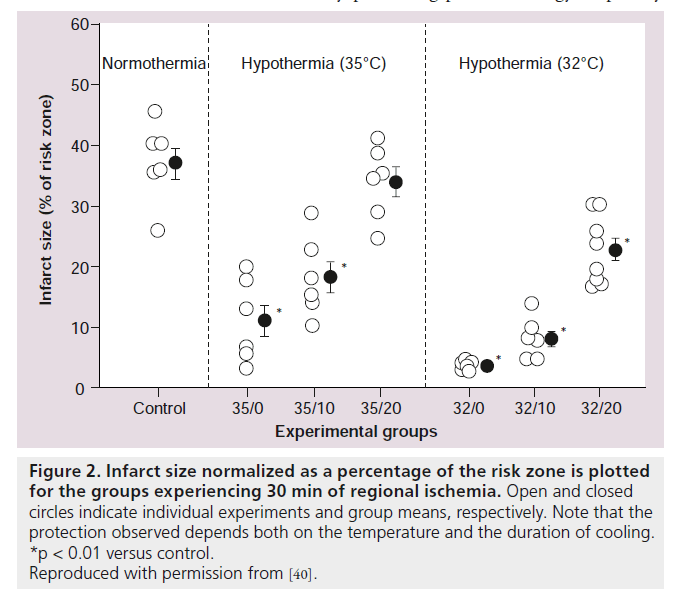
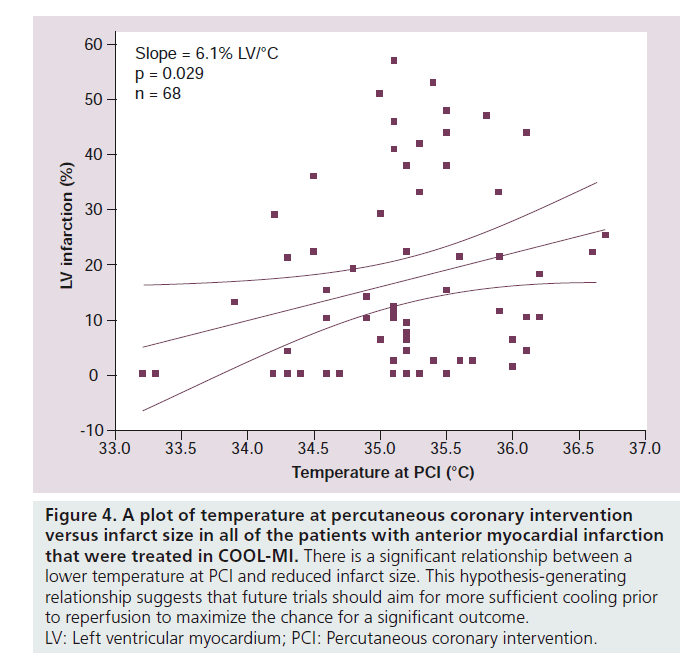
Two major clinical trials investigated hypothermia using endovascular cooling catheters (COOL‑MI and ICE‑IT) as adjunctive therapy to PCI for treatment of AMI [50,51]. In COOL‑MI, 392 patients with anterior or inferior MI less than 6 h duration were randomized to cooling versus control. Patients were cooled for 3 h followed by rewarming at 1°C per h. In ICE-IT, 228 patients were enrolled and randomized to either cooling or control groups. Patients were cooled for 6 h in ICE-IT, followed by rewarming. The target temperature was 33°C in both trials, and shivering was successfully controlled with p.o. buspirone and iv. meperidine. The primary outcome was infarct size measured by SPECT imaging at 30 days. Both studies failed to show an overall reduction in infarct size. Significant differences between the COOL‑MI trial and the preclinical study [42] that used the same endovascular cooling technique were: the animals were cooled sooner during the ischemic period; and the animals were reperfused at target temperature (4°C D compared with controls), whereas the patients were on average only 1°C below baseline during reperfusion (Figure 3). Average temperature in COOL‑MI at PCI was 35°C. This attests to the difficulty in cooling the human body very rapidly and is likely an important reason for the failure of the clinical trials to show a decrease in infarct size. Another consideration is that a fast rate of reperfusion therapy in the catheterization laboratory may not allow time for sufficient cooling to develop. The average duration of cooling in the catheterization laboratory, prior to reperfusion, was 18 min in COOL‑MI. Also, inclusion of inferior infarcts with a smaller area at risk, may have diminished the ability to detect a change in infarct size. A scatter plot of temperature at PCI versus infarct size from all of the anterior MI patients that were treated in COOL‑MI is shown in Figure 4. A correlation between lower temperature at PCI and smaller infarct size is evident. Post‑hoc analysis of the data in the COOL-MI trial showed that patients with anterior MI that were cooled to less than 35°C at the time of reperfusion had smaller infarcts. Similar results were obtained in the ICE-IT trial. These findings are supported by a recent pilot clinical trial (Rapid-MI‑ICE), where cooling was instituted with a bolus infusion of cold saline, followed by endovascular cooling, resulting in a reduction of core body temperature of <35°C (mean 34.7) prior to reperfusion in all of the test patients [52]. An additional difference from the earlier clinical trials is that infarct size was compared with the area at risk, measured concurrently using MRI [52]. Infarct size normalized to myocardium at risk was reduced by 38% in the hypothermia group compared with the control group (29.8 ± 12.6% vs 48.0 ± 21.6%, p = 0.041, n = 8 patients per group). This was supported by a significant decrease in cumulative release of troponin T (p = 0.03). These preliminary results are encouraging and support moving forward with a larger clinical trial, currently recruiting, CHILL‑MI (ClinicalTrials.gov Identifier: NCT01379261 [101]). In all of the hypothermia clinical trials to date, shivering in the awake patient was effectively controlled with administration of iv. meperidine and p.o. buspirone [53]. Further, there has been no reported increased incidence of arrhythmia or other adverse events.
Figure 3. Temperature versus time for cooling and percutaneous coronary intervention. (A) Temperature curves from [42] where endovascular cooling was performed in human-sized animals to reduce infarct size. Of note is the fact that reperfusion was done at target temperature (34°C) in the treatment group compared with 38°C in the control group. (B) A summary temperature curve from the COOL-MI trial, where the same endovascular cooling technique was used as in the preclinical study. The average temperature at reperfusion was 35°C, only 1°C from baseline. Patients received an average of 18 min of cooling in the catheterization laboratory prior to reperfusion. Target temperature (33°C) was reached in the majority of patients, but required approximately 75 min to be reached. Thus, the hypothermia ‘dose’ was much less in the clinical trial compared with the preclinical study.
LA: Left atrial blood pool; LV: Left ventricular myocardium; PCI: Percutaneous coronary intervention.
Figure 4. A plot of temperature at percutaneous coronary intervention versus infarct size in all of the patients with anterior myocardial infarction that were treated in COOL-MI. There is a significant relationship between a lower temperature at PCI and reduced infarct size. This hypothesis-generating relationship suggests that future trials should aim for more sufficient cooling prior to reperfusion to maximize the chance for a significant outcome.
LV: Left ventricular myocardium; PCI: Percutaneous coronary intervention.
Future perspective
There are multiple mechanisms whereby hypothermia can provide cell protection. This ‘poly pharmacy’ approach may provide significant advantages over various pharmacologic interventions that are typically aimed at individual specific mechanisms. That hypothermia is cardioprotective in human myocardium is supported by the findings of a reduction in infarct size in clinical trials in patients that were cooled to less than 35°C prior to reperfusion. Experimental studies suggest that the greatest protection will likely occur with cooling to a sufficient depth, initiated during the ischemic period, prior to reperfusion. Future clinical outcomes may be improved by the development of more powerful cooling devices, or devices that allow cooling to be instituted effectively, prior to arrival to the catheterization laboratory. It is anticipated that mild hypothermia will become the standard of care for adjunctive therapy for reperfusion of AMI.
Executive summary
Cell death in myocardial infarction
▪ Major determinants of infarct size are size of the area at risk, collateral blood flow, duration of ischemia and temperature.
▪ Cell death can result from necrosis, apoptosis and autophagy.
▪ Rates of cell death are innately slower in human and primate hearts compared with other species.
▪ The mitochondrion and opening of the mitochondrial permeability transition pore is central to the fate of the ischemic myocyte.
▪ Reperfusion injury contributes a significant fraction of the ultimate size of myocardial infarction.
Mechanisms of protection with hypothermia
▪ Mild hypothermia (32–25°C) has consistently been shown to be cardioprotective in preclinical studies.
▪ Mechanisms of hypothermia protection during ischemia are:
– Reduction in metabolism and preservation of ATP;
– Stabilization of mitochondrial membrane;
– Inhibition of generation of reactive oxygen species.
▪ Mechanisms of hypothermia protection during reperfusion are:
– Decrease in microvascular injury;
– Induction of heat shock proteins;
– Activation of Akt and nitric oxide production;
– Activation of protective kinases ERK;
– Inhibition of apoptosis;
– Inhibition of inflammation and platelet aggregation.
Effects of hypothermia on infarct size
▪ Hypothermia before ischemia:
– Cold-seeking behavior during hypoxia is used to enhance survival in many species;
– There is a linear relationship between core body temperature and infarct size in experimental animals from various species.
▪ Hypothermia during ischemia before reperfusion:
– Numerous studies have shown a reduction in infarct size when mild hypothermia is instituted after coronary occlusion before reperfusion;
– Hypothermia initiated after reperfusion does not reduce infarct size;
– Microvascular injury (no-reflow) is reduced by mild hypothermia, distinct from a reduction in myocyte necrosis.
Clinical trials of hypothermia during myocardial infarction
▪ Two large clinical trials (COOL-MI and ICE-IT) failed to show an overall reduction in infarct size with mild hypothermia.
▪ Patients that were below 35°C at the time of reperfusion showed a reduction in infarct size.
▪ A recent pilot study (Rapid-MI-ICE) used cold saline infusion and endovascular catheter cooling to reduce core temperature to a mean of 34.7°C, and showed a 38% reduction in infarct size (p = 0.04) and a decrease in cumulative release of troponin T (p = 0.03).
▪ A large pivotal trial (CHILL-MI) is underway to confirm the ability of mild hypothermia to reduce infarct size.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Claeys M, Bosmans J, Veenstra L et al. Determinants and prognostic implications of persistent ST‑segment elevation after primary angioplasty for acute myocardial infarction. Circulation 99, 1972–1977 (1999).
- Wu K, Zethouni E, Judd R et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97(8), 765–772 (1998).
- Choi C, Haji-Momenian S, Dimaria J et al. Infarct involution and improved function during healing of acute myocardial incarction: the role of microvascular obstruction. J. Cardiovasc. Magn. Reson. 6(4), 917–925 (2004).
- Tissier R, Chenoune M, Ghaleh B et al. The small chill: mild hypothermia for cardioprotection? Cardiovasc. Res. 88, 406–414 (2010).
- Hale S, Kloner R. Mild hypothermia as a cardioprotective approach for acute myocardial infarction: laboratory to clinical application. J. Cardiovasc. Pharmacol. Ther. 16(2), 131–139 (2011).
- Reimer K, Jennings R. The ‘wavefront phenomenon’ of myocardial ischemic cell death. Lab. Invest. 40, 633–644 (1979).
- Shen Y-T, Fallon J, Iwase M, Vatner S. Innate protection of baboon myocardium: effects of coronary artery occlusion and reperfusion. Am. J. Physiol. 270, H1812–H1818 (1996).
- Hedstrom E, Engblorm H, Frogner F et al. Infarct evolution in man studied in patients with first-time coronary occlusion in comparison to different species – implications for assessment of myocardial salvage. J. Cardiovasc. Magn. Reson. 11, 38 (2004).
- Whelan R, Kaplinskiy V, Kitsis R. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu. Rev. Physiol. 72, 19–44 (2010).
- Allison T, Ramey C, Holsinger J. Transmural gradients of left ventricular tissue metabolites after circumflex artery ligation in dogs. J. Mol. Cell. Cardiol. 9(10), 837–852 (1977).
- Flameng W, Vanhaecke J, Van Belle H et al. Relation between coronary artery stenosis and myocardial purine metabolism, histology, and regional function in humans. J. Am. Coll. Cardiol. 9, 1235–1242 (1987).
- Yang X, Liu Y, Liu Y et al. Attenuation of infarction in cynomogus monkeys: preconditioning and postconditioning. Basic Res. Cardiol. 105(1), 119–128 (2010).
- Stone G, Dixon S, Grines C et al. Predictors of infarct size after primary coronary angioplasty in acute myocardial infarction from pooled analysis from four contemporary trials. Am. J. Cardiol. 100, 1370–1375 (2007).
- Anversa P, Cheng W, Liu Y et al. Apoptosis and myocardial infarction. Basic Res. Cardiol. 93(Suppl. 3), 8–12 (1998).
- McCully J, Hidetaka W, Hsieh Y et al. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 286, H1923–H1935 (2004).
- Halestrap A. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 61, 372–385 (2010).
- Ong S, Gustafsson A. New roles for mitochondria in cell death in the reperfused myocardium. Cardiovasc. Res. doi:10.1093/cvr/cvr312 (2011) (Epub ahead of print).
- Frangogiannis N. The immune system and cardiac repair. Pharmacol. Res. 58(2), 88–111 (2008).
- Marchant D, Boyd J, Lin D et al. Inflammation in myocardial disease. Circ. Res. 110(1), 126–144 (2012).
- Jennings R. Commentary on selected aspects of cardioprotection. J. Cardiovasc. Pharmacol. Ther.16, 340–348 (2011).
- Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am. J. Physiol. 301, H1723–H1741 (2011).
- Hausenloy D, Yellon D. The therapeutic potential of ischemic conditioning: an update. Nat. Rev. Cardiol. 8, 619–629 (2011).
- Schwartz B, Kloner R. Coronary no reflow. J. Mol. Cell. Cardiol. doi:10.1016/j. yjmcc.2011.06.009 (2011) (Epub ahead of print).
- Jones R, Reimer K, Hill M, Jennings R. Effect of hypothermia on changes in high-energy phosphate production and utilization in total ischemia. J. Mol. Cell. Cardiol. 14(Suppl. 3), 123–130 (1982).
- Riess M, Camara A, Kevin L et al. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc. Res. 61, 580–590 (2004).
- Ning X, Xu C, Song Y et al. Hypothermia preserves function and signaling for mitochondrial biogenesis during subsequent ischemia in isolated rabbit heart. Am. J. Physiol. Heart Circ. Physiol. 274, H786–H793 (1998).
- Shao Z, Sharp W, Wojcik et al. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am. J. Physiol. 298, H2164–H2173 (2010).
- Beiser D, Wojcik K, Zhao D et al. Akt1 genetic deficiency limits hypothermia cardioprotection following murine cardiac arrest. Am. J. Physiol. Heart Circ. Physiol. 298(6), H1761–H1768 (2010).
- Yang X, Yanping L, Xi-Ming Y et al. Cardioprotection by mild hypothermia during ischemia involves preservation of ERK activity. Basic Res. Cardiol. 106, 421–430 (2011).
- Ning X, Chen S, Xu C et al. Hypotheric protection of the ischemic heart via alterations in apoptotic pathways as assessed by gene array analysis. J. Appl. Physiol. 92, 2200–2207 (2002).
- Sutcliffe I, Smith H, Stanimirovic D, Hutchison J. Effects of moderate hypothermia on IL‑1b-induced leukocyte rolling and adhesion in pial microcirculation of mice and on proinflammatory gene expression in human cerebral endothelial cells. J. Cereb. Blood Flow Metab. 21, 1310–1319 (2001).
- Wang G, Deng H, Maier C et al. Mild hypothermia reduces ICAM‑1 expression, neutrophil infiltration and microglia/ monocyte accumulation following experimental stroke. Neuroscience 114, 1081–1090 (2002).
- Michelson A, MacGregor H, Barnard M et al. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb. Haemost. 71, 633–640 (1994).
- Frelinger A, Furman M, Barnard M et al. Combined effects of mild hypothermia and glycoprotein IIb/IIIa antagonists on platelet-platelet and leukocyte-platelet aggregation. Am. J. Cardiol. 92, 1099–1101 (2003).
- Gordon C. The therapeutic potential of regulated hypothermia. Emerg. Med. J. 18, 81–89 (2001).
- Boutilier RG. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 204, 3171–3181 (2001).
- Duncker D, Klassen C, Ishibashi Y et al. Effect of temperature on myocardial infarction in swine. Am. J. Physiol. 270, H1189–H1199 (1996).
- Chien G, Wolff R, Davis R, Winkle D. ‘Normothermic range’ temperature affects myocardial infarct size. Cardiovasc. Res. 28(7), 1014–1017 (1994).
- Hamamoto H, Sakamoto H, Leshnower B et al. Very mild hypothermia during ischemia and reperfusion improves postinfarction ventricular remodeling. Ann. Thorac. Surg. 87(1), 172–177 (2009).
- Miki T, Liu G, Cohen M, Downey J. Mild hypothermia reduces infarct size in the beating rabbit heart. Basic Res. Cardiol. 93, 372–383 (1998).
- Hale S, Dave R, Kloner R. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res. Cardiol. 92, 351–357 (1997).
- Dae M, Gao D, Sessler D et al. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am. J. Physiol. Heart Circ. Physiol. 282, H1584–H1591 (2002).
- Shao Z, Chang W, Wojcik K et al. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am. J. Physiol. 292, H1995–H2003 (2007).
- Maeng M, Mortensen U, Kristensen J et al. Hypothermia during reperfusion does not reduce myocardial infarct size in pigs. Basic Res. Cardiol. 101, 61–68 (2006).
- Gotberg M, van der Pals J, Gotberg M et al. Optimal timing of hypothermia in relation to myocardial reperfusion. Basic Res. Cardiol. 106, 697–708 (2011).
- Tissier R, Cohen M, Downey J. Does mild hypothermia protect against reperfusion injury? The debate continues. Basic Res. Cardiol. 106, 691–695 (2011).
- Hale S, Dae M, Kloner R. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc. Res. 59, 715–722 (2003).
- Gotberg M, Van der Pals J, Olivecrona G et al. Mild hypothermia reduces acute mortality and improves hemodynamic outcome in a cardiogenic shock pig model. Resuscitation 81(9), 1190–1196 (2010).
- Tissier R, Couvreur N, Bruneval P et al. Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left ventricular dysfunction. Cardiovasc. Res. 83(2), 345–352 (2009).
- O’Neil WW. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at: Transcatheter Cardiovascular Therapeutics. Washington, DC, USA, 27 September–1 October 2004.
- Grines C. Intravascular cooling adjunctive to percutaneous coronary intervention for acue myocardial infarction. Presented at: Transcatheter Cardiovascular Therapeutics. Washington, DC, USA, 27 September–1 October 2004.
- Gotberg M, Olivecrona G, Koul S et al. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ. Cardiovasc. Interv. 3, 1–8 (2010).
- Mokhtarani M, Mahgoub A, Morioka N et al. Buspirone and meperidine synergistically reduce the shivering threshold. Anesth. Analg. 93, 1233–1239 (2001).
▪ Excellent recent review of mild hypothermia.
▪ Excellent recent review of mild hypothermia.
▪ Comprehensive study of evolution of myocardial infarction in various species and man.
▪▪ Excellent review of mechanisms of cell death.
▪ Perspectives on cardioprotection from a pioneer researcher.
▪▪ Superb study that addresses timing and levels of hypothermia needed for cardioprotection.
▪ Precursor experimental study to the COOL-MI trial.
▪▪ Promising results of hypothermia therapy with good methodology for initiation of cooling and measurement of infarct size using MRI.
▪ Website
101. CHILL-MI ClinicalTrials.gov Identifier: NCT01379261. http://clinicaltrials.gov/ct2/show/NCT01379261