Review Article - Journal of Medicinal and Organic Chemistry (2022) Volume 5, Issue 6
Hydrocarbons: Chemical Properties of Carbon Black Stable Dispersions
Cruz Ferreira*
Department of Chemistry, Federal University of Sao Carlos, Washington, Sao Carlos, Brazil
Department of Chemistry, Federal University of Sao Carlos, Washington, Sao Carlos, Brazil
E-mail: Ferreiracruz@edu.thi.org
Received: 02-Dec -2022, Manuscript No. JMOC-22-83161; Editor assigned: 05- Dec-2022, PreQC No. JMOC-22- 83161 (PQ); Reviewed: 19-Dec-2022, QC No. jm JMOC-22-83161; Revised: 26-Dec-2022, Manuscript No. JMOC- 22-83161 (R); Published: 30-Dec-2022 DOI: 10.37532/jmoc.2022.5(6).106- 112
Abstract
Carbon black (CB) is a diverse and interesting material from an industrial point of view, mainly as component of composites, reinforcing material, and as a pigment. These matrices need suitable techniques for establishing morphology, chemical and physical properties, and potential transformations of CB in order to ensure a proper performance in several scenarios of use. With increasing global applications, CB flows into the environment during its lifecycle. Thus, sample analysis and CB characterization in environmental and health matrices are mandatory. One of the key parameters for analysis is sample preparation, mainly focused to achieve CB stable dispersions. Measurement techniques are generally based on image analysis and spectroscopy. The growing application and the need to fully understand CB performance, have led to the development of separation methods. This review summarizes the main aspects of CB spotlighted in the wide variety of matrices and analytical techniques. A discussion about the achievements and goals in the field is done.
Introduction
Carbon Black (CB) is a manufactured amorphous material consisting of a fine black powder of nearly elemental carbon in the form of spherical particles and their aggregates and agglomerates [1]. CB is produced mainly by the incomplete combustion of hydrocarbons under reduced presence of oxygen. Its excellent ability to color, electrical conductivity and weather and chemical resistances, make it in a powerful material used in a wide variety of areas. Similarly, the nature of CB surface and the characteristics with respect to structural organization, porous structure, surface area and chemical composition are of outstanding importance according to the different uses of CB in specific applications. The main difference with elemental carbon is the lower content of extractable organic and inorganic compounds [2]. Likewise, CB is physically and chemically distinct from black carbon (BC), which is generated as a byproduct from incomplete combustion, being the amount of carbon content in its structure lesser than 50%.
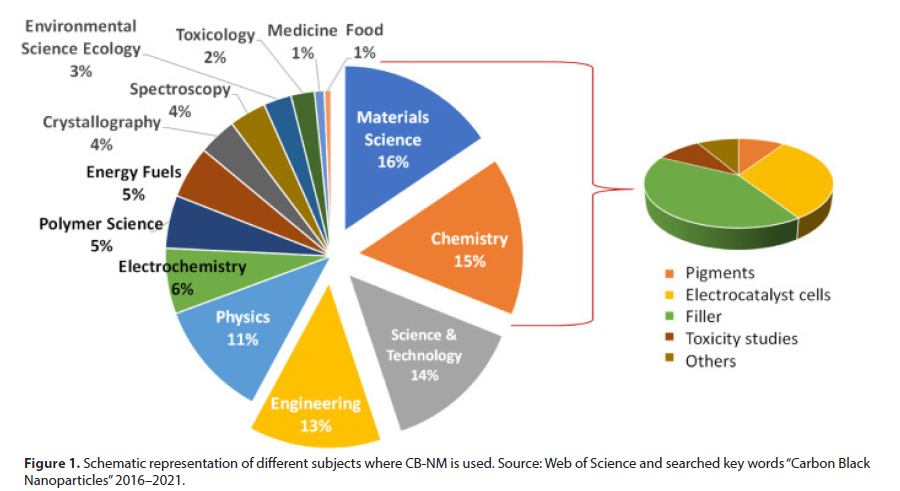
As can be seen in (Figure 1) science of materials, chemistry, science & technology and engineering are the subjects where CB nanomaterial (NM) exhibits a higher presence. These items cover more than 58% of global applicability. Focusing in materials science and chemistry, a revision about the different uses of CB-NM showed that the utility as composite or reinforcing filler in tires and other rubber products, electrochemical sensors, toxicity studies or pigments are predominant [3]. Furthermore, CB-NM has also a role in environmental science, ecology, food and medicine subjects, which cover a percentage up to 10% of applicability. This is an evidence of the increasing interest of CB-NM in biological samples.
The main health concerns associated with CB and other poorly soluble, low-toxicity (PSLT) particles are lung effects resulting from inhalation exposure, besides the overall weight of evidence indicates that CB should not be considered a direct genotoxicant or reproductive toxicant. The International Agency for Research on Cancer (IAARC) has classified CB as possible carcinogenic to humans. The sizes of CB primary particles range from 10 nm to 100 nm. A few to many tens of particles immediately form highly branched chains of primary particles called aggregates, which can agglomerate. CB can cause lung tumours, chronic inflammation and epithelial hyperplasia, depending on the particle size, level of aggregation and/or agglomeration [4]. Agglomerates can reach up to many micrometers in diameter, which decrease cancer risk in humans if their sizes are higher than 10 μm, which is the respirable particle size. Thus, the knowledge of the aggregation/ agglomeration stage is useful to evaluate the potential toxicological issues. Other diseases associated with exposure to CB in animals, specifically mice, are the cardiovascular dysfunctions, sexual and neuro inflammatory changes, lower sperm production and disorder male reproductive system.
An analysis provides chemical or physical information about a sample. The component in the sample of interest called the analyte, in this case a solid as CB, and the remainder of the sample is the matrix. In an analysis the identity, the concentration, or the properties of an analyte is or are stablished (Table 1). Summarizes the most studied CB matrices, including functions for industrial matrices or studies carried out for environmental and health related matrices, and CB used concentrations and sizes. Considering the high efficiency in radiation absorption of the pigment CB, toners, inks, and paints were analyzed [5]. CB was used for modifying working glassy carbon electrodes (GC), for example GC modified by casein-CB (CAS-CB) was applied for improving the detection limit of bisphenol A (BPA) sensing in environmental and milk samples. Functionalized CB nanospheres and MoS2 nanoclusters were proposed for the effective electrocatalytic reduction of chloramphenicol. Moreover, CB was proposed as filler in nanoclays, resins or polymers and rubber tires. Note that the industrial matrices containing CB are solid and solid-liquid dispersions as (Table 1) shows, which are very different in CB sizes and amounts, and compositions. It is interesting to note that for CB dispersions, stability is a relevant factor [6].
| Empty Cell | Matrix/Ref | CB Phase | Function/∗Studies | Concentration | Size (nm) |
|---|---|---|---|---|---|
| INDUSTRIAL MATRICES | Toners | Solid | Pigment | 4–20% | 3000–10000 |
| Inks | Solid- Liquid dispersion | Pigment | 0.2–20% | 70–270 | |
| Paints | Solid-Liquid dispersion | Pigment | 20–25% | 10–6800 | |
| Electrodes | Solid | Determination of | 0.10% | <510 | |
| Bisphenol A | 0.50% | ||||
| chloramphenicol | |||||
| Nanoclays | Solid | Filler | 3% | <2000 | |
| Resins | Solid | Filler | 14–33% | <500 | |
| Polymers | Solid | Filler | 10–50% | 40–50 | |
| Rubber tires | Solid | Reinforce and filler | 20–35% | 15–100 | |
| Plastics | Solid | Reinforce and filler | 2.5–5% | 16, | |
| 100–200 | |||||
| ENVIRONMENTAL MATRICES | Air | Solid-Gas | Particle size determination and toxicological studies (as model)∗ | 1.08mgmL−1; 50μL instillation volume per mouse | 150 |
| dispersion | |||||
| Soils | Solid | Particle size determination and toxicological studies∗ | 10–1000mg CB kg−1of soil | 20 -70 (aggregates) | |
| Solid-Liquid dispersion | (CB spikes in dispersion) | ||||
| Sea water | Solid-Liquid dispersion | Particle size determination and toxicological studies∗ | 1–10mgL−1; invitro assays of mussel hemocytes | 35–400 | |
| HEALTH related MATRICES | Cell media | Solid-Liquid dispersion | Characterization and toxicological studies∗ | 10–500mgL−1 | 60–270 |
| Invitro assays | |||||
Considerations on Carbon Black Nanomaterial
CB is used in several areas of modern life, considering the range of relevant physical and chemical features, which are showed in (Table 1). The most cited applications including its use in printer ink, reinforcements for rubber, active agent in electrically conductive plastic, and pigments in paints. Additionally, CB can be used as coatings, papers ink and cosmetics [7]. CB can be produced by the following processes: “furnace,” “channel,” and “acetylene”.
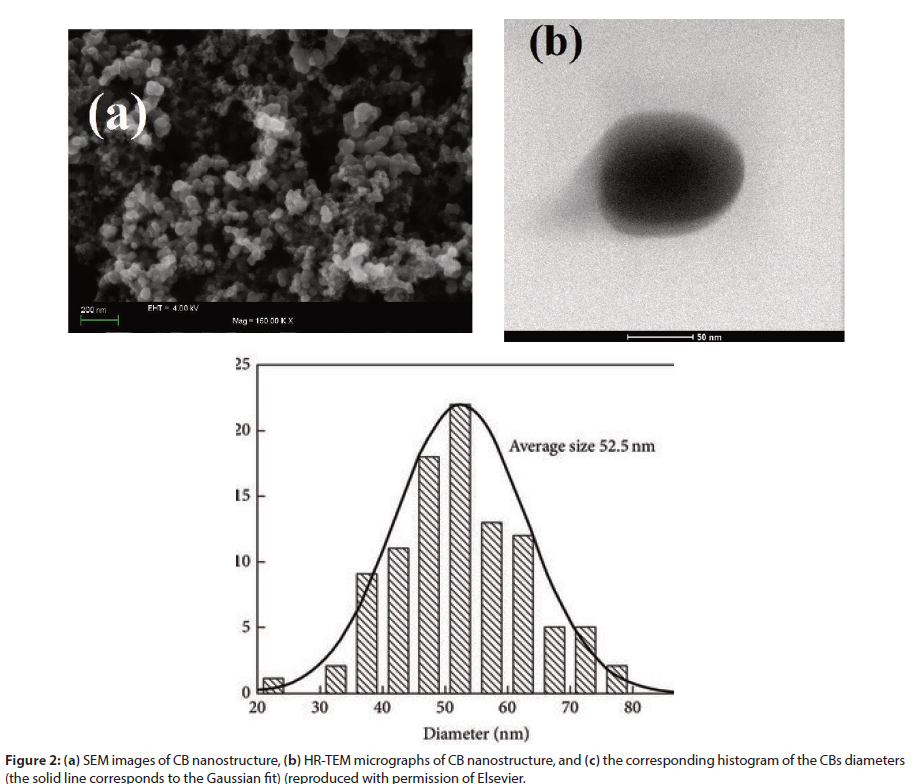
The most useful process is the “furnace,” which produces more than 80% of the CB in the world. Depending on the process, CB can present different characteristics. In special, “acetylene” process produces small CB particles with high complexity of the agglomerates and higher graphitic content than those produced by furnace process. CB primary particle is formed by elemental carbon arranged in fine particles, which has an amorphous and quasigraphitic structure. The average CB particle size ranges from 3.0 to 100 nm. However, one of the characteristics of CB is the formation of aggregates nanostructures, by presenting semispherical groupings. Moreover, these aggregates groups, with a distinctly long dimension, can form agglomerates. Figure 1 was adapted from the Kohjiya et al. article and schematically displays the CB particle, CB aggregates, and agglomerates. Figure 2(a) shows typical scanning (SEM) and transmission (TEM) electron microscopy images recorded for CB particles. From that, it is possible to verify the morphological aspects that confirm the scheme shown in (Figure 1). CB formed porous structure based on beads (Figure 2(b)) and the spherical particles (Figure 2(c)) present an average diameter of 52.5 nm. CB prepared from heat treatment up to 700° C may increase the number of conjugated carbons in the sp2 hybridization, leading to a progressive increase in the conductivity of this material.
This factor can be associated with electrons with delocalized π-bonds that are available for charge storage. In addition, oxygenates groups are preferentially formed at the edges of the graphite-like micro crystallites. This behavior may be interesting to create an environment to immobilize organic molecules, such as enzymes and other biological material. The presence of a great number of sp2 edge plans, the oxygenate species displayed over the CB particles, and the CB aggregation forms are intrinsic linked to the CB electrochemical behavior [8]. These characteristics show that CB has the ability to intercalate ions on graphite layers, which affects electrical conduction between particles by tunneling and the surface reaction. In particular, the surface area, the number of edges planned, and the structural defects depend on the synthesis or functionalization processes, which create several functional groups that can be useful for the immobilization of various species, such as enzymes, genetic material, antibodies, and nanoparticles. The sum of structural and electrical/electronic characteristics, ability to produce stable dispersion without the need for sophisticated procedures, and the fact that it is an extremely cheap material make the CB an interesting nanostructured material for the development of electrochemical devices for sensing and bio sensing [9].
Raw Materials and Industrial Matrices
One of the most characteristic aspects of CB materials is the oil absorption number as mentioned in section 2, which is related with the ability of a given CB to absorb liquids. Besides, the structural information, crystallinity and graphitic content are key parameters in order to evaluate its possible modification, functionalization and applicability. In this sense, different analytical techniques such as Fourier transform infrared spectrocopy (FTIR), thermogravimetric analysis (TGA), Raman spectroscopy, X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), among others, are employed. Furthermore, other image techniques like Transmission electron microscopy (TEM) or Scanning Electron Microscopy (SEM) are also used for establishing the morphology of samples and to characterize the particle core sizes and shapes [10]. Analysis of CB-NM is completed with techniques, which need to disperse the raw material, as dynamic light scattering (DLS) and several separation techniques such as asymmetrical flow field flow fractionation (AF4) and sedimentation field flow fractionation (SdF3) coupled to different detectors. DLS is employed for studying the size of aggregates and the mono or polidispersity of the whole dispersion and, AF4 and SdF3 give the possibility of separating different nanoparticles populations of different sizes of the studied dispersion. Those all mentioned techniques appear in (Table 2), including calculated sizes from these ones for raw CB-NMs and relative standard deviation (%) reported in some papers [11].
| Empty Cell | CB phase measured | Dispersant/assisted dispersion by | Analytical technique | Size (nm) | RSD (%) |
|---|---|---|---|---|---|
| RAW CB ANALYSIS | Dispersion | Ethanol | DLS | 178–233 | – |
| Dried dispersion | Idem | FTIR, TGA, SEM, TEM | 24 | – | |
| Aqueous dispersion | Triton X-100, tween 20 and FL-70 | SdF3 | 78–300 | 20 | |
| Aqueous dispersion | Styrene and acrylic acid, cell culture medium and Tween 20/Sonication 2h | AF4-DLS, DLS | 387 and 175a, 404 and 175b | 2–5 | |
| Solid | – | TEM, SEM, Raman | 40 | – | |
Biosensors Based on Nanostructured Carbon Black
CB is a relatively novel material in the field of electro analytical chemistry. The first studies related to investigation of CB as modifier for sensors preparation were reported only in the last decade. One of the highlight pioneering works was the electrochemical sensors reported by Dang et al. and Zhang. In these cases, CB was designated with acetylene black for the voltammetric sensing of tetracycline, colchicine, and rutin. The origin and/or type of CB can affect directly the electro analytical performance of the designed electrochemical biosensor [12]. In this sense, our research group reported recently a complete study about the electro analytical performance of GCE modified with different CB structures. The properties of this low cost material include fast charge transfer kinetics and high analytical sensitivity. Therefore, the identification of the appropriated supplier and kind of CB is an important factor in the preparation of high analytical performance CB-based electrochemical biosensing.
Glucose enzymatic biosensing is a research topic that has been explored widely in the literature, due to the relevance of continuous diabetes monitoring. The use of CB as a carbon support for immobilization of glucose oxidase was investigated by Xiao-He et al. The better approach for preparation of the biosensor using poly (PSS) grafted on the CB surface was studied. The obtained results demonstrated that CB-g-PSS showed a good environment for glucose oxidase immobilization; however, selectivity and applicability tests were not provided [13]. Carbon nanomaterials are widely explored to design biosensors based on glucose oxidase. Thus, it is an interesting approach to provide a comparison of analytical features recorded by using biosensors for glucose constructed with carbon nanomaterials, in order to verify the real advantages of low cost and widely available CB nanoparticles. The great part of works explored direct electron transfer (DET) between GOx enzyme and the nanostructured electrode surface. In these cases, a typical redox process is verified for the biosensor response in buffer solution, which is attributed to the enzyme active centre. Therefore, this constant provides information about the electron transfer kinetic between immobilized GOx and electrode surface. CB nanoparticles were able to ensure a fast DET, with ks constant higher than those obtained using SWCNTs, MWCNTs, graphene, GO, RGO, and SWCNHs with only two exceptions among twenty-five revised works. From an analytical point of view, the CB-based GOx biosensor showed comparable linear range and limit of detection [14]. In some cases, it is recorded limits of detection at micromolar levels. However, it should be observed that the glucose concentration in human blood is typically located in the millimolar range and, therefore, the linear range and limit of detection provided by the CB biosensor are adequate. A very important analytical parameter is the sensitivity. In this case, the most sensitive biosensors are those designed with MWCNTs, C60, and RGO. On the other hand, the sensitivity of CB biosensor is higher than graphene biosensor and presented a high sensitivity than several biosensors constructed with SWCNTs, MWCNTs, pristine graphene, GO, RGO, and SWCNHs. Besides that, from repeatability studies, low relative standard deviations (RSD) were obtained for the biosensor response during consecutive measurements in all the cases. The longterm stability was evaluated as being the percentage of signal variation after some days of storing in a refrigerator at 4°C. There is not a standard in the works in terms of number of storing days and, in general, the biosensors maintained its initial response after many days, in special, for the CB biosensor case, which maintained 94% of its initial signal after ten days of storing [15].
Conclusions
It is shown that CB and functionalized CB represent an important carbon nanomaterial group with a wide range of applications in different fields and in a high variety of matrices. Size, structure, characteristic chemistry surface and intrinsically properties as mechanical, thermal and electrical, allow the possible uses as a pigment in toners, inks or paints, as composites or reinforcing filler in tires and other industrial products, or as electrochemical sensors. Moreover, its wide application gave rise to different analytical scenarios as pollutant and toxic agent.
Matrices are solid and solid-gas and solidliquid dispersions of different nature and with different amounts and sizes of CB or functionalized CB. On the other hand, the different kind of CB synthesis provided several raw materials. For these matrices surface chemistry, particle sizes and structure are the most relevant needed measurements. For testing these properties, solid directly and dispersions were used and for solid the main sample treatment proposed is dispersion. The particularity of this material is derived of the several raw materials containing also several forms of NMs.
The dispersion process is a critical step in the analysis of CB according to its particular chemistry. The most used dispersants are polymeric compounds and cellular culture media, which can be modified with organic and inorganic substances, in order to improve the dispersive behavior.
For environmental and health samples few in-vitro and in-vivo assays were carried out using prepared dispersions and studied CB effects in vitro or in-vivo. Soils spiked with CB were tested for toxicological studies. Present studies only use a CB known concentrations for carry out toxicity tests, which are based on the contact between the CB with the organism studied. The study of CB in animal organs is referred to the toxicity assays developed in earthworms and mussels. In these studies, the effect of CB in the organisms was evaluated. Isolation and quantitation of the CB is not carried out in our knowledge.
Regarding the analysis, study, characterization and separation of this material, different analytical techniques are employed. Microscopic technique provides morphological and structural information, such as size, orientation, porous structure, interparticle distances or surface charges. In the case of spectroscopic techniques, DLS, FTIR, XRD, EIS and BET are used to obtain information about particle size, structure and chemical composition in inks, catalysts or forensic samples, among others. DLS works with dispersions and provides information about CB aggregates sizes and if the dispersion is mono o multimodal, but gives global information. Separation techniques such as AF4 and SdF3 provide information allowing to evaluate the presence or not of several NPs distributions according to particle properties, such as size, composition or electrophoretic mobility. These techniques cover a great size range, from a few nm to μm.
Tools to determine CB mainly in industrial matrices have been proposed, however, environmental and biological samples are still an unexplored field in terms of isolation and separation methods for CB determination. Therefore, more efforts are necessary to advance in the knowledge of CB as potential target analyte in the environmental and health fields. Characterization, study and evaluation of the possible transformations of CB in environmental and health matrices are other topic that needs new efforts.
References
- Van der Does AM,Hiemstra PS,Mookherjee N. Antimicrobial host defence peptides: immunomodulatory functions and translational prospects. Adv Exp Med Biol.1117,149-171(2019).
- S.Bobone,L.Stella. Selectivity of antimicrobial peptides: a complex interplay of multiple equilibria. Adv Exp Med Biol.1117, 175-214 (2019).
- Matsuzaki K. Membrane permeabilization mechanisms. Adv Exp Med Biol.1117,9-16 (2019).
- Melo MN, Ferre Castanho R. Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat Rev Microbiol.7,245-247 (2019).
- Gazit E, Miller IR, Biggin PC et al. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J Mol Biol.258,860-870 (1996).
- Roversi D, Luca V, Aureli S et al. How many antimicrobial peptide molecules kill a bacterium? The case of PMAP-23. ACS Chem Biol.9,2003-2007 (2014).
- Savini F, Luca V, BocediR et al. Cell-density dependence of host-defense peptide activity and selectivity in the presence of host cells. ACS Chem Biol.12,52-56 (2012).
- Savini F,Bobone S, Roversi D et al. From liposomes to cells: filling the gap between physicochemical and microbiological studies of the activity and selectivity of host-defense peptides. Pept Sci.110, 345-349(2018).
- Snoussi M, Talledo JP,Del Rosario NA et al. Heterogeneous absorption of antimicrobial peptide LL37 in Escherichia coli cells enhances population survivability. E Life.7, 45-48(2018).
- Starr CG, He J, Wimley WC et al. Host cell interactions are a significant barrier to the clinical utility of peptide antibiotics. ACS Chem Biol.11, 3391-3399 (2016).
- Zhu Y, Mohapatra S, Weisshaar JC et al. Rigidification of the Escherichia coli cytoplasm by the human antimicrobial peptide LL-37 revealed by super resolution fluorescence microscopy. Proc Natl Acad Sci.116,1017-1026 (2019).
- Wu F, Tan C. Dead bacterial absorption of antimicrobial peptides underlies collective tolerance. J R Soc Interface.16, 3457-3459 (2019).
- Bocchinfuso G, Palleschi A, Orioni B et al. Different mechanisms of action of antimicrobial peptides: insights from fluorescence spectroscopy experiments and molecular dynamics simulations. J Pept Sci. 15,550-558 (2009).
- Orioni B, Bocchinfuso G, Kim JY et al. Membrane perturbation by the antimicrobial peptide PMAP-23: a fluorescence and molecular dynamics study. Biochim Biophys Acta – Biome.1788,1523-1533 (2009).
- Steiner H, Andreu D, Merrifield RB et al. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta – Biome.939,260-266 (1998).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref