Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Histogenous Hypoxia in Depression: A Cross-Sectional Paired Study into Venous Blood Gases in Outpatients with Depression
- Corresponding Author:
- Yang Zhimin
Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou 510120, China
Tel: +8620-81887233-3206
Fax: +8620-81887233
Abstract
Abstract
Background: It has been suggested that oxidative stress is increased and antioxidant defenses are decreased in depression, and oxidative stress has a complex relationship with hypoxia. Is there a definite hypoxia index in patients with depression? Also, any other correlations between hypoxia indicators and the symptoms of depression remain unreported.
Methods: In the present study, a cross-sectional design was undertaken with 1:1 pairs. Patients with depression and healthy participants were recruited from the Guangdong Province Hospital of Traditional Chinese Medicine. Both groups were assessed using the Hamilton Depression Scale (including five factors: anxiety/somatization, weight, cognition impairment, hysteresis and sleep) and had their venous blood gases analyzed. All data were analyzed using the PASW Statistics 18.0 data statistical package. Results were analyzed statistically using the paired t test, Pearson correlation analysis and multiple linear regression analysis.
Results: In total 52 cases were recruited to each study group, including 15 (28.8%) males and 37 (71.2%) females. The values of the mean venous pH, PvO2, SvO2 and CvO2 in the depressed group of patients were higher than those of the control group. PvCO2 was also lower in the depressed than in the control group, and this difference was statistically significant. The total depressive score and cognitive impairment factor were related to PvO2, while the anxiety/somatization and sleep factors were related to the value of the venous pH. Age was positively correlated with venous pH in both study groups. Venous pH had a positive association with PVO2 in the depressed group (multiple linear regression analysis), but there was no significant correlation
between these two factors in the non-depressed control group.
Conclusion: Histogenous hypoxia is present in patients with depression and this is related to elevated venous pH; both of these are related to depression. Elevated venous pH and decreased PvCO2 may explain histogenous hypoxia in depression and venous blood gas concentrations potentially represent a biomarker for depression.
Keywords
Depression, Venous blood gas analysis, Histogenous hypoxia, Partial venous oxygen pressure, Venous pH
Introduction
The common features of a depression disorder are the presence of a sad, empty, or irritable mood, accompanied by somatic and cognitive changes that significantly affect an individual’s capacity to function [1]. According to an epidemiological survey conducted in Guangzhou, China [2], the point prevalence rate and lifetime prevalence rate of major depression disorder were 0.8% and 4.42%, respectively, in 2006. The prevalence rates were higher than those of the National epidemiological investigation in China in 1992 [3]. Depressive disorders were determined to be the second most common cause of years lived with a disability (YLDs) in 2010, and depressive disorders were also a leading cause of disability adjusted life years (DALYs), even though mortality was not attributed to them as an underlying cause [4].
The pathophysiology of depression has not been fully expounded. A recent meta-analysis that pooled data from studies with different oxidative stress markers suggested that oxidative stress is increased and antioxidant defenses are decreased in depression [5]. In another review, it was indicated that depression is characterized by higher levels of oxidative biomarkers and lower levels of antioxidant defense biomarkers [6]. So, Michel and colleagues considered that the hypothesis of oxidative stress represented a novel explanation for the etiology of depression [7].
There exists a complex relationship between hypoxia and oxidative stress. Severe hypoxia may induce an increase in the production of reactive oxygen species (ROS), particularly the generation of oxygen (O2) by oxygen-dependent enzymes such as cytochrome C oxidase, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and uncoupled endothelial nitric oxide synthase [8,9].
The variability in ROS production in response to changes in the ambient oxygen partial pressure has been linked to the activation of the hypoxia inducible factor (HIF) α-subunits, and there is evidence for feedback regulation between ROS production and the HIF pathway [10]. So Görlach, et al. [11] proposed that an intricate interplay between hypoxia and ROS production exists, and that this involves feed-forward and feed-back mechanisms involving the functioning of HIFs.
It has been suggested that oxidative stress is increased and antioxidant defenses are decreased in depression, and the relationship between oxidative stress and hypoxia is complicated; is there a relationship between hypoxia and depression?
Hypoxia is a pathological process in which there is an insufficient oxygen supply or a disorder of oxygen utilization, and these results in abnormalities of metabolism and function, and changes in tissue morphology [12]. Blood gas analysis has long been used to evaluate the hypoxic status of the human body. Arterial blood is mainly reflective of alveolar gas exchange, as the drawing of blood occurs following alveolar ventilation [13]. It is likely that arterial blood gases seldom change, except during serious diseases that affect alveolar gas exchange. On the contrary, venous blood mainly reflects gas exchange in the tissues [13]. Mixed venous blood is drawn prior to alveolar ventilation, thus reflecting the respiratory and metabolic status of bodily tissues more accurately [14]. The partial oxygen pressure in terminal arterial capillaries is equal to the arterial partial pressure of oxygen (PaO2). The surrounding tissue continuously absorbs oxygen when blood flows through the capillaries, and the partial pressure of oxygen in capillary blood declines during this process. When blood reaches the venule end of the capillary, the partial pressure of oxygen is equal to the venous partial pressure of oxygen (PvO2) meaning that venous blood gas analysis is considered to be a valid indicator of tissue oxygenation [15]. So, here we have designed a cross-sectional study with a 1:1 pairing in order to compare the venous blood gases in patients with depression and in healthy controls.
Methods
▪ Participants
The research included patients with depression and healthy controls. Patients with depression were recruited from among the outpatients of the Department of Psychology and Sleep in Guangdong Province Hospital of TCM. The healthy controls were recruited from the physical examination center of Guangdong Province Hospital of TCM, and were matched with patients with depression according to gender, age and body mass index (BMI).
Inclusive criteria for the depressive group were: (1) current symptoms met the diagnosis of depression according to ICD-10 (the international statistical classification of diseases and related health problems, 10th revision; ICD coding: F32) [16]; (2) age≧18 years; (3) scores on the Hamilton Depression Scale (HAMD) (17 items) [17] ≧17. Exclusion criteria for the depressive group were: (1) have fatty liver disease, hypertension, arthritis, renal failure, diabetes or other medical diseases; (2) abuse of alcohol and psychoactive substances; (3) pregnant or lactating female patients; (4) confined to bed or a wheelchair; (5) infection or a history of alcohol intake within the previous week.
Inclusion criteria for the healthy group were: (1) a good health record, no physical illness or psychiatric disorder; (2) test results have been normal in most recent examination; (3) no sleeping disorders; (4) a score in HAMD (17 items) < 7 points; (5) no history of infection or alcohol intake within the previous week.
▪ Clinical interviews
Each group was required to attend a clinical interview. The patients with depression were interviewed by an experienced psychiatrist to determine whether the patients met the screening criteria. The controls were paired with patients by age±2 years, gender, and BMI±1 following the interview.
All participants were informed of the purpose, procedure, and possible benefits and risks of participation in the study. The participants who agreed to join the trial were asked to sign informed consent forms and to register their general information. These data included: (1) background information, such as their name, address, company, telephone number, etc. (2) demographic items such as their age, sex, nationality, marital status, education level, occupation, height, weight, etc. (3) previous history such as their previous medical history, treatments received for depression, current medication and smoking status.
▪ Scale assessment
Both groups were assessed by an experienced psychiatric worker with the Hamilton Depression Scale (HAMD; version with 17 items), the most widely used evaluation tool in China, the reliability and validity of which has been tested (reliability coefficient is 0.88~0.99, p<0.01; validity coefficient is 0.92). Items are divided into five factors: anxiety/somatization, weight, cognition impairment, hysteresis and sleep. The total score of the HAMD reflects the severity of depression and the effects of intervention [17].
Assessment of the biochemical measures
After completing scale assessments, all participants were led to the emergency department by members of the project and 2 ml elbow vein fasting blood was withdrawn with a single-use arterial blood gas needle in the morning. The samples were collected by members of the project team and tested with a fully-automatic blood gas analyzer within 15 minutes. The test indices included the venous pH value, venous partial pressure of oxygen (PvO2), venous partial pressure of carbon dioxide (PvCO2), venous oxygen saturation (SvO2), venous oxygen content (CvO2) and total venous carbon dioxide (TvCO2) .
▪ Statistical analysis
All statistical analyses were performed with the PASW Statistics 18.0 data statistical package. The data measured are presented as means±standard deviations ( ͞X ± S ) after the data normality test. Enumeration data were presented with constituent rates and ratios. The test level was set to alpha = 0.05. For normally distributed data a comparison between two matched-pair groups was performed using a paired sample t test. The relationship between two variables was determined using Pearson correlation analysis and multiple linear regression analysis. Enumeration data: a comparison of constituent rates and ratios between two groups was carried out with the Chisquared test or Fisher’s exact probability.
Results
▪ Analysis of general data
There were 52 subjects in each study group, 15 male (28.8%) and 37 female (71.2%).
Comparisons of age, years of education, height, weight and BMI
The two groups showed no significant differences in age, height or weight, but showed differences in number of years spent in education and BMI. The years in education and BMI in the depression group were lower than those in the control group (Table 1). BMI in some patients with depression was so low (BMI<17) that it was hard to find matches for them within the normal population, so the matching requirements for BMI had to be loosened slightly.
| Variable | Depression Group(N=52) | Control Group(N=52) | T | P |
|---|---|---|---|---|
| age | 35.75±11.04 | 35.73±10.94 | 0.087 | 0.931 |
| years of education | 11.96±3.50 | 14.23±3.87 | -3.485 | 0.001** |
| height | 162.13±7.70 | 162.54±8.74 | -0.362 | 0.719 |
| weight | 54.00±9.20 | 55.17±9.82 | -1.406 | 0.166 |
| BMI | 20.49±2.87 | 20.80±2.61 | -2.122 | 0.039* |
Table 1: Comparison of age, years of education, height, weight and BMI (͞X ± S).
▪ Comparison of other conditions
There was no significant difference in marital status between the two groups.
In the depression group, there were 51 cases (98.08%) of Han, 1 (1.92%) Mongolian; 5 cases (9.62%) with a religious belief and 47 cases (90.38%) without. There were 5 cases (9.62%) with a smoking habit, 34 cases (65.38%) suffering from their first depressive episode and 18 cases (34.62%) of a recurrent episode (two of these cases being bi-polar). There were 17 cases (32.69%) undergoing treatment (including fluoxetine, venlafaxine, paroxetine, citalopram, sertraline, fluvoxamine, mirtazapine and trazotone), while 35 cases (67.31%) were not, and 25 cases had never been treated. The average withdrawal period (from drug withdrawal until the time of the study) was 17.7 months (1,60). The course of the disease was 32.38±34.95 months. The course of western medical treatment was 10.69±24.99 months, and Chinese medical treatment was 3.71±8.71 months.
In the control group, there were 51 cases (98.08%) of Han, 1 (1.92%) Manchu; 3 cases (5.77%) with religious belief with 49 cases (94.23%) without. There were 6 cases (11.54%) with a smoking habit. There was no significant difference found in the distribution of nationality, religious belief and smoking status.
▪ Scale assessment
The scores for the depressive scale and factors in the depression group were higher than those of the control group (Table 2).
| Variable | Depression Group(N=52) | Control Group(N=52) | T | P |
|---|---|---|---|---|
| anxiety/somatization factor | 7.13±1.61 | 0.67±0.90 | 34.297 | <0.001** |
| cognition impairment factor | 4.90±2.06 | 0.10±0.30 | 16.614 | <0.001** |
| hysteresis factor | 7.96±1.85 | 0.27±0.53 | 28.509 | <0.001** |
| weight factor | 0.87±0.91 | 0.00±0.00 | 6.872 | <0.001** |
| sleep factor | 4.83±1.31 | 0.44±0.80 | 20.759 | <0.001** |
| total depressive score | 27.38±5.10 | 1.73±1.62 | 34.297 | <0.001** |
Table 2: Comparison of scores of total depressive scale and factors(͞X ± S).
▪ Comparison of the venous blood gas analyses
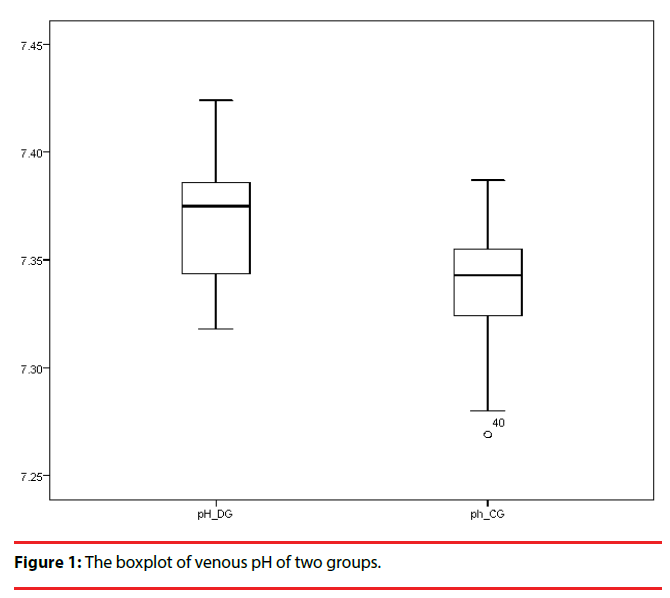
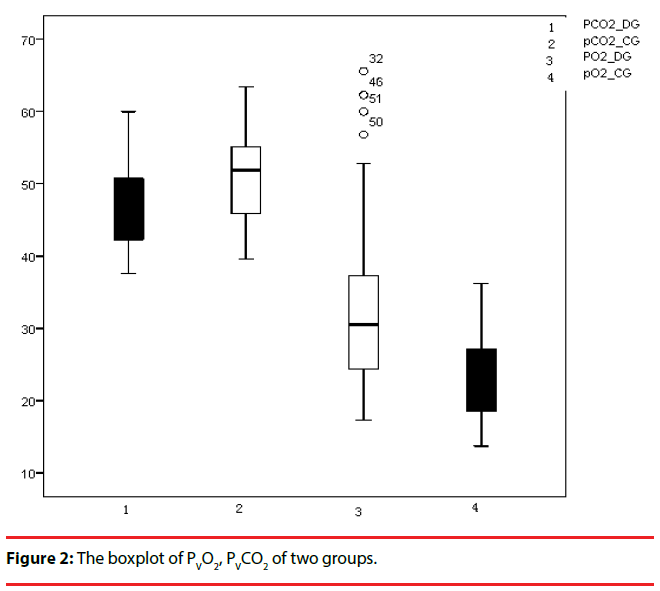
The values of venous pH, PvO2, SvO2, and CvO2 in the group with depression were higher than those of the control group, whereas the levels of PvCO2 and TvCO2 in the depression group were lower than those of the control group, and these differences were statistically significant (Table 3) (Figure 1 & 2).
| Variable | Depression Group(N=52) | Control Group(N=52) | T | P |
|---|---|---|---|---|
| pH | 7.368±0.026 | 7.341±0.024 | 5.132 | <0.001** |
| PvCO2(mmHg) | 46.635±5.708 | 51.117±5.410 | -4.061 | <0.001** |
| PvO2(mmHg) | 32.523±11.518 | 22.646±5.383 | 5.516 | <0.001** |
| SvO2(%) | 52.162±21.559 | 29.552±12.748 | 6.483 | <0.001** |
| CvO2(mmol/L) | 4.679±2.277 | 2.625±1.109 | 5.784 | <0.001** |
| TvCO2(mmol/L) | 27.534±2.317 | 28.433±2.246 | -2.240 | 0.030* |
Table 3: Comparison of venous blood gas analysis (͞X ± S).
▪ Correlation analysis of the total depressive score in the depressive group
To explore the relevance between the depressive score and other variables, a Pearson relevance analysis was performed. It was found that PvCO2 was negatively correlated with the total depressive score, while the venous pH value, PvO2, SvO2 and CvO2 were positively correlated with the total depressive score (Table 4). Based on these results, a multiple linear regression analysis was implemented with venous pH, PvCO2 and PvO2 as the independent variables and the total depressive score as the dependent variable. A “backward” method was used (removal criterion=0.100). As a result, PvO2 was the only variable remaining in the regression model (t=2.727, p=0.009, unstandardized coefficients=0.158, 95% CI=[0.042, 0.275]).
| Variable | Variable | Correlation Coefficient | P |
|---|---|---|---|
| Total depressive score | Venous pH | 0.355 | 0.010* |
| PvCO2 | -0.291 | 0.036* | |
| PvO2 | 0.360 | 0.009** | |
| SvO2 | 0.337 | 0.015* | |
| CvO2 | 0.353 | 0.010* | |
| TvCO2 | -0.161 | 0.254 | |
| age | 0.253 | 0.071 | |
| BMI | 0.066 | 0.642 | |
| education years | -0.098 | 0.491 | |
| whether first-episode | 0.073 | 0.606 | |
| Course of disease | -0.058 | 0.681 | |
| whether administration nowadays | -0.216 | 0.123 | |
| course of administration | 0.060 | 0.673 |
Table 4: Correlation analysis of total depressive score.
The same method was used to explore the relationship between five depressive factors and other variables. It was found that the anxiety/somatization factor and sleep factor were closely linked to venous pH and the cognition impairment factor was correlated with PvO2. Anxiety/somatization factor was negatively correlated to years of education (Table 5).
| Dependent Variable | Independent Variable | Unstandardized Coefficients | T | P | 95%ci | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Total depressive score | PVO2 | 0.158 | 2.727 | 0.009** | 0.042 | 0.275 |
| anxiety/somatization factor | Education years | -0.136 | -2.240 | 0.030* | -0.258 | -0.014 |
| Venous pH | 17.791 | 2.212 | 0.032* | 1.626 | 33.957 | |
| cognition impairment factor | PvO2 | 0.066 | 2,818 | 0.007** | 0.019 | 0.113 |
| sleep factor | Venous pH | 20.829 | 3.279 | 0.002** | 8.069 | 33.589 |
Table 5: Multiple regression analysis of five factors of depression.
▪ Correlation between venous blood-gases and the general situation
A Pearson relevance analysis was performed to analyze the venous blood-gas data (the most important three factors= pH, PVO2 and PVCO2) and the general situation in both groups. It was found that age showed a significant positive correlation with venous pH values in both groups, while BMI showed a significant positive correlation with venous pH and PVO2, and a negative correlation with PVCO2 in the depressive group, but there was no significant correlation with the results of venous blood-gas analysis in the healthy control group. There was no significant correlation between venous bloodgas analysis and smoking, the course of the depressive disease, whether drug administration was current and the course of administration (Table 6).
| Group | Variable | Age | Smoking | BMI | Course of disease | Whether administration nowadays | Course of administration |
|---|---|---|---|---|---|---|---|
| Depression group | pH | 0.313* | -0.080 | 0.401** | -0.238 | -0.055 | -0.041 |
| PVCO2 | -0.201 | 0.148 | -0.391** | 0.226 | -0.105 | 0.129 | |
| PVO2 | 0.231 | 0.053 | 0.353* | -0.042 | -0.017 | 0.089 | |
| Control group | pH | 0.364** | -0.141 | -0.012 | / | / | / |
| PVCO2 | -0.107 | 0.217 | 0.122 | / | / | / | |
| PVO2 | 0.004 | -0.130 | 0.031 | / | / | / |
Table 6: Correlation analysis of the results of venous blood-gas analysis.
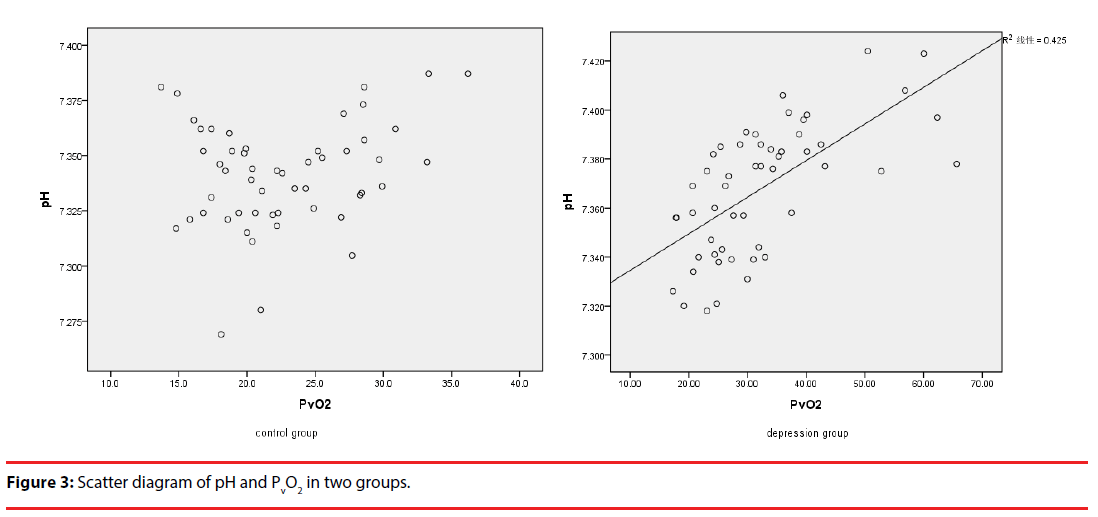
A Pearson relevance analysis within the results of the venous blood-gas analysis was also performed. The venous pH showed a significant negative correlation with PVCO2 in both groups. Interestingly, the venous pH was positively correlated significantly with PVO2 in the depressive group, but there was no correlation in the healthy control group (Table 7) (Figure 3).
| Group | variable | Venous pH | PVCO2 | PVO2 |
|---|---|---|---|---|
| Depression group | Venous pH | 1 | -0.766** | 0.652** |
| PVCO2 | -0.766** | 1 | -0.614** | |
| PVO2 | 0.652** | -0.614** | 1 | |
| Control group | Venous pH | 1 | -0.697** | 0.245 |
| PVCO2 | -0.697** | 1 | -0.460** | |
| PVO2 | 0.245 | -0.460** | 1 |
Table 7: Correlation analysis within the results of the venous blood-gas analysis.
Discussion
▪ Histogenous hypoxia exists in depression
In the present study, patients with depression exhibited higher PvO2/SvO2/CvO2levels and lower PvCO2 levels as compared with healthy control individuals, after subjects were matched for sex, age, and BMI. These results indicate that patients with depression have significant differences in their oxygen utilization compared to healthy controls.
Exchange of metabolic materials continuously exists between the circulating blood and tissue fluid. Oxygen and nutrients move from the blood to the tissue fluid, and carbon dioxide and metabolic products from tissue fluid into the blood. This is essential in order that cells bathed in tissue fluid remain alive [18]. Diffusion is the primary process by which O2 and CO2 exchange. The rate of diffusion is partially determined by the difference in the concentration of a substance inside and outside of the capillary walls [18]. When the arterial blood flows through capillaries and the partial pressure of O2 in the blood is higher than that in tissue fluid, O2 will diffuse into the tissue fluid. The partial pressure of CO2 in tissue fluid, however, is higher than that in blood, so it will diffuse into the blood. Diffusion rate is positively correlated with the difference in partial pressure between the arterial and venous O2 and CO2 partial pressures. The difference in arterial and venous oxygen saturation may reflect the conditions of O2 release, uptake and utilization in tissues; therefore, it can be used to estimate tissue circulation and metabolism [19].
Arterial gas analysis was not performed in the current research, but in a similar study by Yuan Tao [20], arterial blood gas analysis was compared in 34 patients suffering from fatigue without strength, and normal controls. It was found that arterial PaO2, CaO2 and SaO2 in patients were lower than in the control group, and these differences were statistically significant. Without considering differences between arterial oxygen partial pressure, and just with the results of decreased PvCO2 and elevated PvO2/SvO2/ CvO2 in this study, the conclusion can be drawn that increased PvO2 and decreased differences in arterial and venous oxygen partial pressure exist in depression.
There are four types of hypoxia, including hypotonic hypoxia, hemic hypoxia, circulatory hypoxia and histogenous hypoxia [12]. Histogenous hypoxia means there is an adequate amount of oxygen delivered to tissues, but the tissues are unable to utilize the oxygen effectively. The symptoms of histogenous hypoxia are an increased PvO2 and a decreased difference between arterial and venous oxygen partial pressure [12]. Hence, histogenous hypoxia exists in depression.
▪ Hypoxia and depression
The present study has also demonstrated that PvO2, SvO2 and CvO2 are positively correlated with the total depressive score. Multiple linear regression analysis showed that the total depressive score and the cognition impairment factor were closely correlated with the PvO2. This indicates that depression can be clearly linked with histogenous hypoxia.
The metabolic processes of many enzymes are affected by brain hypoxia, including tryptophan hydroxylase, the rate-limiting enzyme of serotonin synthesis. It has been found that mean serotonin synthesis is 50% higher when the respiratory oxygen concentration reached 60%, compared to 15% oxygen concentration [21]. Young [22] explored the relationship between hypoxia and the risk of suicide. He found that the suicide rate at high altitudes was higher than that at low altitudes, and that other conditions related to hypoxia increased the suicide risk. The most likely explanation is that mild hypoxia leads to a decrease in serotonin synthesis.
Zhuang, et al. [23] found that acute, mild and moderate hypoxia had a negative impact on human mood and emotion, by further increasing its effects. Apart from affecting emotions, hypoxia influences metabolism and so energy levels in many ways. Ma, et al. [24] found that hypoxia affected the metabolism of rat liver cells, with ATP content in liver cells declining, and ADP and AMP content increasing with hypoxia, suggesting lower energy production. Gao, et al. [25] found that cerebral mitochondrial metabolic dysfunction occurred under conditions of acute hypoxia and partial functional compensation occurred under conditions of chronic hypoxia. These results indicate that metabolism, and so energy, is affected when the extracellular pH declines and conditions become hypoxic, generating feelings of tiredness, weakness and a lack of motivation to perform tasks. This reflects one of the core symptoms of depression, which is exhaustion or fatigue.
In contrast to other published studies, here we excluded populations with sleep disorders from the healthy controls, as clients who complain of a sleep disorder, but who produce normal test results in clinics are not totally healthy. In the process of collecting case studies, we came across an interesting index which might be useful, PvO2, a particularly sensitive indicator. A person with PvO2≤22mmHg exhibited a healthy appetite and sleep pattern, and was energetic during the daytime. With an increase in PvO2, a person gradually develops hypersomnia, then insomnia, then anxiety and even suicidal ideation if PvO2 >40mmHg. The fluctuation of PvO2 from normality to somnolence, insomnia, anxiety and depression reflected the severity of hypoxia.
▪ Factors correlated with histogenous hypoxia
It was found that BMI correlated with the results of venous blood-gas analysis in the depressive group, but there was no correlation in the healthy control group. This means that BMI can be used to evaluate the severity of histogenous hypoxia in patients with depression, but would not be suitable for healthy people. There was no significant correlation between venous blood-gas analysis and smoking, course of disease, whether drug administration is current and the course of administration. This means that the results of venous blood-gas analysis are not influenced by smoking, course of administration and drugs used during depression.
The venous pH showed a positive correlation with PVO2 in the depressive group, but no correlation in the healthy control group. This means that histogenous hypoxia can be affected by venous pH in patients with depression, but is unsuitable for healthy people. The PvCO2 showed a negative correlation with PVO2 in both groups. The reason for these may be the influence of pH and PvCO2 of venous blood on the oxygen dissociation curve. The oxygen dissociation curve will move to the right when there is a decreased pH or increased PvCO2, with a decreased affinity of hemoglobin for O2. The curve will move to the left when pH increases or PvCO2 decreases, with an increased affinity of hemoglobin for O2 [26]. The venous blood pH is significantly higher and PvCO2 is significantly lower in patients with depression than in healthy controls. So the oxygen dissociation curve would be expected to move to the left, with an increase in the affinity of hemoglobin for O2, with oxygen less easily released [12]. This may be a reason underlying histogenous hypoxia in patients with depression.
▪ The significance of an elevated venous pH
In the present study, it was found that venous pH increased in patients with depression in comparison with healthy controls, and the venous pH was significantly positively correlated with the total depressive score, the anxiety/ somatization factor and the sleep factor. Under normal physiological conditions, the arterial blood pH is slightly higher than that of venous blood by about 0.03–0.04 [19]. This indicates that some acidic metabolites are discharged from the tissue fluid into blood in the capillaries. Therefore, the larger the difference in pH between the arterial and venous blood, the stronger the acidic excretion in the tissues is, and the less prone to accumulating acidic metabolites a person would be. In the present study the patient’s arterial blood gases were not analyzed, but Yuan Tao [20] could determine no significant difference in arterial pH values between the two groups studied. Therefore, the higher the venous blood pH value is, the less the ability for acid excretion, and an individual would be expected to be more prone to the accumulation of acidic metabolites.
The difference in venous pH was 0.027 between patients with depression and controls. One reason may lie in the measurement error and another may be that acidic metabolites accumulate in depression. A study with a larger sample is required to confirm the latter.
Conclusion
In this cross-sectional study, we compared venous blood gases between patients with depression and healthy controls. We found that there were significantly higher PvO2/SvO2/CvO2 levels and significantly lower PvCO2 levels in patients with depression compared with the control group. From our data it can be deduced that histogenous hypoxia exists in depression. This histogenous hypoxia is correlated positively with elevated venous pH, and both of these indicators are correlated with depression. We conclude that elevated venous pH and a decrease in PvCO2 may underlie histogenous hypoxia in depression, and venous blood-gas levels may be potential biomarkers of depression.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Guangdong Provincial Hospital of Traditional Chinese Medicine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards. Informed consent of taking part in and publishing study result were obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Consent for publication
Informed consent for taking part in the study, and for publishing the results were obtained from each individual participant.
Competing interests
The authors declare that they have no competing interests.
Funding
This research was funded by the Guangdong Province Administration of Traditional Chinese Medicine (program number: 20171209), the National Science and Technologic Program of China (2015BAI13B02), Guangdong Province Hospital of Traditional Chinese Medicine and Guangzhou Huiai Hospital.
▪ Limitations and suggestions for future research
The conclusion of histogenous hypoxia was drawn based on the venous blood-gas analysis and other author’s result of arterial blood-gas analysis in patients suffering from fatigue without strength, but not the arterial and venous bloodgas analysis simultaneous. The results would be more credible if arterial and venous blood-gas analysis were detected simultaneously. Larger sample studies are needed to confirm whether the accumulation of acidic metabolites exists in depression. Whether histogenous hypoxia and elevated venous pH are the state variables or the trait variables remains unclear. Followup blood gas results are needed after patients have received treatment. Further confirmation of data using case-control studies or cohort studies is required.
Acknowledgements
Thanks for the supports of Department of Psychology and Sleep and physical examination center of Guangdong Province Hospital of TCM. Thanks for the direction by Kangguang Lin and Dewei Shang during revision.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-Ⅴ) (2013).
- Zhao ZH, Huang YQ , Li J, et al. An epidemiological survey of mental disorders in Guangzhou area. Chin. J. Nerv. Mental. Dis 35(9), 530–534 (2009).
- Zhang WX, Shen YC, Li SR, et al. Epidemiological investigation on mental disorders in 7 areas of China. Chin. J. Psychiatry 31(2), 69–71 (1998).
- Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS. Med 10(11), 1–12 (2013).
- Palta P, Samuel LJ, Miller ER, et al. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom. Med 76(1), 12–19 (2014).
- Smaga I, Niedzielska E, Gawlik M, et al. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep 67(3), 569–580 (2015).
- Michel TM, Frangou S, Thiemeyer D, et al. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder – a postmortem study. Psychiatry. Res 151(1-2), 145–150 (2007).
- Clanton TL. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol (6), 2379-2388 (1985).
- Sylvester JT, Shimoda LA, Aaronson PI, et al. Hypoxic pulmonary vasoconstriction. Physiol. Rev 92(1), 367–520 (2012).
- Hervouet E, Cizkova A, Demont J, et al. HIF and reactive oxygen species regulate oxidative phosphorylation in cancer. Carcinogenesis 29(8), 1528–1537 (2008).
- Görlach A, Dimova EY, Petry A, et al. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox. Biol 6(1), 372–385 (2015).
- Jin HM, Wang JZ. Pathophysiology. 7th version. People's Medical Publishing House 69–82 (2008).
- Yu QJ. Clinical apply of venous blood gas analysis. Chin. Crit. Care. Med 6(3), 183–186 (1994).
- Zhang DP. Venous blood gas analysis and its clinical significance. J. Med. Postgrad 17(8), 741–743 (2004).
- Dantzker DR. Oxygen transport and utilization in ARDS. Eur. Respir. J. Suppl 11(1), 485s–489s (1990).
- World Health Organization. The international statistical classification of diseases and related health problems 10th revision. In: Liu P, Xu YX (translation) People's Medical Publishing House, China (1995).
- Zhang MY, He YL. Handbook of psychiatric rating scale. 1st version. Hunan Science and Technology Press, 145–148 (2015).
- Lin MZ. Clinical Renal Physiology. People's Military Medical Press (2004).
- Piao ZE. Rapid interpretation of arterial blood gases analysis. China Medical Science and Technology Press, 14–186 (2013).
- Yuan T. The study of relationship between channel Qi and oxygen metabolism. Master Thesis. Hubei Medical College of Chinese Medicine (2007).
- Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J. Neurochem 78(6), 1185–1200 (2001).
- Young SN. Elevated incidence of suicide in people living at altitude, smokers and patients with chronic obstructive pulmonary disease and asthma: possible role of hypoxia causing decreased serotonin synthesis. J. Psychiatry. Neurosci 38(6), 423–426 (2013).
- Zhuang Y, Li XY, Wu XY, et al. The effects of acute mild and moderate hypoxia to human mood. J. Fourth Milit. Med. Univ 21(6), 667–669 (2000).
- Ma ZW, Wang SL, Wang FJ, et al. Changes of energy metabolism in rat BRL cells after hypoxia. J. Dig. Surg 1(5), 311–314 (2002).
- Gao WX, Liu JZ, Wu LP, et al. The effects of acute and chronic hypoxia on energy metabolism of rat brain chondriosome. Chin. J. Pathophysiol 16(10), 879–882 (2000).
- Zhou GJ. Physiology, China Union Medical University Press, 95–127 (2011).