Research Article - Imaging in Medicine (2017) Volume 9, Issue 6
High-resolution diffusion-weighted MRI of the breast using readout-segmented EPI and single-shot EPI
Shotaro Kanao*1, Masako Kataoka1, Mami Iima1, Ayami Ohno1, Rena Sakaguchi1, Akane Ohashi1, Maya Honda1, Masakazu Toi2, David A Porter3 & Kaori Togashi11Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University Graduate School of Medicine, Japan
2Department of Breast Surgery, Kyoto University Graduate School of Medicine, Japan
3Fraunhofer Mevis Institute for Medical Image Computing, Bremen, Germany
- Corresponding Author:
- Shotaro Kanao
Department of Diagnostic Imaging and Nuclear Medicine
Kyoto University Graduate School of Medicine, Japan
E-mail: kanaos@kuhp.kyoto-u.ac.jp
Abstract
Objective: Compared to dynamic contrast enhanced MRI (DCE-MRI), image quality in diffusion-weighted MR imaging (DW-MRI) is generally poor because of low image spatial resolution and distortion. Readout-segmented EPI (RS-EPI) is a recently-developed technique to obtain high resolution DW-MRI with less distortion. The study aimed to evaluate the feasibility of high-resolution DW-MRI of the breast, using RS-EPI and single-shot EPI (SS-EPI).
Methods: With IRB approval, 26 patients with clinically suspected breast cancer were prospectively enrolled. Breast MRI was performed on a 3.0 T scanner using a 16-channel breast coil. Sequences include T2-weighted imaging (T2WI) with fat saturation, a 3D DCE-MRI series and DW-MRI (b values of 0 and 850 s/mm2 for RS-EPI and SS-EPI). To evaluate image distortion, the antero-posterior (AP) extent of the mammary gland on DW-MRI with RS-EPI and SS-EPI were measured and differences from values obtained from T2WI were calculated. The artefacts on both sequences were scored. Diagnostic performance of lesions on both sequences was assessed using BI-RADS classification and the category was compared to that determined by DCE-MRI.
Results: The average difference in the measured AP extent on DW-MRI with RS-EPI was significantly less than that on DW-MRI with SS-EPI (2.3 ± 2.0 versus 12.5 ± 4.7 mm, respectively, p<0.01). Distortion artefacts were significantly lower on DW-MRI with RS-EPI than that with SS-EPI in all cases. The sensitivity of DW-MRI with RS-EPI was higher than that with SS-EPI (88.9 and 77.8%, respectively).
Conclusion: DW-MRI with RS-EPI has an advantage of less distortion compared to that with SS-EPI.
Keywords
breast cancer ▪ magnetic resonance imaging ▪ diffusion weighted imaging ▪ readout segmented echo planner imaging ▪ single shot echo planner imaging
Introduction
MRI of the breast is a study that requires the administration of a gadolinium-containing contrast agent during the study. Non-contrast diffusion-weighted MR imaging (DW-MRI) is expected to increase the diagnostic accuracy of detecting breast cancer due to excellent lesion conspicuity [1,2]. However, compared to dynamic contrast enhanced MRI (DCE-MRI), the image quality of DW-MRI is generally poor because of low spatial resolution and image distortion, particularly around the nipple or breast-air interface. According to the Guidelines of the European Society of Breast Imaging, the spatial resolution of DCE-MRI should be at least 1.0 × 1.0 × 2.5 mm [3]. If DW-MRI is used to evaluate breast lesion, it would be ideal to achieve a high spatial resolution equivalent to that of DCE-MRI, with minimal image distortion.
Single-shot echo-planar imaging (SS-EPI) has been established as the method of choice for performing DW-MRI [4]. SS-EPI has the advantage of a short scan time and high signal-to-noise ratio compared to other techniques, but has the disadvantage of geometric distortion, signal dropout, and image blurring from field inhomogeneity and eddy currents [5]. On the other hand, readout segmented EPI (RS-EPI), with 2D navigator echoes for reduced sensitivity to motion-induced phase errors, is a recentlydeveloped technique to obtain high resolution DW-MRI with less geometric distortion, image blurring, and ghosting artefacts [6]. The advantages of RS-EPI have already been reported for a number of anatomical regions, including the breast [7-9], the skull base [10] and the head and neck region [11], demonstrating that the reduction of distortion is important to identify anatomical structure correctly.
The objective of our study was to evaluate the feasibility of high resolution DW-MRI (special resolution of 1.0 × 1.0 × 2.5 mm) of the breast, using RS-EPI and SS-EPI. Image distortion and artefacts on RS-EPI and SS-EPI images were evaluated. Diagnostic performances of these two high resolution DW-MRI sequences were estimated and compared with that of DCE-MRI.
Materials and methods
■Patient population
With IRB approval, 26 patients who underwent DCE-MRI of the breast with clinically suspected breast cancer were prospectively enrolled between January and November 2011. General written informed consent was obtained for each patient. Exclusion criteria were as follows: those who had received presurgical systemic therapy; those who had already had an MR scan due to post-treatment follow-up; those whose diagnosis was not pathologically confirmed.
■Image acquisition
Breast MRI was performed on a 3.0 T scanner (MAGNETOM Trio, A Tim System, Siemens AG) with a 16-channel breast coil. Sequences were as follows: axial T1-weighted images (T1WI), T2-weighted images (T2WI) with fat saturation, a 3D dynamic DCEMRI series and DW-MRI. The 3.0-T MRI parameters were set as follows: T2-weighted images (whole breast; axial orientation; 2D turbo spin echo with fat suppression; repetition time/echo time [TR/TE], 5500/77 ms; FOV, 330 × 330 mm; matrix, 448 × 336; thickness, 3.0 mm), T1-weighted images (whole breast; axial orientation; 3D volumetric interpolated breath-hold examination [VIBE]; TR/TE, 4.83/2.45; FOV, 330 × 330 mm; matrix, 448 × 399; thickness, 1.5 mm), T1-weighted DCE images scanned pre-contrast and at 0-1, 1-2 and 5-6 min after gadolinium injection (whole breast; axial orientation; 3D-VIBE with fat suppression; TR/TE, 3.70/1.36 ms; flip angle [FA], 15; FOV, 330 × 330 mm; matrix, 384 × 346; thickness, 1.0 mm), contrast-enhanced T1-weighted images at high spatial resolution at 2-4.5 min after gadolinium injection (whole breast; coronal orientation; 3D-VIBE with fat suppression; TR/TE, 4.01/1.63 ms; FA, 15; FOV, 330 × 330 mm; matrix, 512 × 461; thickness, 0.8 mm). Sagittal, unilateral breast DW-MRI at b values 0 and 850 s/mm2 were obtained using the following parameters:
1) RS-EPI: TR/TE=6800/60 ms, FOV=200 × 150 mm, matrix=200 × 150, 2.5 mm thickness, 40 slices, resolution of 1.0 × 1.0 × 2.5mm NEX=1, integrated parallel acquisition techniques (iPAT) × 2, 3 min 32 s, seven shots.
2) SS-EPI: TR/TE=8400/69 ms, FOV=200 × 156 mm, matrix=200 × 156, 2.5 mm thickness, 40 slices, resolution of 1.0 × 1.0 × 2.5 mm, NEX=5, iPAT × 2, 3 min 30 s. Both DW-MRI sequences were work-in-progress sequences and used a modified monopolar diffusion scheme [12] and fat suppression with gradient reversal technique [13]. A summary of sequence parameters used for DW-MRI is shown in TABLE l. The infused Gadolinium contrast medium was Gadoteridol (ProHance, Eisai Inc., Tokyo, Japan) for 0.2 ml/kg power injected at the speed of 2.0 ml/s and flashed with 20 ml of saline at the same rate.
| Single-Shot Echo-planar Imaging | Readout-Segmented Echo-planar Imaging | |
|---|---|---|
| Diffusion directions | Three-direction trace | Three-direction trace |
| Diffusion schema | modified monopolar | modified monopolar |
| b value (s/mm2) | 0, 850 | 0,850 |
| Fat suppression | SPAIR with gradient reversal | SPAIR with gradient reversal |
| Repetition time (ms) | 8400 | 6800 |
| Echo time (ms) | 69 | 60 |
| Field of view (mm) | 200 × 156 | 200 × 150 |
| Matrix | 200 × 156 | 200 × 150 |
| No. of sections | 40 | 40 |
| Section thickness (mm) | 2.5 | 2.5 |
| Intersection gap (%) | 0 | 0 |
| Parallel Imaging Factor | 2 | 2 |
| Phase-encoding direction | Antero-posterior | Antero-posterior |
| Readout segments | 1 | 7 |
| Average | 5 | 1 |
| Acquisition time | 3 min 32 s | 3 min 32 s |
Table 1: Sequence parameters for single-shot and readout-segmented echo-planar imaging.
■Image evaluation
Image distortion and artefacts
To evaluate the degree of image distortion, the antero-posterior (AP) extent of the mammary gland was measured on sagittal DW-MRI acquired with RS-EPI and SS-EPI (b=0 s/mm2). The AP extent measured on sagittal T2WI was used as a reference. The AP extent was measured as the maximum distance between the mammary gland border on DWI image and T2WI.
Distortion artefacts and ghost artefacts was also scored separately using RS-EPI and SSEPI (b=0 and 850 s/mm2), and evaluated by one board-certified radiologist using a fourpoint scale (0=no artifact, 1=small artifact, 2=moderate artefact, 3=severe artefact).
■Diagnostic performance
To evaluate the diagnostic performance of each sequence in breast cancer, lesions on RSEPI/ SS-EPI DW-MRI (b=850 s/mm2) and DCE-MRI were categorized based on Breast Imaging Report and Data System (BI-RADS) MRI. ADC maps were calculated on a pixel-bypixel basis, according to the equation: ADC=ln [S1/S0]/(b1-b0), where S0 and S1 are the signal intensities in the region-of-interest (ROI) obtained at two b-values, b1 and b0 (b0=0 s/ mm2 and b1=850 s/mm2). For quantitative evaluation, the apparent diffusion coefficient (ADC) of background mammary gland and lesions were measured by one board-certified radiologist.
■Statistical analysis
Statistical Analysis was performed using EZR (Saitama Medical Center, Jichi Medical University [14] which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.2.2). The image quality scores of the two DW-MRI sequences were compared using the Wilcoxon matched-pairs signed rank test. The ADC values of lesions and background parenchyma were compared using paired Student t test. Statistical significance was set at p<0.05 for all tests.
For diagnostic performance based on BIRADS classification, the five categories were used for Receiver Operating Characteristic (ROC) analysis. Sensitivity and specificity of three sequences were evaluated using category 4 and 5 as positive for malignancy.
Results
Among 26 patients, 18 patients were pathologically confirmed as having breast cancer including 15 invasive ductal carcinoma (IDC), one mucinous carcinoma and two ductal carcinomas in situ (DCIS).
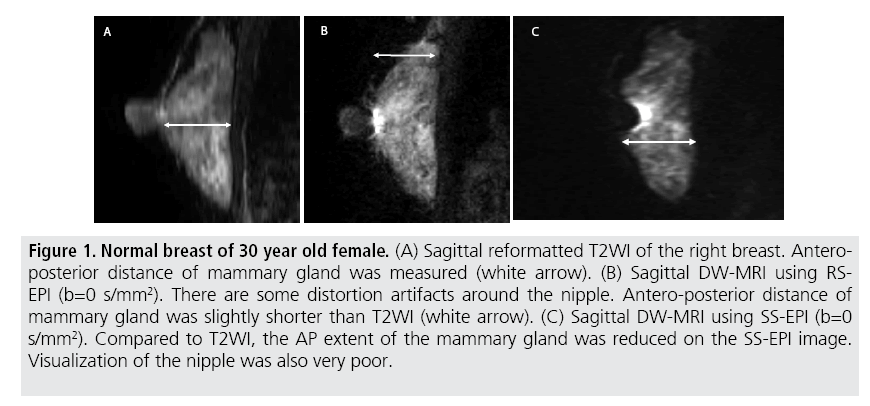
Relative to the reference value provided by T2WI, the average difference in the AP extent of the mammary gland on DW-MRI with RSEPI (2.3 ± 2.0 mm) was significantly less than that on DW-MRI with SS-EPI (12.5 ± 4.7 mm, p<0.01) (TABLE 2 and FIGURE 1). Distortion artefacts were significantly lower on DW-MRIs with RS-EPI than those with SS-EPI in all cases, while no significant difference of ghost artefacts was observed between the two sequences (p=0.83) (TABLE 2).
| RS-EPI | SS-EPI | ||
|---|---|---|---|
| AP distance of mammary gland (compared to T2WI) | 2.3 ± 2.0 mm | 12.5 ± 4.7 mm | p<0.01 |
| Distortion Artifact | 1.04 ± 0.20 | 2.15 ± 0.37 | p<0.01 |
| Ghost Artifact | 1.31 ± 0.47 | 1.31 ± 0.55 | p=0.83 |
| Abbreviations DW-MRI: diffusion weighted MRI; RS-EPI: readout segmented echo planner imaging; SS-EPI: single shot echo planner imaging; AP: antero-posterior Distortion and ghost artifacts using a four-point score (0=no artifact, 1=small artifact, 2=moderate artifact, 3=severe artifact) |
|||
Table 2: Evaluation of the image distortion on DW-MRIs with RS-EPI and SS-EPI.
Figure 1: Normal breast of 30 year old female. (A) Sagittal reformatted T2WI of the right breast. Anteroposterior distance of mammary gland was measured (white arrow). (B) Sagittal DW-MRI using RSEPI (b=0 s/mm2). There are some distortion artifacts around the nipple. Antero-posterior distance of mammary gland was slightly shorter than T2WI (white arrow). (C) Sagittal DW-MRI using SS-EPI (b=0 s/mm2). Compared to T2WI, the AP extent of the mammary gland was reduced on the SS-EPI image. Visualization of the nipple was also very poor.
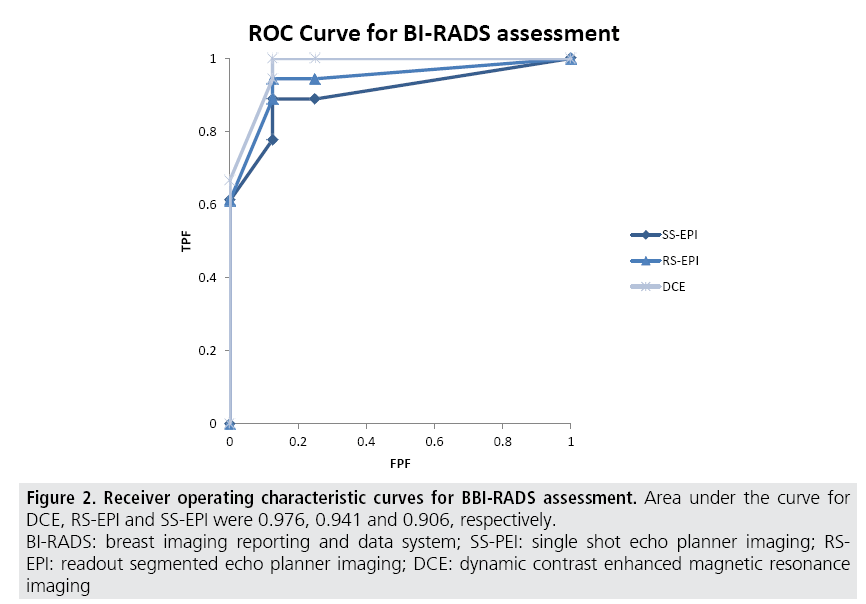
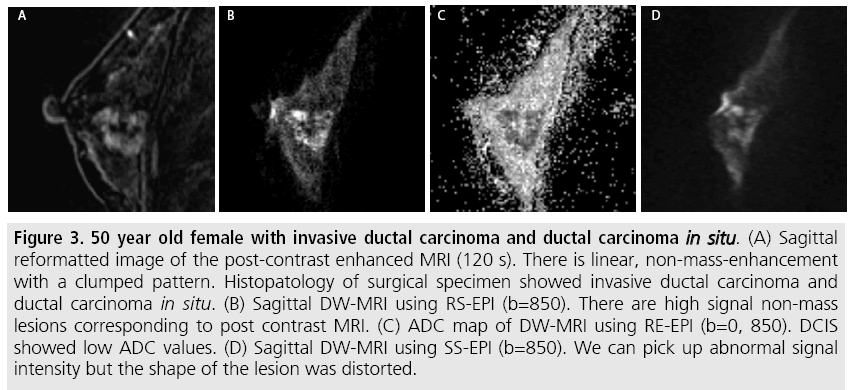
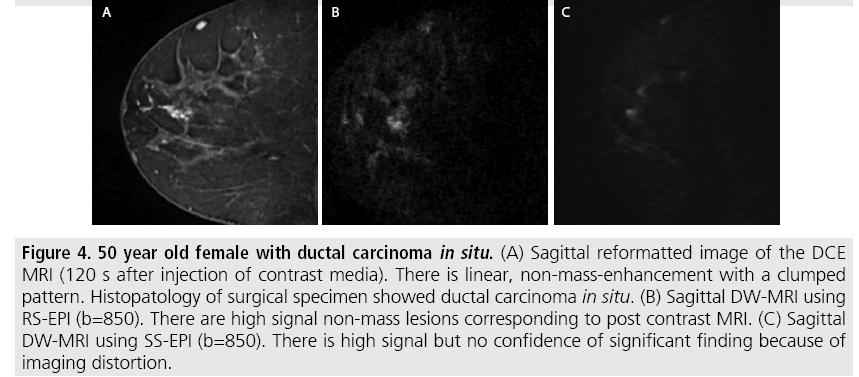
The sensitivity of DW-MRI with RS-EPI was higher than that with SS-EPI (88.9 versus 77.8%), while that of DCE-MRI was 94.4%. Specificity of these three sequences was 85.7 % for all. ROC analysis was performed (FIGURE 2) and the area under the curve for RS-EPI and SS-EPI was 0.941 and 0.906 respectively, while that of DCE-MRI was 0.976. The false negative case on DW-MRI with RS-EPI and SS-EPI, and DCE-MRI was mucinous carcinoma with minimal enhancement and very high ADC. Typical case of IDC with DCIS was shown in (FIGURE 3). The false negative cases only by DW-MRI with SS-EPI were DCIS with small volume (FIGURE 4).
Figure 2: Receiver operating characteristic curves for BBI-RADS assessment. Area under the curve for
DCE, RS-EPI and SS-EPI were 0.976, 0.941 and 0.906, respectively.
BI-RADS: breast imaging reporting and data system; SS-PEI: single shot echo planner imaging; RSEPI:
readout segmented echo planner imaging; DCE: dynamic contrast enhanced magnetic resonance
imaging
Figure 3: 50 year old female with invasive ductal carcinoma and ductal carcinoma in situ. (A) Sagittal reformatted image of the post-contrast enhanced MRI (120 s). There is linear, non-mass-enhancement with a clumped pattern. Histopatology of surgical specimen showed invasive ductal carcinoma and ductal carcinoma in situ. (B) Sagittal DW-MRI using RS-EPI (b=850). There are high signal non-mass lesions corresponding to post contrast MRI. (C) ADC map of DW-MRI using RE-EPI (b=0, 850). DCIS showed low ADC values. (D) Sagittal DW-MRI using SS-EPI (b=850). We can pick up abnormal signal intensity but the shape of the lesion was distorted.
Figure 4: 50 year old female with ductal carcinoma in situ. (A) Sagittal reformatted image of the DCE MRI (120 s after injection of contrast media). There is linear, non-mass-enhancement with a clumped pattern. Histopatology of surgical specimen showed ductal carcinoma in situ. (B) Sagittal DW-MRI using RS-EPI (b=850). There are high signal non-mass lesions corresponding to post contrast MRI. (C) Sagittal DW-MRI using SS-EPI (b=850). There is high signal but no confidence of significant finding because of imaging distortion.
Average ADC values of the mammary gland by RS-EPI and SS-EPI were 2.12 ± 0.24 and 1.47 ± 0.40 × 10-3 mm2/s, respectively (p<0.01) and those of breast cancer were 1.04 ± 0.36 and 0.87 ± 0.32 × 10-3 mm2/s, respectively (p<0.01).
Discussion
In our study, most of the breast cancers were picked up by both high-resolution DW-MRI of the breast using RS-EPI and SS-EPI with the same specificity. We also showed that DW-MRI with RS-EPI has less image distortion, which is probably related to the improved sensitivity compared to DW-MRI with SS-EPI. The results suggest that there is a promising role for RSEPI, despite known advantages of SS-EPI over RS-EPI including established clinical experience with extensive data relating to ADC for various lesion types [4,15,16].
Our results regarding the lower distortion with RS-EPI agree with previous reports that RS-EPI tends to delineate anatomic detail better than SS-EPI because T2* blurring is reduced [7]. There are several previous DWI studies in the breast using RS-EPI [7-9,17]. In 2012, Bogner et al. reported the advantage of RS-EPI compared to SS-EPI. In this study, spatial resolution of both images was about 2.0 mm in plane [7]. In 2014, Wisner et al. reported the advantage of RS-EPI with spatial resolution about 1.8 mm in plane [17]. In 2015, one study reported the advantage of unilateral Zoomed EPI (single shot EPI with reduced FOV technique) compared to RS-EPI of both breasts. But in this study, spatial resolution of RS-EPI and Zoomed EPI were not matched (1.7 mm, 0.58 mm, respectively) [8]. Recently, Bogner et al. reported the 7.0 T breast DW-MRI using RS-EPI with spatial resolution about 0.9 mm [9]. These papers have a common point that diagnostic performance is better with higher spatial resolution and if the spatial resolution is the same, RS-EPI has the advantage of less distortion. Our study is, to our knowledge, the first paper that compares SS-EPI and RS-EPI in breast MRI using the same high spatial resolution of 1.0 mm for both sequences.
For quantitative evaluation, the ADC value is recognized as an important parameter in the interpretation of DW-MRI images. Regarding the comparison of SS-EPI and RS-EPI, some papers have reported no significant difference among them [7,11]. But Wisner et al. reported a higher ADC value in background breast parenchyma that is similar to the result of our study [17]. Ihalainen et al also reported a higher ADC using RS-EPI in a phantom study [18]. With these mixed results, we should be cautious when performing quantitative evaluation of DW-MRI using RS-EPI in comparison to those obtained using SS-EPI. One reason for the difference is likely to be due to the effect of calculating modulus images before signal averaging to avoid motion-induced phase errors with SS-EPI.
There are some limitations to this study. One limitation is the small population, making it difficult to identify significant differences in diagnostic performance between the two sequences. Another limitation is related to the version of the sequences used in the study. Because we used works-in-progress sequences, we have the latitude of choice about TR/TE. To the contrary, in the commercial version of DWMRI, we cannot scan with high resolution or very long time. In this situation, there are some solution about two sequence that is the zoomed EPI technique [8] for SS-EPI and partial Fourier technique [19] for RS-EPI.
The ultimate goal of breast DW-MRI is to allow breast MR examinations to be performed without contrast medium. For patient with renal dysfunction, past allergic history for gadolinium contrast medium and asthma, breast MR examination without contrast medium is desirable. Meta-analysis of diagnostic performance comparing DWMRI with SS-EPI and DCE-MRI showed lower sensitivity despite higher specificity of DW-MRI [16], with problems in diagnosing non-mass lesions [20]. In order to improve the diagnostic performance of DW-MRI, high resolution DW-MRI with less distortion using RS-EPI is a possible way to achieve this goal. Another important role of DWI is to provide complementary information about the lesion compared to that provided by DCE-MRI, such as cell density. Again, less-distorted images are essential in order to match cell density information from DW-MRI with perfusion information from DCE-MRI.
Conclusion
High resolution DW-MRI using RS-EPI has the advantage of reduced distortion compared to SS-EPI.
References
- Guo Y, Cai YQ, Cai ZL et al. Differentiation of clinically benign and malignant breast lesions using diffusion weighted imaging. J. Magn. Reson. Imaging. 16, 172-178 (2002).
- Partridge SC, Rahbar H, Murthy R et al. Improved diagnostic accuracy of breast MRI through combined apparent diffusion coefficients and dynamic contrast-enhanced kinetics. Magn. Reson. Med. 65, 1759-1767 (2011).
- Mann RM, Kuhl CK, Kinkel K et al. Breast MRI: guidelines from the European Society of Breast Imaging. Eur. Radiol. 18, 1307-1318 (2008).
- Wenkel E, Geppert C, Schulz WR et al. Diffusion weighted imaging in breast MRI: Comparison of two different pulse sequences. Acad. Radiol. 14, 1077-1083 (2007).
- Porter DA, Calamante F, Gadian DG et al. The effect of residual Nyquist ghost in quantitative echo-planar diffusion imaging. Magn. Reson. Med. 42, 385-392 (1999).
- Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn. Reson. Med. 62, 468-475 (2009).
- Bogner W, Pinker DK, Bickel H et al. Readout-segmented echo-planar imaging improves the diagnostic performance of diffusion-weighted MR breast examinations at 3.0 T. Radiology. 263, 64-76 (2012).
- Park JY, Shin HJ, Shin KC et al. Comparison of readout segmented echo planar imaging (EPI) and EPI with reduced field-of-VIew diffusion-weighted imaging at 3t in patients with breast cancer. J. Magn. Reson. Imaging. 42, 1679-1688 (2015).
- Bogner W, Pinker K, Zaric O et al. Bilateral diffusion-weighted MR imaging of breast tumors with submillimeter resolution using readout-segmented echo-planar imaging at 7 T. Radiology. 274, 74-84 (2015).
- Iima M, Yamamoto A, Brion V et al. Reduced-distortion diffusion MRI of the craniovertebral junction. AJNR. Am. J. Neuroradiol. 33, 1321-1325 (2012).
- Koyasu S, Iima M, Umeoka S et al. The clinical utility of reduced-distortion readout-segmented echo-planar imaging in the head and neck region: initial experience. Eur. Radiol. 24, 3088-3096 (2014).
- Morelli JN, Runge VM, Feiweier T et al. Evaluation of a modified Stejskal-Tanner diffusion encoding scheme, permitting a marked reduction in TE, in diffusion-weighted imaging of stroke patients at 3 T. Invest. Radiol. 45, 29-35 (2010).
- Park HW, Kim DJ, Cho ZH. Gradient reversal technique and its applications to chemical-shift-related NMR imaging. Magn. Reson. Med. 4, 526-536 (1987).
- Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452-458 (2013).
- Tsushima Y, Takahashi TA, Endo K. Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J. Magn. Reson. Imaging. 30, 249-255 (2009).
- Chen X, Li WL, Zhang Y et al. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC. Cancer. 10, 693 (2010).
- Wisner DJ, Rogers N, Deshpande VS et al. High-resolution diffusion-weighted imaging for the separation of benign from malignant BI-RADS 4/5 lesions found on breast MRI at 3T. J. Magn. Reson. Imaging. 40, 674-681 (2014).
- Ihalainen T, Kuusela L, Soikkeli M et al. A body-sized phantom for evaluation of diffusion-weighted MRI data using conventional, readout-segmented, and zoomed echo-planar sequences. Acta. Radiol. 57, 947-954 (2015).
- Frost R, Porter DA, Miller KL et al. Implementation and assessment of diffusion-weighted partial Fourier readout-segmented echo-planar imaging. Magn. Reson. Med. 68, 441-451 (2012).
- Tozaki M, Fukuma E. 1H MR spectroscopy and diffusion-weighted imaging of the breast: Are they useful tools for characterizing breast lesions before biopsy? AJR. Am. J. Roentgenol. 193, 840-849 (2009).