Mini Review - Interventional Cardiology (2021)
Extracellular matrix proteins in aortic stenosis
- Corresponding Author:
- Rihab Bouchareb
Cardiovascular Research Institute,
Icahn School of Medicine at Mount Sinai,
New York, NY 10029,
USA,
E-mail: rihab.bouchareb@mssm.edu/rihabdahou@gmail.com
Received date: August 09, 2021; Accepted date: August 23, 2021 Published date: August 30, 2021
Abstract
Extracellular Matrix Proteins (ECM) are the major protein component of the aortic valve. ECM proteins ensure the normal function of the valve by sensing and transducing mechanical stress signals to the Interstitial Valve Cells (VICs). The fibrosa, spongiosa, and ventricularis are the three layers making up the aortic valve. Each layer in the AV has a distinguished ECM composition. Indeed, the fibrosa is mainly composed of collagen type I and III. In contrast, the spongiosa is composed of Proteoglycans (PGs) and Glycosaminoglycan (GAG). Finally the ventricularis is composed of elastin. A disturbance in the ECM matrix may subsequently affect valve cell signaling and promote valve calcification. The use of proteomic approaches have helped to identify differences in expression levels of ECM proteins in the different layers of the aortic valve. This review will discuss the role of changes in the composition of ECM proteins in aortic stenosis and explore the latest findings regarding the ECM network in valvular heart disease.
Keywords
Aortic stenosis • Transcatheter aortic valve replacement • Interstitial cell • Extracellular matrix • Proteoglycans
Introduction
Aortic Stenosis (AS) is a fibrocalcific disease characterized by both fibrosis and calcification. AS was considered as an age-related degenerative disease, however, accumulating evidence is showing AS to be an active process [1,2]. Indeed, AS is a progressive disease that might be influenced by inflammation, lipid infiltration and osteoblastic transition [3,4]. Currently there are no pharmacological treatments to reverse calcification or to stop its progression. The only effective treatment for AS is surgical or Transcatheter Aortic Valve Replacement (TAVR) [5].
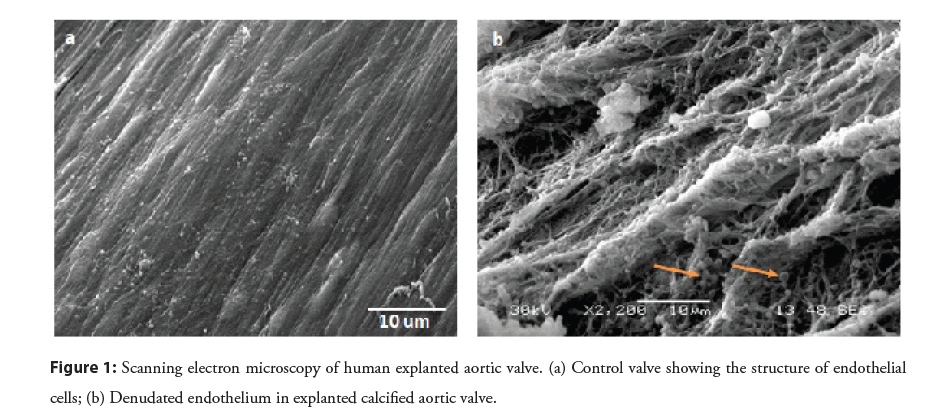
In non-pathological states, the presence of collagen type I in the fibrosa is crucial to prevent valvular prolapse, whereas elastin in the ventricularis layer insures the expandability of the valve during systole and diastole [2,6]. The spongiosa layer is composed of a structure of hydrophilic proteoglycans proteins that oppose the mechanical stress applied to the valve during closure in diastole [2,7]. However, under pathological aortic valve disease, collagen fibers bundles are degraded and disorganized [2]. Our previous microscopic analysis of calcified valve tissue showed an extensive denudation of the endothelial cell layer exposing collagen fibers to the mechanical forces of shear stress (Figure 1). Moreover, we have observed the presence of calcified vesicles interacting with the exposed collagen fibers (Figure 1). We have recently used a proteomics approach to examine the nature and the distribution of ECM proteins in stenotic valves with or without calcification in vitro [4]. Our data confirmed the disturbance in collagen that was accompanied by the overexpression of metalloproteinase proteins. Aortic valve tissue is also rich in Proteoglycans (PGs) e.g. Versican, decorin and biglycan [8,9]. PGs play a role in collagen fibrillogenesis and their overexpression has been observed surrounding calcium nodules in pathological aortic valve tissue [8].
Literature Review
The implication of ECM proteins in valve structure
The aortic valve is the outflow valve for the left ventricle [10]. The aortic valve must have proper closing and opening of three leaflets or cusps [11]. Each leaflet is composed of an outer endothelial cell layer and an inner connective tissue i.e. Interstitial Cell (VIC) layer. The VIC layer is composed of a heterogeneous cell population [10]. It is composed of interstitial cells, smooth muscle cells, fibroblasts, myofibroblast and recently identified resident stem cells originating from circulating hematopoietic stem cells [12]. Valve cells are embedded in the Extracellular Matrix (ECM) proteins [10]. The VIC layer is further divided into three compartmental layers based on the distinct composition and orientation of ECM proteins, thus defining mechanical properties [10,13]. A correct organization and composition of ECM in the leaflets enables the stress bearing repetitive valve motion [10]. On the aortic side, the fibrosa layer that bears intense tensile shear stress is mostly composed of type I collagen fibers that are circumferentially oriented with a small amount of elastin fibers [4,6,10,14]. In the diseased valve, this layer is often the target site of major pathophysiological alteration that leads to valve dysfunction. The middle layer or spongiosa is the thickest layer and is rich in proteoglycan [10,14]. The main molecular component of proteoglycans is glycosaminoglycan that provides hydration and expansive pressure to the tissue functioning as a damper that absorbs shocks and pressure gradients generated by hemodynamic mechanical stress [10,13,15]. The elastin fiber rich ventricularis is the layer closest to the ventricle providing pliability to the aortic valve [10]. In addition to these major ECM layers, fibronectin, osteonectin and periostin in the fibrosa layer provide valvular stiffness [10]. A recent proteomics study in combination with network connectivity analysis identified ECM proteins critical for normal valve function. There were newly discovered ECM proteins including Nidogen 1 (Nid1), Biglycan (Bgn), Elastin microfibril interface-located protein 1 (Emmilin-1) and Milk fat globule-EGF factor 8 (Mfge8) that have been validated with functional distribution by immunofluorescence [8].
Association of ECM proteins and aortic valve dysfunction
Aortic valve calcification was once considered a degenerative disease resulting from excess calcium deposition [16]. However, current consensus is that it is a progressive disease associated with tissue remodeling accompanied by a dynamic alteration in ECM proteins [7]. An increasing number of studies have discovered the importance of ECM proteins in maintaining the structure of aortic valves during development and during pathophysiological states [10,14]. Elucidating the precise mechanism(s) of the role of ECM proteins in disease progression will allow us to develop new treatment strategies for aortic valve calcification.
It has been reported that diseased human aortic valves exhibit thickening and increased stiffness in the leaflets resulting from changes in the composition of ECM proteins and tissue calcification [7]. Healthy human aortic valve structure is assured by VICs’ role in secretion and degradation of ECM proteins under physiological conditions. Cross talk between Valve Endothelial Cells (VECs) and VICs is also crucial for maintenance of proper ECM architecture [14]. The number of myofibroblast-like cells in non-diseased adult human aortic valves (~ 2.5%) are relatively low compared to numbers reported in neonates and pediatric children (~ 20% and ~ 6% respectively) [14] reflecting their role in valvular tissue undergoing a dynamic change in structure and function during the course of growth and development. However, in pathological conditions in addition to VICs and VECs other cell types including immune related cells such as resident macrophages and dendritic cells are activated [17]. Furthermore, circulating macrophages, T cells and B cells infiltrate valve tissue as a result of endothelial cell damage and participate in disease development [17].
Discussion
Stimulation of isolated porcine VICs with transforming growth factor β TGF-β induces stress fibers containing αSMA expression that enhance contractility of activated myofibroblasts and inhibits proliferation of valve cells with apoptosis in a dose-dependent manner [18,19]. Furthermore, TGF-β promotes the expression of proteolytic enzymes such as Matrix Metalloproteinase (MMPs) [1]. Indeed, histological analysis of explanted calcified aortic valves revealed the overexpression of TGF-β, MMP9, and Alkaline Phosphatase (ALP) [20]. The pro-inflammatory cytokine Tumor Necrosis Factor alpha (TNFα) may activate myofibroblast differentiation, stimulate proliferation, and affect the composition of MMPs [21]. Likewise, immunohistochemical analysis of human explanted valve tissue revealed an increase in leukocyte infiltration and intense co-localization signals of TNFα and MMP1 in calcific aortic valves while Tissue Inhibitor of Metalloproteninase1 (TIMP-1) was reported to be unchanged [21]. Recently, an in vitro study showed that the stimulation of isolated human valve cells with recombinant MMP10 induces inflammatory, fibrosis as well as over-expression of osteogenic markers [1]. Furthermore, an overexpression of MMP10 in explanted human calcified valve tissue has been reported [1].
The disruption of ECM organization and ECM protein expression and composition have been reported to be affected in calcified aortic valve tissue which may impact normal valve function [10]. Indeed, specific types of proteoglycans i.e. decorin and biglycan have higher electrostatic affinity to LDL particles resulting in subendothelial retention of oxLDL [9,22]. The retention of ox-LDL activates an inflammatory state in the valves through cytokine overexpression and the recruitment of macrophages [3]. Likewise, the secreted cytokines promote the differentiation of VICS into a myofibroblast phenotype, which consequently leads to ECM protein release [3,23,24]. Interestingly, Second Harmonic Generation microscopy studies have identified layerspecific changes in the microarchitecture of the ECM in calcific aortic valves [10,25]. Although type 1 collagen is predominantly present in the fibrosa layer in healthy valve leaflets, image analysis revealed that major collagen fiber changes occur in the spongiosa where the quantity of collagen fibers was increased by more than 2-fold. In addition, collagen fiber width and density also increased significantly while in the fibrosa the collagen fibers became significantly shorter [2].
Current Findings and Future Perspective
A recent analysis of ECM proteins using mass spectrometry using human calcified aortic valves by Bouchareb, et al. [4] discovered novel ECM proteins that can serve as biomarkers for valve calcification i.e. Fibronectin Type III Domain Containing 1 (FNDC1) and Matrix-Remodeling Associated 5 (MXRA5) [4]. Interestingly, these novel markers were also identified and validating in an RNA-seq dataset obtained from an independent human cohort study of calcified valves in Canada [4]. The study also revealed a potential association between altered expression of ECM proteins and changes in metabolic pathway genes. MXRA5 is a secretory glycoprotein also known as an adhesion protein [4]. A study of its role in tissue injury and fibrosis using HK-2 human proximal tubular epithelial cells identified MXRA5 as a TGFbeta regulated protein with anti-inflammatory and anti-fibrotic properties [19]. Thus, elevated levels of MXRA5 expression may reflect a compensatory mechanism in response to inflammation in the early stages of the valve calcification process [4]. FNDC1 has been implicated in multiple cancers including breast, gastric and prostate cancers [26,27]. The roles of FNDC1 have been demonstrated in the regulation of proliferation, apoptosis and cell migration [28]. A gene expression profiling study using breast cancer tissue revealed an upregulation in FNDC1 [29]. Bouchareb’s study independently confirmed that the disruption in ECM proteins is significantly associated with different stages of the disease progression (early fibrotic stage vs. late calcified stage) in aortic valve calcification. It is particularly important to identify early-stage disease biomarkers that can be used for early diagnosis and treatment and that could help prevent/slow the progression of the disease.
Metabolic syndrome has been linked to valvular calcification together with other risk factors such as age, sex, smoking, hypertension, obesity as well as type 2 diabetes [30]. The Multi- Ethnic Study of Atherosclerosis (MESA) reported that the risk for aortic valve calcification increased with an increase in the number of metabolic syndrome components [31]. Recent advancement in high-throughput techniques including RNA-seq and massspectrometry and data analysis tools have allowed us to ask more complex biological questions. Indeed, Bouchareb’s study reported the downregulation of metabolic pathway proteins such as Superoxide Dismutase 1 (SOD1), Aldehyde Dehydrogenase 1 (ALDH1), and Enolase 1 (ENO1). Furthermore, ECM proteins and metabolic pathway proteins were the most enriched in the Kyoto Encyclopedia of Genes and Genomes (KEGG) network. This analysis indicates a potential strong interaction between metabolic syndrome pathways and altered ECM protein expression in valve calcification [4].
Discovering ECM proteins that are strongly correlated with aortic valve calcification through the application of proteomic studies and RNA-seq data analysis might be a first step toward understanding the disease process. Future studies validating causal mechanisms between identified EMC proteins and valve calcification are necessary to develop pharmacological prevention/ treatment protocols for valve calcification.
Conclusion
Extracellular matrix proteins are crucial to insure normal valve motion and hemodynamics. Excessive ECM proteins remodeling will affect valve structure, promote lipid retention and inflammation and subsequently calcification or fibrosis of the valve. Discovering the specific ECM protein network(s) involved will enhance our understanding of valve calcification.
References
- Matilla L, Roncal C, Ibarrola J, et al. A role for MMP-10 (Matrix Metalloproteinase-10) in calcific aortic valve stenosis. Arterioscler Thromb Vasc Biol. 40(5): 1370-1382 (2020).
- Hutson HN, Marohl T, Anderson M, et al. Calcific aortic valve disease is associated with layer-specific alterations in collagen architecture. PLoS One. 11(9): e0163858 (2016).
- Nsaibia MJ, Boulanger MC, Bouchareb R, et al. OxLDL-derived lysophosphatidic acid promotes the progression of aortic valve stenosis through a LPAR1-RhoA-NF-kappaB pathway. Cardiovasc Res. 113(11): 1351-1363 (2017).
- Bouchareb R, Guauque-Olarte S, Snider J, et al. Proteomic architecture of valvular extracellular matrix: FNDC1 and MXRA5 are new biomarkers of aortic stenosis. JACC Basic Transl Sci. 6(1): 25-39 (2021).
- Bashir M, Harky A, Bleetman D, et al. Aortic valve replacement: Are we spoiled for choice? Semin Thorac Cardiovasc Surg. 29(3): 265-272 (2017).
- Walker GA, Masters KS, Shah DN, et al. Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res. 95(3): 253-60 (2004).
- Otto CM, Kuusisto J, Reichenbach DD, et al. Characterization of the early lesion of 'degenerative' valvular aortic stenosis: Histological and immunohistochemical studies. Circulation. 90(2): 844-53 (1994).
- Angel PM, Nusinow D, Brown CB, et al. Networked-based characterization of extracellular matrix proteins from adult mouse pulmonary and aortic valves. J Proteome Res. 10(2): 812-23 (2011).
- Neufeld EB, Zadrozny LM, Phillips D, et al. Decorin and biglycan retain LDL in disease-prone valvular and aortic subendothelial intimal matrix. Atherosclerosis. 233(1): 113-21 (2014).
- Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 73: 29-46 (2011).
- Simionescu DT, Chen J, Jaeggli M, et al. Form follows function: Advances in trilayered structure replication for aortic heart valve tissue engineering. J Healthc Eng. 3(2): 179-202 (2012).
- Visconti RP, Ebihara Y, LaRue AC, et al. An in vivo analysis of hematopoietic stem cell potential: Hematopoietic origin of cardiac valve interstitial cells. Circ Res. 98(5): 690-6 (2006).
- Huang R, Merrilees MJ, Braun K, et al. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 98(3): 370-7 (2006).
- Aikawa E, Whittaker P, Farber M, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: Implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 113(10): 1344-52 (2006).
- Dupuis LE, McCulloch DR, McGarity JD, et al. Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease. Dev Biol. 357(1): 152-64 (2011).
- Rajamannan NM, Evans FJ, Aikawa E, et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 124(16): 1783-91 (2011).
- Passos LSA, Lupieri A, Becker-Greene D, et al. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis. 306: 59-67 (2020).
- Varshney R, Murphy B, Woolington S, et al. Inactivation of platelet-derived TGF-beta1 attenuates aortic stenosis progression in a robust murine model. Blood Adv. 3(5): 777-788 (2019).
- Poveda J, Sanz AB, Fernandez-Fernandez B, et al. MXRA5 is a TGF-beta1-regulated human protein with anti-inflammatory and anti-fibrotic properties. J Cell Mol Med. 21: 154-164 (2017).
- Clark-Greuel JN, Connolly JM, Sorichillo E, et al. Transforming growth factor-beta1 mechanisms in aortic valve calcification: Increased alkaline phosphatase and related events. Ann Thorac Surg. 83(3): 946-53 (2007).
- Kaden JJ, Kilic R, Sarikoc A, et al. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: A potential regulatory mechanism of valvular calcification. Int J Mol Med. 16(5): 869-72 (2005).
- Mohty D, Pibarot P, Despres JP, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol. 28(1): 187-93 (2008).
- Bouchareb R, Boulanger MC, Tastet L, et al. Activated platelets promote an osteogenic programme and the progression of calcific aortic valve stenosis. Eur Heart J. 40(17): 1362-1373 (2019).
- Bouchareb R, Mahmut A, Nsaibia MJ, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 132(8): 677-90 (2015).
- Ayoub S, Ferrari G, Gorman RC, et al. Heart valve biomechanics and underlying mechanobiology. Compr Physiol. 6(4): 1743-1780 (2016).
- Jiang T, Gao W, Lin S, et al. FNDC1 promotes the invasiveness of gastric cancer via Wnt/beta-catenin signaling pathway and correlates with peritoneal metastasis and prognosis. Front Oncol. 10: 590492 (2020).
- Yunwen C, Shanshan G, Zhifei B, et al. The silencing of FNDC1 inhibits the tumorigenesis of breast cancer cells via modulation of the PI3K/Akt signaling pathway. Mol Med Rep. 23(6): 479 (2021).
- Sato M, Hiraoka M, Suzuki H, et al. Protection of cardiomyocytes from the hypoxia-mediated injury by a peptide targeting the activator of G-protein signaling 8. PLoS One. 9(3): e91980 (2014).
- Shin SU, Lee J, Kim JH, et al. Gene expression profiling of calcifications in breast cancer. Sci Rep. 7(1): 11427 (2017).
- Briand M, Lemieux I, Dumesnil JG, et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 47(11): 2229-36 (2006).
- Katz R, Budoff MJ, Takasu J, et al. Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes. 58(4): 813-9 (2009).