Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 4
Endothelial dysfunction and subclinical atheromatosis in gout: A Spanish multicenter cross-sectional study
- Corresponding Author:
- Enrique Calvo Aranda
Department of Rheumatology
Hospital Universitario HM Sanchinarro, Madrid, Spain
E-mail: ecalvoa@hotmail.com
Abstract
Objectives: To assess subclinical vascular features in gout patients using carotid ultrasound and flow mediated vasodilation as measures of cardiovascular risk. Methods: Cross-sectional study over a cohort of 150 patients diagnosed with gout, on whom a vascular study was performed to assess angiodynamic parameters measured by flow mediated vasodilation in brachial artery and pathological findings in carotid ultrasound: carotid intima-media thickness and atherosclerotic plaques. Classical cardiovascular risk factors were also assessed, as well as main features of gout in terms of joint involvement and inflammation parameters. Results: 150 patients with gout were recruited. 81% of patients had endothelial dysfunction, being severe in 51%. Carotid intima-media thickness was pathological in 66% and atherosclerotic plaques were present in 38%. 73% of patients had low vitamin D levels and elevation of homocysteine was present in 38% of the sample. A negative correlation between vitamin D levels and endothelial dysfunction was obtained (OR= 0.27; CI 95% 0.09-0.83). No other features related to gout (serum uric acid levels, joint involvement, inflammatory parameters or treatments) were associated to vascular damage in this cohort. Conclusions: Patients with gout have a great prevalence of subclinical vascular involvement, mainly endothelial dysfunction. So a correct characterization of vascular damage is important in these patients to better assessment and management of cardiovascular risk.
Keywords
gout • uric acid • cardiovascular risk • endothelial dysfunction • atherosclerotic plaques
Introduction
Gout is the most frequent cause of arthritis in developed countries. Its prevalence and incidence have increased globally in recent decades, possibly owing to changes in factors such as lifestyle and eating habits [1-3]. Damage caused by gout and hyperuricemia is not only limited to joints, and it also may affect the vascular endothelium, so several studies performed in recent years have revealed a major increase in Cardio Vascular Risk (CVR) and even in cardiovascular-related mortality in patients with gout [4-7] as occurs in other inflammatory rheumatic diseases [8-15].
Despite its leading position among the tools used to estimate CVR in rheumatic diseases such as rheumatoid arthritis, ankylosing spondylitis or systemic lupus erythematosus [16-18] in recent years, it is striking that research with vascular ultrasound in patients with gout has received little attention [19-27], especially given that the prevalence of gout is estimated to be much higher than that of other rheumatic diseases.
Nevertheless, the management of CVR in gout remains suboptimal in that the disease is underestimated or not correctly stratified using appropriate tools [14,28]. For this reason, the objective of this study was to assess vascular hemodynamics in patients with gout to optimize the cardiovascular diagnostic-therapeutic approach and, therefore, improve morbidity and mortality.
Methods
In this cross-sectional study, we evaluated a cohort of 150 adult patients diagnosed with gout (American College of Rheumatology criteria) [29] attended in the Rheumatology Departments of a hospital group (Madrid, Spain) between May 2014 and September 2015. CVR assessment was performed according to the established protocol between Rheumatology and Vascular Surgery Units in our Center. The study was developed pursuant to the Declaration of Helsinki principles and informed consent was obtained from all patients.
Clinical features were collected in Rheumatologist baseline visit. We evaluated the presence of traditional Cardio Vascular Risk Factors (CVRF) such as hypertension, diabetes mellitus, dyslipidemia, and smoking, as well as personal and family history of Cardio Vascular Disease (CVD), i.e. acute myocardial infarction, stroke, or peripheral arterial disease. We also recorded heart rate, blood pressure, and anthropometric data. Regarding to gout, we evaluated the disease duration, treatments received, clinical joint involvement and also joint ultrasound examination (MyLab® 25 Gold, by Esaote®) was performed to determine the microcrystalline aggregates following the four-joint protocol proposed by Peiteado et al [30]. Blood sample were obtained to determine a series of variables: complete blood count, biochemistry for detection of liver or kidney disorders, Erythrocyte Sedimentation Rate (ESR), C-reactive protein (CRP), homocysteine, vitamin D, ferritin, and serum Uric Acid (sUA).
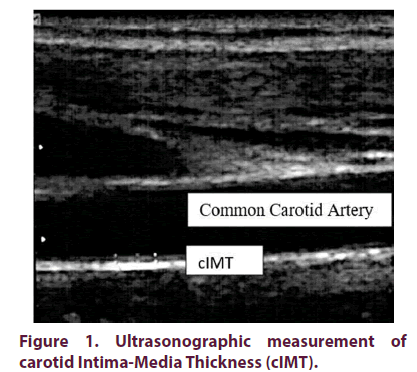
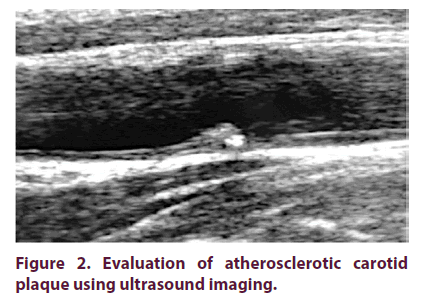
A preliminary ultrasonographic protocol was designed for the vascular study, which established the CVR variables to be studied: carotid Intima- Media Thickness (cIMT) (pathological if >0.9 mm) (Figure 1), Atherosclerotic Plaques (AP) (cIMT greater than 1.5 mm) (Figure 2), and Endothelial Dysfunction (ED) measured by Flow Mediated Vasodilation (FMD) of post-ischemic Brachial Artery (BA) (considered pathological <10% and severe <5%). Ultrasonographic measurements were performed in all patients by a vascular surgeon experienced in these techniques and blinded for patient clinical information, using a high frequency linear transducer (>7.5 MHz; Samsung US System SONOACE R7®), following international recommendations [31- 35]. The examination is performed in a quiet environment with dimmed light, with the patient lying down in a supine position. First, with the neck extended and slightly rotated to the opposite side of the carotid axis to be explored, a morphological examination is performed in B mode with the probe, placing it in a transverse position to obtain a preliminary impression on the anatomy and possible morphological alterations. Subsequently, a longitudinal scan is performed from the clavicle to the jaw, as distally as possible. A complete visualization is performed in which the Common Carotid Artery (CCA), the carotid bifurcation, the Internal Carotid Artery (ICA) and the External Carotid Artery (ECA) are identified, locating the plaques and areas of obstruction, if any. IMT of CCA and carotid bulb is measured, describing AP morphologically, if any. IMT is measured manually on the posterior side of the CCA, in its middle segment, 1 cm from the bulb. Three measurements are taken in a 1 cm segment and the average is calculated. Subsequently, the IMT is measured in both carotid axes at the level of the bulb, on the posterior side, with a single measurement. After that, the non-invasive study of endothelial function was performed, evaluating the vasodilation response of the endothelium in the BA after a period of transient ischemia of 5 minutes. With the patient in a comfortable supine position, right upper limb extended, the scan of the BA is performed in the longitudinal plane, 4 cm proximal to the cubital fossa, marking the place of application of the probe. At baseline, blood flow is measured with pulsed Doppler and with an angle of incidence of 60°. To measure the arterial diameter, we set the image in B mode, manually taking three measurements of the intima-media interface at three nearby points and the mean is calculated. To create the stimulus of the flow in the BA we place the pressure cuff above the cubital fossa, which is inflated above the systolic pressure to occlude the artery. When the cuff is deflated, the diameter of the BA is measured again to assess the response to hyperemia. Two measurements are made: one immediately after the cuff is deflated and another about 60-75 seconds later. All measurements are made in diastole. FMD, as a percentage change from baseline measurements, is calculated using the following formula: FMD (%) = ([Post-ischemic Peak Diameter - Baseline Diameter] / Baseline Diameter) × 100.
Statistical analysis
Descriptive analysis was made using mean and Standard Deviation (SD) or median and Inter Quartile Range (IQR) for quantitative variables and frequencies for qualitative variables. The association between different vascular and clinical variables defined by the different result measures was studied using Chi-square test for categorical data and t-test for continuous variables. Significant variables from bivariate analysis were selected to assemble a multivariate logistic regression model and linearity was checked in the logistic function using a linear trend test over included continuous variables. Differences were considered significant at p<0.05. Statistics software was STATA version 14.Results
Out of the 150 patients included, 98% were middle-aged men (55.9 ± 12.7 years) and the duration of gout was 6 years (IQR 1-15).
Classic CVRF distribution was: 50.6% hypertension, 5.3 % diabetes mellitus, 32% obesity, 57.3 dyslipidemia and 59.3% eversmokers. Regarding CVD history, one patient previously suffered stroke and 18 patients’ ischemic coronary disease. Suboptimal levels of vitamin D were observed in 73% of patients, high levels of homocysteine in 38% and regarding to acute phase reactants, CRP was >5 mg/dl in 12.7% and ESR >20 mm/h in 10.9%. Mean sUA levels of 6.8 ± 1.7 mg/dl. 78% of patients received urate-lowering therapy (ULT). Table 1 shows the clinical characteristics of patients and Table 2 treatments received.
| N=150 | N (%) |
|---|---|
| Male gender | 147 (98%) |
| Family history of cardiovascular disease | 65 (43.3%) |
| Obesity (BMI>30.0) | 47 (32%) |
| Hypertension | 76 (50.6%) |
| Diabetes mellitus | 8 (5.3%) |
| Smoking habit | |
|
59 (39.3%) |
|
30 (20%) |
| Previous stroke | 1 (0.7%) |
| Ischemic heart disease | |
|
11 (7.3%) |
|
7 (4.7%) |
| Thrombotic phenomena | 4 (2.6%) |
|
1 (0.7%) |
|
1 (0.7%) |
|
1 (0.7%) |
|
1 (0.7%) |
| Hyperhomocysteinemia | 49 (37.7%) |
| Family history of gout | 67 (44.6%) |
| Renal lithiasis caused by uric acid | 17 (11.3%) |
| Presence of tophi | 15 (10%) |
| Articular involvement pattern | |
|
99 (66%) |
|
42 (28%) |
| Ultrasound elementary lesions in gout | |
|
33 (26.6%) |
|
91 (73.4%) |
| Study of synovial fluid with PLM | 92 (61.3%) |
| Elevated CRP levels > 5 mg/dl | 18 (12.7%) |
| Levels of low 25-hydroxy vitamin D (<30) | 96 (73.8%) |
| Elevated ESR > 20 mm/h | 14 (10.9%) |
BMI: Body Mass Index; PLM: Polarized Light Microscope; CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate
Table 1. Baseline patients characteristics and cardiovascular risk factors.
| Vascular features (N=150) | N (%) |
|---|---|
| Atherosclerotic plaques | |
|
28 (18.7%) |
|
29 (19.3%) |
| Atherosclerotic plaques | |
|
57 (38%) |
| Pathological carotid intima-media thickness | 99 (66%) |
| Endothelial dysfunction | 120 (80.5%) |
| Endothelial dysfunction: categories of involvement | |
|
77 (51.6%) |
|
43 (28.8%) |
|
29 (19.5%) |
Table 2. Vascular hemodynamics features.
The vascular study revealed ED in 80.5% of the patients, which was severe (FMVD <5%) in 51.6%. Mean value of FMD was -0.60 ± 0.52. A negative association (OR= 0.27; CI 95% 0.09- 0.83) was found between ED and vitamin Dlevels. This difference was statistically significant (p=0.023). cIMT presented pathological values in 66% and AP were observed in 38% of patients, which were bilateral in half of the cases (Table 3).
| Treatment | N (%) |
|---|---|
| Use of corticosteroids (N=150) | |
|
117 (78%) |
|
25 (16.7%) |
|
8 (5.3%) |
| Doses of corticosteroids (N=33) | |
|
14 (42.4%) |
|
12 (36.4%) |
|
7 (21.2%) |
| Duration of treatment with corticosteroids (N=33) | |
|
12 (36.4%) |
|
20 (60.6%) |
|
1 (3%) |
| Use of NSAIDs (N=148) | |
|
11 (7.3%) |
|
134 (89.3%) |
|
3 (1.8%) |
| Doses of NSAIDs (N=118) | |
|
6 (4%) |
|
112 (74.7%) |
| Duration of treatment with NSAIDs (N=142) | |
|
57 (40.7%) |
|
59 (42.1%) |
|
16 (11.4%) |
| Use of urate-lowering treatments (N=150) | |
|
15 (10%) |
|
98 (65%) |
|
20 (13.3%) |
|
17 (11.3%) |
| Doses of urate-lowering treatments (N=135) | |
|
63 (46.7%) |
|
50 (37%) |
|
2 (1.5%) |
|
6 (4.4%) |
|
14 (10.4%) |
NSAIDS: Non-Steroidal Anti-Inflammatory Drugs
Table 3. Treatments.
Inflammatory parameters (CRP and ESR), hyperhomocysteinemia and sUA levels were not significantly associated with any of the vascular variables studied.
Discussion
Our study provides a complete clinical, cardiovascular and laboratory characterization of patients with different degrees of gout severity by multidisciplinary cooperation. Patients were included from everyday clinical practice, so our cohort was not extent from several patients with comorbid conditions such as chronic kidney disease, established CVD or patients aged >65 despite the influence of age on vascular parameters. These features were considered as exclusion criteria in patient selection in some studies [19-21,27] but in others, as in ours, were included [22,23,25]. While our sample size could be larger, it contains the second highest number of vascular examinations at present (after Gancheva et al cohort
Our findings revealed the high prevalence of subclinical cardiovascular involvement in patients with gout, as well as classic CVRF and CVD. The most relevant result of this study was the elevated percentage of ED (81% of patients), which was severe in 51.6%. The mean value of FMD was negative, thus indicating, albeit paradoxically, a vasoconstrictive response. In our patients, the only factor that presented correlation with ED was deficiency of vitamin D, no other laboratory parameter such as hyperuricemia was associated with this finding, which is in line with Brook et al, although several studies observed that hyperuricemia may damage endothelium and reduce arterial responsiveness leading to ED [36,37]. Other studies reported a higher rate of ED in gout, [26] not only due to hyperuricemia, but also associated to elevated CRP in untreated patients [38]. In our study, only 12.7% of patients presented CRP levels >5 mg/dl, so it could be the reason why CRP is not associated to ED in our cohort.
Regarding cIMT, we found lower values than Gancheva et al, Cukurova et al and Uyanik et al, which reported comparable results. But we found a higher percentage of patients with pathological cIMT (66%) than Andrés et al (45.1%) and Il´ina et al (41.6%). In our cohort, cIMT was not related to any parameter, in contrast with the studies of Lapkina et al and Andres et al, which found a significant correlation between serum uric acid levels and cIMT. On the other side, the percentage of AP in our study was 38%, which has also heterogeneous results between different studies, Gancheva et al reported AP in 50% of non-tophaceous gout patients and 46.7% in tophaceous gout patients, and Andrés et al reported 46.5%. However, Cukurova et al and Uyanik et al reported respectively 29.5% and 28.9% of patients with AP (Table 4).
| Raw Estimates (Bivariate Study) | Adjusted Model (Multivariate Study) | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Serum uric acid levels | 0.54 (0.27-1.08) | 0.084 | 0.74 (0.31-1.76) | 0.505 |
| Presence of tophi | 1.47 (0.44-4.86) | 0.529 | ||
| CRP | 1.44 (0.48-4.33) | 0.508 | ||
| Waist circumference | 2.02 (0.99-4.12) | 0.052 | ||
| Homocysteine | 0.66 (0.32-1.38) | 0.277 | 0.46 (0.18-1.15) | 0.097 |
| 25-OH-Vitamin D | 1.01 (0.44-2.27) | 0.99 | ||
| SCORE indicating CVR | 10.79 (4.46-26.07) | <0.0001 | 13.02 (5.05-33.5) | <0.0001 |
| Use of corticosteroids previous/current | 0.53 (0.24-1.17) | 0.119 | ||
| Use of urate-lowering treatment previous/current | 1.05 (0.85-1.30) | 0.651 | ||
| Ultrasound gout lesions | 1.20 (0.53-2.72) | 0.665 | ||
| Use of colchicine previous/current | 0.46 (0.94-2.28) | 0.344 | ||
| Ferritin | 0.13 (0.65-2.67) | 0.436 | ||
| Time since onset | ||||
|
1 | 0.05 | ||
|
2.02 (0.99-4.11) | |||
OR: Odds Ratio; CI: Confidence Interval; CRP: C-Reactive Protein; CVR: Cardio Vascular Risk
Table 4. Analysis of associations with pathological IMT.
Other interesting point is the fact that 38% of patients presented hyperhomocysteinemia, which has been classically related to CVR [39], but in our study, homocysteine levels were not related to any vascular parameter.
Our results should be interpreted with caution, because this study is limited by its crosssectional design, and no causal relationship can be established between the development of CV events in patients with gout. The absence of an age-matched control group, which is also important, mostly when studying cIMT and carotid plaques. However, this has been partially compensated by benchmarking against samples from other studies, some of which were performed in our Country [40,41]. Nevertheless, there is a prospective ongoing follow-up of patients to fill data gaps over time and determine the evolution of CV parameters in gout.
Conclusion
Vascular assessment in patients with gout enables us to establish a correct characterization of the scope of CVR. Ultrasound study provides a more comprehensive estimation of CVR, mainly due to the early identification of pathologic vascular features (ED and cIMT) which are considered the previous stage to AP. This can be useful to establish better preventive strategies and prescribe more individualized and comprehensive treatment for these patients because up to date, CVR may be underestimated when classic tools were used.
Acknowledgments
Grant support
This work was the result of a project of cardiovascular risk in gout, which development was possible thanks to a research grant from Fundación Española de Reumatología.
Disclosure of interest
Dr. Enrique Calvo Aranda has received speaking and consulting fees from: Menarini and Grünenthal labs. None of the other authors has any conflict of interest.
References
- Arromdee E, Michet CJ, Crowson CS et al. Epidemiology of gout: is the incidence rising? J. Rheumatol. 29, 2403–2406 (2002).
- Rai SK, Avina-Zubieta JA, McCormick N et al. The rising prevalence and incidence of gout in British Columbia, Canada: Population-based trends from 2000 to 2012. Semin. Arthritis. Rheum. 46(4), 451–456 (2017).
- Kuo CF, Grainge MJ, Zhang W et al. Global epidemiology of gout: prevalence, incidence and risk factors. Nat. Rev. Rheumatol. 11(11), 649–662 (2015).
- Perez-Ruiz F, Martinez-Indart L, Carmona L et al. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann. Rheum. Dis. 73(1), 177–182 (2014).
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N. Engl. J. Med. 359(17), 1811–1821 (2008).
- Clarson LE, Chandratre P, Hider SL et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 22(3), 335–343 (2015).
- Lottmann K, Chen X, Schadlich PK. Association between gout and all-cause as well as cardiovascular mortality: a systematic review. Curr. Rheumatol. Rep. 14(2), 195–203 (2012).
- Wang H, Jacobs DR Jr., Gaffo AL et al. Longitudinal association between serum urate and subclinical atherosclerosis: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J. Intern. Med. 274(6), 594–609 (2013).
- Cicero AF, Salvi P, D'Addato S et al. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J. Hypertens. 32(1), 57–64 (2014).
- Andres M, Quintanilla MA, Sivera F et al. Silent Monosodium Urate Crystal Deposits Are Associated With Severe Coronary Calcification in Asymptomatic Hyperuricemia: An Exploratory Study. Arthritis. Rheumatol. 68(6), 1531–1539 (2016).
- Roman MJ, Shanker BA, Davis A et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med. 349(25), 2399–2406 (2003).
- Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR et al. Endothelial dysfunction, carotid intima-media thickness, and accelerated atherosclerosis in rheumatoid arthritis. Semin. Arthritis. Rheum. 38(2), 67–70 (2008).
- Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA et al. The high prevalence of subclinical atherosclerosis in patients with ankylosing spondylitis without clinically evident cardiovascular disease. Medicine (Baltimore). 88(6), 358–365 (2009).
- van der Burg LR, van Amelsvoort LG, Boonen A et al. Cardiovascular morbidity and mortality among working individuals with rheumatic disease: results from a large prospective cohort study. J. Clin. Rheumatol. 21(7), 359–363 (2015).
- Castaneda S, Martin-Martinez MA, Gonzalez-Juanatey C et al. Cardiovascular morbidity and associated risk factors in Spanish patients with chronic inflammatory rheumatic diseases attending rheumatology clinics: Baseline data of the CARMA Project. Semin. Arthritis. Rheum. 44(6), 618–626 (2015).
- Corrales A, Gonzalez-Juanatey C, Peiro ME et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann. Rheum. Dis. 73(4), 722–727 (2014).
- Rueda-Gotor J, Llorca J, Corrales A et al. Carotid ultrasound in the cardiovascular risk stratification of patients with ankylosing spondylitis: results of a population-based study. Clin. Exp. Rheumatol. 34, 885–892 (2016).
- Wu GC, Liu HR, Leng RX et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Autoimmun. Rev. 15(1), 22–37 (2016).
- Andres M, Bernal JA, Sivera F et al. Cardiovascular risk of patients with gout seen at rheumatology clinics following a structured assessment. Ann. Rheum. Dis. 76(7), 1263–1268 (2017).
- Brook RD, Yalavarthi S, Myles JD et al. Determinants of vascular function in patients with chronic gout. J. Clin. Hypertens (Greenwich). 13(3), 178–188 (2011).
- Cukurova S, Pamuk ON, Unlu E et al. Subclinical atherosclerosis in gouty arthritis patients: a comparative study. Rheumatol. Int. 32(6), 1769–1773 (2012).
- Gancheva R, Kundurdjiev A, Ivanova M et al. Evaluation of cardiovascular risk in stages of gout by a complex multimodal ultrasonography. Rheumatol. Int. 37(1), 121–130 (2017).
- Gancheva RN, Kundurdjiev AI, Ivanova MG et al. Ultrasonographic measurement of carotid artery resistive index and diastolic function of the heart in gout patients. Rheumatol. Int. 35(8), 1369–1375 (2015).
- Il'ina AE, Varfolomeeva EI, Volkov AV et al. Relationship between the intima-media complex thickness, the risk factors of cardiovascular diseases, and the level of C-reactive protein in gouty patients. Ter. Arkh. 81, 45–49 (2009).
- Lapkina NA, Baranov AA, Barskova VG et al. Markers of vascular endothelium activation in gout. Ter. Arkh. 77, 62–65 (2005).
- Shelest BA. Peripheral vessel wall changes in hypertensive patients with gout. Ter. Arkh. 88(5), 43–46 (2016).
- Uyanik MS, Pamuk GE, Pamuk ON et al. Tissue factor pathway inhibitor and thrombin-activatable carboxypeptidase B for prediction of early atherosclerosis in gouty arthritis. Thromb. Res. 134(2), 526–530 (2014).
- Fisher MC, Rai SK, Lu N et al. The unclosing premature mortality gap in gout: a general population-based study. Ann. Rheum. Dis. 76(7), 1289–1294 (2017).
- Wallace SL, Robinson H, Masi AT et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis. Rheum. 20(3), 895–900 (1977).
- Peiteado D, De Miguel E, Villalba A et al. Value of a short four-joint ultrasound test for gout diagnosis: a pilot study. Clin. Exp. Rheumatol. 30, 830–837 (2012).
- Stein JH, Korcarz CE, Hurst RT et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 21(2), 93–111 (2008).
- Touboul PJ, Hennerici MG, Meairs S et al. Mannheim intima-media thickness consensus. Cerebrovasc. Dis. 18(4), 346–349 (2004).
- Corretti MC, Anderson TJ, Benjamin EJ et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 39(2), 257–265 (2002).
- Celermajer DS, Sorensen KE, Gooch VM et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 340(8828), 1111–1115 (1992).
- Harris RA, Nishiyama SK, Wray DW et al. Ultrasound assessment of flow-mediated dilation. Hypertension. 55(5), 1075–1085 (2010).
- Kato M, Hisatome I, Tomikura Y et al. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am. J. Cardiol. 96(11), 1576–1578 (2005).
- Mercuro G, Vitale C, Cerquetani E et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am. Cardiol. 94(7), 932–935 (2004).
- Krasnokutsky S, Romero AG, Bang D et al. Impaired arterial responsiveness in untreated gout patients compared with healthy non-gout controls: association with serum urate and C-reactive protein. Clin. Rheum. 37(7), 1903–1911 (2018).
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 288(16), 2015–2022 (2002).
- Fernandez-Friera L, Penalvo JL, Fernandez-Ortiz A et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation. 131(24), 2104–2113 (2015).
- Coll B, Betriu A, Feinstein SB et al. The role of carotid ultrasound in assessing carotid atherosclerosis in individuals at low-to-intermediate cardiovascular risk. Rev. Esp. Cardiol. 66(12), 929–934 (2013).