Review Article - Imaging in Medicine (2013) Volume 5, Issue 5
Digital breast tomosynthesis: a concise overview
Nooshin Kiarashi*and Ehsan Samei1Department of Radiology, Duke University Medical Center, Durham, NC, USA
- *Corresponding Author:
- E-mail: nooshin.kiarashi@duke.edu
Abstract
Keywords
3D breast imaging; breast cancer; contrast-enhanced breast imaging; diagnosis; digital breast tomosynthesis; screening
According to WHO, breast cancer is the most common cancer in women, both in the developed and the developing world; the incidence of breast cancer is rising in the latter. Some suggested reasons for this include increased life expectancy, increased urbanization and adoption of the western lifestyle. Although some risk reduction may be achieved with prevention, these strategies cannot eliminate the majority of breast cancers that develop in low- and middle-income countries where breast cancer is diagnosed in very late stages. Therefore, early detection for improved breast cancer outcome and prognosis remains the cornerstone of breast cancer control.
The recommended early detection strategies are awareness of early symptoms and screening by clinical breast examination in low- and middle-income countries, and mammography in countries with better health infrastructure that can afford a long-term program. The WHO, with the support of the Komen Foundation, is currently conducting a 5-year breast cancer cost–effectiveness study in ten low- and middleincome countries. It is expected that the results of this project will help to provide evidence for shaping adequate breast cancer policies in less-developed countries [1].
Mammography screening is the only screening method that has proven to be effective. It can reduce breast cancer mortality by approximately 20% in women over 40 years old in high-income countries [2,3]. The American Cancer Society recommends annual screening mammography for women aged 40 years and older. While providing the highest spatial resolution, the patients might be called back for further examination due to the limited accuracy and poor image quality of mammography, which also leads to its limited sensitivity and specificity. Unfortunately, the sensitivity of both analog and digital mammography remains as low as 36–79%, depending partly on breast tissue density and heterogeneity of tumor growth patterns, with recall rates remaining well above 5–10% for some practitioners [4–7]. The low sensitivity is caused by breast tissue superposition, which can conceal malignancies. Furthermore, breast tissue superposition can cause false positives, lowering the specificity of mammography to 90–98%, depending on breast tissue density, and resulting in follow-up imaging, additional radiation exposure, increased expenses and unnecessary anxiety [4–8]. The clinical work-up can include diagnostic mammography with additional views or magnification, breast ultrasound imaging, breast MR imaging, fine-needle aspiration biopsy, core biopsy or surgical excisional biopsy. There are also several other established or experimental technologies that could be helpful in re-examining suspicious areas but have not proven to be a substitute for screening mammography. These technologies include scintimammography [9], thermography [10], electrical impedance imaging (T-scans) [11], positron emission mammography (PEM) [12], molecular imaging [13] and optical imaging [14].
To overcome the loss of information in the third dimension due to tissue superposition in mammography, new imaging techniques have emerged with the advent of digital detectors [15–17]: dedicated breast CT [18] and digital breast tomosynthesis [19]. Dedicated breast CT is an imaging technique that provides tomographic images of the breast by scanning the pendant breast during a patient’s breath-hold. The technique offers lower spatial resolution and higher contrast resolution compared with mammography, presenting challenges for microcalcification detection and axillary tail and chest wall coverage. Currently, there is no dedicated breast CT system available clinically. Digital breast tomosynthesis is an imaging technique that creates cross-sectional images of the breast at mammographic in-plane resolution through limitedangle tomography of the compressed breast. Due to uncertainties that result from the limited angle tomography, the z-resolution is distorted and expanded. In-depth reviews of the history and development of tomosynthesis have been published [20–23]. Digital breast tomosynthesis has the potential to reduce false positives and provide equal or better sensitivity compared with mammography. Currently, there are a number of digital breast tomosynthesis systems available clinically worldwide.
Image acquisition
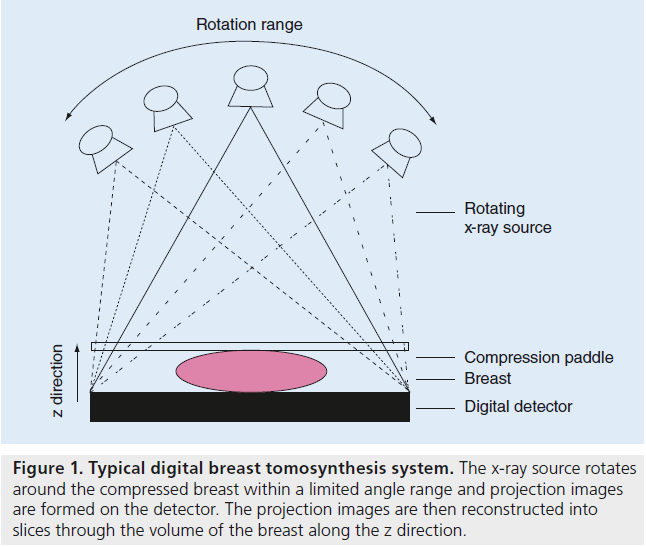
Digital breast tomosynthesis is a high-resolution limited-angle tomography technique. The original theoretical concept of tomosynthesis was first introduced almost 80 years ago and the term tomosynthesis was coined in a journal article almost 40 years ago [24–26]. However, since effective implementation of the theory necessitates employing digital detectors, digital tomosynthesis has only recently become feasible and has developed over the past 15 years [19]. The digital breast tomosynthesis system consists of a rotating x-ray tube that acquires a series of lowdose projections of the compressed breast over a range of angles with a static or moving detector ( Figure 1). The low-dose projection images are reconstructed into a semitomographic volume of the breast with typically 1-mm thick slices. Tomosynthesis systems use the basic mammography system structure. The patient can be imaged at standing, seated or lying positions with the breast compressed between the compression paddle and the detector. The applied compression is comparable to mammography. There is, however, some room for a reduction in compression due to the 3D nature of tomosynthesis, compensating for tissue overlap in mammography and resulting in more patient comfort [27]. The radiation dose for a single tomosynthesis examination is comparable to a traditional single mammography examination [20,24]. While the compression paddle and detector cover remain static, the x-ray tube can move with continuous or step-and-shoot motion around the breast to acquire craniocaudal or mediolateral oblique views. Different commercial and prototype systems currently acquire between 11 and 50 projections over a range of 15–60° in 5–25 s. The x-ray tube generates voltages of 23–49 kVp with tungsten, rhodium or molybdenum targets coupled with aluminum, silver, rhodium, copper or titanium filtering. The flat panel digital detectors used for tomosynthesis typically have a faster reading time, minimal detector ghosting and lag, and minimal reduction of detective quantum efficiency at low exposure compared with digital mammography detectors, and thus allowing acquisition of several images over a short scanning time. These detectors are made of amorphous selenium or amorphous silicon for direct conversion, or cesium iodide for indirect conversion. Since tomosynthesis is a relatively new technique, these specifications are subject to change.
The detector pixel size currently ranges from 50 to 140 μm considering detector binning. Reconstructed voxel sizes are typically around 100 μm × 100 μm × 1 mm. However, the 1-mm slice definition in the z direction is not indicative of resolution in that direction. The limited so-called z-resolution is due to limited angle tomography and can be a function of the angular range [28].
In addition to the commercial and prototype systems explained above, a couple of nontraditional prototype tomosynthesis systems currently exist. One of these prototype tomosynthesis systems employs scanning-slit photon counting detectors [29,30]. Advantages of this system include low scatter signal, no electronic noise, high quantum efficiency and low radiation dose requirements. In addition, a new version of this photon-counting detector includes energy resolution, allowing for simultaneous acquisition of two images at low and high energies. Another alternative prototype tomosynthesis system employs multibeam field emission x-ray source array to avoid focal spot blurring due to the x-ray tube motion during acquisition and to potentially shorten total acquisition time [31,32]. In a recent work, the x-ray tube and gantry of a commercial tomosynthesis system (Selenia Dimensions, Hologic Inc., MA, USA) was replaced with a carbon nanotube array with 31 x-ray sources, spanning 370 mm, resulting in an angular coverage of 30° [33]. The stationary x-ray source was shown to yield an improved modulation transfer function compared with that with a standard rotating x-ray tube. However, detector readout rate, and x-ray tube current and exposure time, still need to be addressed to optimize total scan time.
Reconstruction & image handling
Tomosynthesis projection images are reconstructed using filtered back projections [34,35] or iterative algorithms [36]. Most iterative reconstruction methods are based on other techniques, such as algebraic reconstruction [37], simultaneous iterative reconstruction [38] and simultaneous algebraic reconstruction [39], and maximum likelihood expectation maximization reconstruction technique [40], which can provide superior image quality and fewer artifacts compared with filtered back projection [41,42]. However, iterative reconstruction requires several orders of magnitude greater computation time compared with filtered back projection. There is active research for optimizing reconstruction algorithms to maximize their performance with lower computational cost. The application of graphic-processing units has also helped significantly reduce the time required for iterative reconstruction.
Tomosynthesis is highly susceptible to artifacts due to its nature of limited-angle acquisition [43]. The most common artifacts are the out-ofplane presence of shifted and repeated versions of high-contrast features or ghosting. The other reconstruction artifact, the embossed appearance of high-contrast features, can sometimes aid in detection. Additional artifacts include the blurring of the breast margin into other planes and truncation artifacts close to the edges of the detector. Various new methods and modifications to existing reconstruction algorithms have been proposed to reduce or eliminate these artifacts [44–46].
The tomosynthesis-reconstructed images can be displayed sequentially as a continuous cine loop, or as one image at a time controlled manually at the user’s discretion and preference of image display rate. The users can magnify the images to full acquisition resolution. The tomosynthesis projection images can also be available for viewing one-by-one or in a loop, depending on the manufacturer.
For microcalcification searches, slab-views have been suggested, which improve microcalcification conspicuity by collecting the entire cluster into one slab, rather than having individual microcalcifications spread over several slices [47]. In this view mode, maximum-intensity projections (MPs) of 5–10 slices are often presented [48].
In 2011, In order to reduce the scan time and patient exposure, the C-View™ (Hologic Inc., MA, USA) software was commercially released to synthetically reconstruct mammograms from tomosynthesis data sets [101]. The US FDA approved the technique for use in the USA in 2013 [102]. Development of such algorithms and their clinical evaluations is an active area of research. There are potentially additional viewing modes for tomosynthesis data (including stereoscopy [49] and multiview stereoscopy [50] among others) that await clinical evaluation.
Figure 1. Typical digital breast tomosynthesis system. The x-ray source rotates around the compressed breast within a limited angle range and projection images are formed on the detector. The projection images are then reconstructed into slices through the volume of the breast along the z direction.
Tomosynthesis data sets are significantly larger than mammography data sets. Each projection image in a tomosynthesis scan is equivalent to a mammogram in size. Therefore, depending on the number of projection angles, a four-view tomosynthesis data set can be 25-times as large as a four-view mammography data set. Thus, storage and networking requirements for these large data sets need to be considered in practice.
Clinical experience & implementation
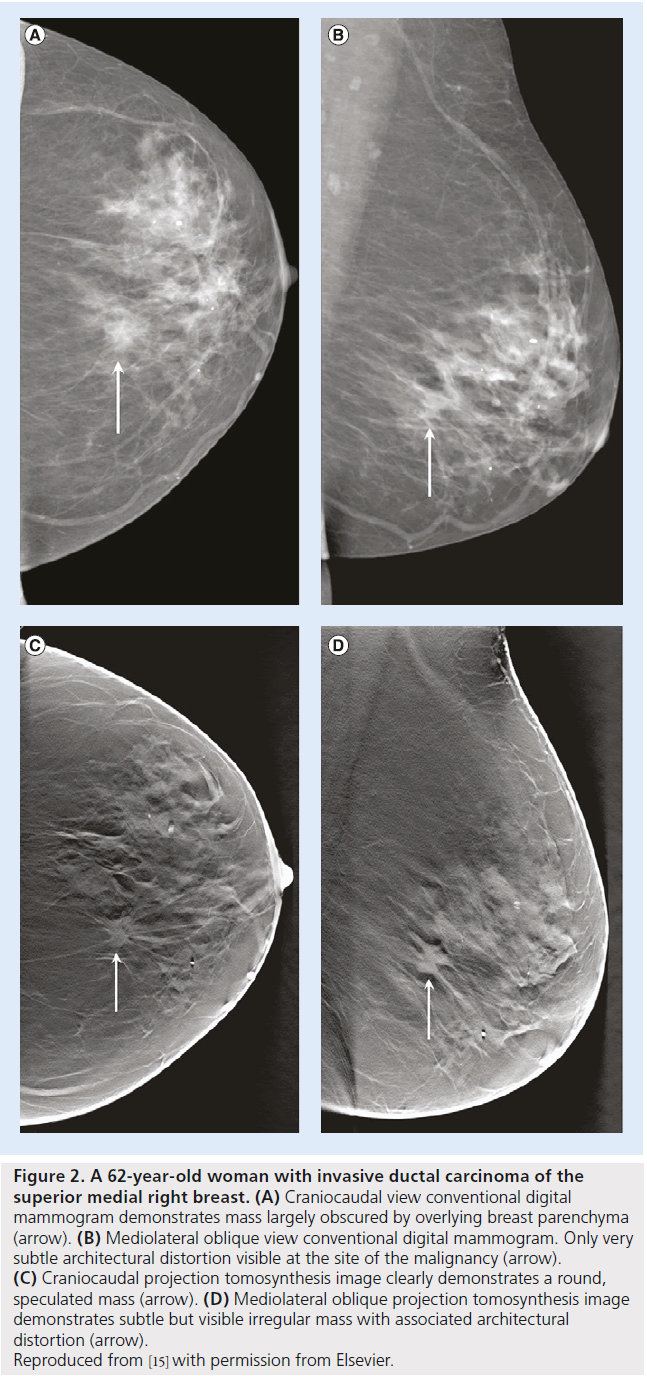
Various clinical studies have been devised to evaluate and assess the relative advantages or disadvantages of tomosynthesis to other technologies for screening and diagnosis. Figure 2 illustrates the potential advantage of two-view digital breast tomosynthesis with two-view digital mammography. In a 2007 study, Poplack et al. followedup 98 women with abnormal digital screening mammography with diagnostic film-screen mammography and tomosynthesis [51]. They reported an equivalent or superior image quality for tomosynthesis compared with diagnostic mammography in 89% of the cases and concluded that if tomosynthesis had also been used for screening, half of these cases would not have been recalled. It should, however, be noted that this study was not properly designed for such inference [52]. In 2008, Good et al. published a pilot study comparing full-field digital mammography, 11 low-dose projections acquired for the reconstruction of tomosynthesis images and the reconstructed digital breast tomosynthesis examinations of 30 subjects read by nine experts [53]. Although observer performance measures were not statistically significant, the authors reported great potentials for tomosynthesis-based breast imaging. In 2008, Andersson et al. published the results of a study conducted on symptomatic or recalled women in Sweden from 2006 to 2007. Their lesion visibility study, within a population of 40 cancers, concluded that cancer visibility in one-view tomosynthesis is superior to digital mammography. This indicated the potential of tomosynthesis to increase sensitivity [54]. In 2010, Teertsrtra et al. published the results of a study of 513 women with an abnormal screening mammogram or clinical symptoms in The Netherlands from 2006 to 2007 [55]. They found that the sensitivity of both techniques for the detection of breast cancer was 92.9%, and the specificity of mammography and tomosynthesis was 86.1 and 84.4%, respectively. They also concluded that tomosynthesis could be used as an additional technique to mammography in patients referred with an abnormal screening mammogram or with clinical symptoms. In 2010, Gennaro et al. published the results of a study of 200 women with at least one breast lesion discovered by mammography and/or ultrasound in Italy from 2007 to 2008 [56]. They concluded that the clinical performance of tomosynthesis in one view at the same total dose as standard screen-film mammography is not inferior to digital mammography in two views. In 2013, Skaane et al. published the results of a comparative study of digital mammography alone and digital mammography plus tomosynthesis in 12,631 women as part of the Oslo screening program from 2010 to 2011 [57]. They reported a 27% increase in the detection rate for invasive and in situ carcinoma cancers with digital mammography plus tomosynthesis, as well as a 15% decrease in false-positive rates. In 2013, Ciatto et al. published the results of investigating the effect of integrated mammography and tomosynthesis in population breast cancer screening [58]. This comparative study, STORM trial, recruited 7292 asymptomatic women aged 48 years or older who attended population-based breast cancer screening and reported that integrated mammography and tomosynthesis improves breast cancer detection and has the potential to reduce false-positive recalls.
Figure 2.A 62-year-old woman with invasive ductal carcinoma of the superior medial right breast. (A) Craniocaudal view conventional digital mammogram demonstrates mass largely obscured by overlying breast parenchyma (arrow). (B) Mediolateral oblique view conventional digital mammogram. Only very subtle architectural distortion visible at the site of the malignancy (arrow). (C) Craniocaudal projection tomosynthesis image clearly demonstrates a round, speculated mass (arrow). (D) Mediolateral oblique projection tomosynthesis image demonstrates subtle but visible irregular mass with associated architectural distortion (arrow). Reproduced from [15] with permission from Elsevier.
In 2007, Rafferty reported an advantage of mammography in detecting microcalcifications in comparison with tomosynthesis [59]. This may be due to the fact that the microcalcifications are visualized in different planes. As a result, it is suggested that tomosynthesis and mammography may be used best in a complementary way. In 2011, in a study of 100 women, Spangler et al. reported mammography to be slightly more sensitive than tomosynthesis for the detection of calcifications [60]. However, since diagnostic performance, as measured by the area under the receiver operating characteristic (ROC) curve, was not significantly different, they envisioned that, with improvements in processing algorithms and display, tomosynthesis could potentially be improved for this purpose. In a 2011 study of 119 women, Kopans et al. showed that microcalcifications can be demonstrated with equal or greater clarity on tomosynthesis than on conventional mammography when the detector pixels are not binned [61].
As noted earlier, research is ongoing in creating a synthetic mammographic image from a tomosynthesis acquisition. In the only study on this matter, Gur et al. reported on an observer study to compare the performance of tomosynthesis combined with either an actual mammogram or a synthetic mammogram and concluded that the synthetic mammogram was not a replacement for the actual mammogram [62].
A number of studies are currently being undertaken to further assess tomosynthesis. For instance, to assess whether tomosynthesis could improve upon digital mammography as a screening tool, particularly in certain groups of women such as those with a family history of breast cancer or those recalled to an assessment clinic following abnormal screening mammography, six centers in the UK are participating in a study, the TOMMY trial, to recruit a total of 7000 women undergoing both standard digital mammography and tomosynthesis [103]. The Malmö Breast Tomosynthesis Screening Trial is also designed to compare tomosynthesis and mammography as screening tools in terms of the number of cancers detected in 15,000 women, aged 40–74 years in Sweden between 2010 and 2016 (NCT01091545) [104].
There are also studies designed to investigate the effect of number of views available in tomosynthesis and mammography. For example, Wallis et al. report that two-view tomosynthesis outperforms mammography, but only for readers with the least experience [63].
It is worth noting that most of these studies acknowledged the potentially limiting impacts of observer experience and training with a new technology as well as not fully optimized acquisition and display settings of the prototype units in the outcome of the studies. Smith et al. reported that radiologists with a range of experience demonstrated improved performance using tomosynthesis in combination with digital mammography, measured using recall rate reduction and the area under the ROC curve metrics [64].
Currently, tomosynthesis units from a few manufacturers are commercially available in Europe. In the USA, however, only one manufacturer has been approved by the FDA to date.
Advanced applications
Digital breast tomosynthesis, similar to digital mammography, can benefit from enhanced lesion detectability by incorporating contrast agents. Intravenous iodine-based contrast agents can improve the visibility of areas with high blood perfusion, such as malignancies, when energies above the k-edge of iodine are used for acquisition. Furthermore, the kinetic patterns of contrast agent-uptake within lesions can be studied if imaging is repeated over the course of a few minutes, as the contrast agent propagates throughout the breast. This kinetics information can serve as an additional tool to characterize a potential lesion. Most lesions, following a washout or a plateau pattern, are reported to be suggestive of malignancy, whereas most benign lesions follow constant enhancement patterns [65,66]. The relative advantage of this technique to contrast-enhanced MRI lies in its high in-plane resolution and short acquisition time, as well as the considerably lower costs. However, the technique can potentially expose the patient to a greater level of ionizing radiation. Contrast-enhanced digital tomosynthesis is extensively studied and continues to be an active area of research [67,68].
To further augment the lesion visibility, postprocessing contrast-enhancement techniques have been introduced that involve multiple acquisitions before and/or after administration of the contrast agent at one or more energies [69–72]. One contrast enhancement technique is temporal subtraction, which involves acquiring images before and after the administration of the contrast agent and then subtracting them. The principal behind this technique is the fact that, after administration of the contrast agent, the areas with most blood infusion show the highest contrast. Hence, when the two images are subtracted, most of the anatomy is subtracted out and the malignant lesions that have blood pooling around them will be what remain. Another contrast-enhancement technique, dual-energy subtraction, involves acquiring images after the administration of the contrast agent at energies below and above the k-edge of iodine. At the higher energy acquisition, areas with the most blood infusion will result in the highest attenuation, and hence, the highest contrast. Therefore, if the two images are subtracted, most of the anatomy could cancel out and the malignancies could remain visible. Technique optimization in contrast-enhanced imaging, using physical and virtual phantoms as well as clinical observer studies, is an ongoing area of research [73,74].
Although tomosynthesis has only been recently and partly introduced to the clinical practice, some multimodality approaches have already been investigated. These approaches include the combination of tomosynthesis with electrical impedance tomography for characterization of suspicious lesions [75] and with ultrasound obtaining a coregistered 3D ultrasound image [76]. The combination of morphological information with functional information is also being developed through integrating tomosynthesis with SPECT [77] or with diffuse optical tomography [78]. Phase-contrast tomosynthesis in order to enhance feature edges was tested on phantoms and was proven to be promising [79]. Tomosynthesis-guided positioning for radiation therapy and interventional biopsy has also been an active area of development [80,81].
Computer-aided detection (CAD) of masses in tomosynthesis has been performed by employing segmentation and gradient and feature analysis, on either the projection images or the reconstructed images, or both [82,83]. CAD methods based on information theoretic metrics are also in existence [84,85]. A number of different algorithms have been proposed for the automated detection of microcalcification clusters in tomosynthesis images, a task that has proven easier for CAD systems than the detection of masses [86,87].
Given the increased risk of cancer in women with denser breasts, there have been attempts in estimating breast density based on tomosynthesis projection and reconstructed images [88,89]. However, it is still unclear whether mammography- based techniques overestimate the density or tomosynthesis-based techniques underestimate the density
Conclusion
The advent of digital detectors facilitated realization of digital breast tomosynthesis systems, which acquire low-dose projection images of the breast from multiple directions to synthesize slices through the volume of the breast parallel to the plane of the projection images. Although still in its clinical infancy, this imaging system has been studied in a multitude of domains. This concise overview introduced digital breast tomosynthesis and elaborated on the state-of-the-art in its applications and performance.
Future perspective
Digital breast tomosynthesis is still in its clinical infancy around the world. Hence, only limited data are available for understanding its advantages and shortcomings. The initial promising clinical results suggest its use as an adjunct to, or in combination with, digital mammography for screening purposes. Whether tomosynthesis systems could be used as a screening tool or as a diagnostic tool is a subject that deserves further development and investigation. Aside from advancements in hardware, which can potentially facilitate faster, more efficient and safer acquisition, there is also a need for advancements in the postprocessing and interpretation arena. Development of smart techniques to synthesize mammograms from tomosynthesis data sets could eventually eliminate the need for a separate mammogram, resulting in faster acquisition and less exposure to the patient. Optimal acquisition protocols and reconstruction techniques for various purposes still need to be determined and examined. Advanced applications have to be tested clinically to justify their proper usage and potential benefits. For translation into wide clinical usage, issues, such as reader training, data handling and storage, and assistive CAD tools, need to be considered and planned for. Overall, given the current evaluated improvements in sensitivity and specificity over digital mammography, digital breast tomosynthesis is considered to be an emerging tool in breast imaging with great potential and a bright future.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
* of interest
- Anderson BO, Yip CH, Smith RA et al. Guideline implementation for breast healthcare in low-income and middle-income countries. Cancer 113, 2221–2243 (2008).
- Boyle P, Levin B. World Cancer Report 2008. International Agency for Research on Cancer, Lyon, France (2008).
- Independent UK panel on breast cancer screening. The benefits and harms of breast cancer screening: an independent review. Lancet 380(9855), 1778–1786 (2012).
- Pisano ED, Gatsonis C, Hendrick E et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 353, 1773–1783 (2005).
- Vernacchia FS, Pena ZG. Digital mammography: its impact on recall rates and cancer detection rates in a small community-based radiology practice. Am. J. Roentgenol. 193, 582–585 (2009).
- Lewin JM, Hendrick RE, D’Orsi CJ et al. Comparison of full-field digital mammography with screen-film mammography for cancer detection: results of 4,945 paired examinations. Radiology 218, 873–880 (2001).
- Rosenberg RD, Yankaskas BC, Abraham LA et al. Performance benchmarks for screening mammography. Radiology 241, 55–66 (2006).
- Bick U, Diekmann F. Digital Mammography. Springer, Berlin, Germany (2010).
- Hisada K, Tonami N, Miyamae T et al. Clinical evaluation of tumor imaging with 201Tl chloride. Radiology 129, 497–500 (1978).
- Lawson, RN. Implication of surface temperatures in the diagnosis of breast cancer. Can. Med. Assoc. J. 75, 309–310 (1956).
- Mitsuyama N, Morimoto T, Kinouchi Y et al. In-vivo measurements of electrical bioimpedance of breast tumors. Nippon Gakkai Zasshi 89, 251–255 (1988).
- Kubota K, Matsuzawa T, Amemiya A et al. Imaging of breast cancer with [18F] fluorodeoxyglucose and positron emission tomography. J. Comput. Assist. Tomogr. 13, 1097–1098 (1989).
- Oude Munnink TH, Nagengast WB, Brouwers AH et al. Molecular imaging of breast cancer. Breast 18(3), S66–S73 (2009).
- Leff D, Warren O, Enfield A, Gibson A et al. Diffuse optical imaging of the healthy and diseased breast: a systematic review. Breast Cancer Res. Treat. 108, 9–22 (2007).
- Mahesh M. Digital mammography: an overview. Radiographics 24, 1747–1760 (2004).
- Spahn M. Flat detectors and their clinical applications. Eur. Radiol. 15, 1934–1947 (2005).
- Pisano ED, Yaffe MJ. Digital mammography. Radiology 234, 353–362 (2005).
- Boone JM, Nelson TR, Lindfors KK, Seibert JA. Dedicated breast CT: radiation dose and image quality evaluation. Radiology 221, 657–667 (2001).
- Niklason LT, Christian BT, Niklason LE et al. Digital tomosynthesis in breast imaging. Radiology 205, 399–406 (1997). n Original realization of a digital breast tomosynthesis unit.
- Dobbins JT 3rd, Godfrey DJ. Digital x-ray tomosynthesis: current state of the art and clinical potential. Phys. Med. Biol. 48, R65–R106 (2003).
- Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad. Radiol. 18, 1298–1310 (2011). & Review of tomosynthesis from the clinical point of view.
- Sechopoulos I. A review of breast tomosynthesis. Part I. The image acquisition process. Med. Phys. 40(1), 014301 (2013).
- Sechopoulos I. A review of breast tomosynthesis. Part II. Image reconstruction, processing and analysis, and advanced applications. Med. Phys. 40(1), 014302 (2013).
- Dobbins JT 3rd. Tomosynthesis imaging: at a translational crossroads. Med. Phys. 36, 1956–1967 (2009).
- Siedses Des Plantes BG. Eine neue methode zur differenzierung in der roentgenographie (planigraphie). Acta. Radiol. 13, 182–192 (1932).
- Grant DG. Tomosynthesis: a threedimensional radiographic imaging technique. IEEE Trans. Biomed. Eng. 19, 20–28 (1972).
- Saunders RS, Samei E, Lo JY, Baker JA. Can compression be reduced for breast tomosynthesis? Monte Carlo study on mass and microcalcification conspicuity in tomosynthesis. Radiology 251, 673–682 (2009).
- Li B, Avinash GB, Uppaluri R, Eberhard JW, Claus BEH. The impact of acquisition angular range on the z-resolution of radiographic tomosynthesis. Proc. CARS 1268, 13–18 (2004).
- Svane G, Azavedo E, Lindman K et al. Clinical experience of photon counting breast tomosynthesis: comparison with traditional mammography. Acta Radiol. 52, 134–142 (2011).
- Aslund M, Cederstrom B, Lundqvist M et al. Physical characterization of a scanning photon counting digital mammography system based on Si-strip detectors. Med. Phys. 34 1918–1925 (2007).
- Qian X, Rajaram R, Calderon-Colon X et al. Design and characterization of a spatially distributed multi-beam field emission x-ray source for stationary digital breast tomosynthesis. Med. Phys. 36, 4389–4399 (2009).
- Sprenger F, Calderon X, Gidcumb E et al. Stationary digital breast tomosynthesis with distributed field emission x-ray tube. Proc. SPIE 7961, pii: 878280 (2011).
- Qian X, Tucker A, Gidcumb E et al. High resolution stationary digital breast tomosynthesis using distributed carbon nanotube x-ray source array. Med. Phys. 39, 2090–2099 (2012).
- Mertelmeier T, Orman J, Haerer W, Dudam MK. Optimizing filtered backprojection reconstruction for a breast tomosynthesis prototype device. Proc. SPIE 6142, 61420F–F61412 (2006).
- Zhao B, Zhao W. Three-dimensional linear system analysis for breast tomosynthesis. Med. Phys. 35, 5219–5232 (2008).
- Herman GT, Lent A. Iterative reconstruction algorithms. Comput. Biol. Med. 6, 273–294 (1976).
- Gordon R, Bender R, Herman GT. Algebraic reconstruction techniques (ART) for three-dimensional electron microscopy and x-ray photography. J. Theor. Biol. 29, 471–481 (1970).
- Gilbert P. Iterative methods for the three-dimensional reconstruction of an object from projections. J. Theor. Biol. 36, 105–117 (1972).
- Andersen AH, Kak AC. Simultaneous Algebraic Reconstruction Technique (SART): a superior implementation of the ART algorithm. Ultrason. Imaging 6, 81–94 (1984).
- Lange K, Fessler JA. Globally convergent algorithms for maximum a posteriori transmission tomography. IEEE Trans. Image Process. 4, 1430–1438 (1995).
- Wu T, Moore RH, Rafferty EA, Kopans DB. A comparison of reconstruction algorithms for breast tomosynthesis. Med. Phys. 31, 2636–2647 (2004).
- Zhang Y, Chan HP, Sahiner B et al. A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis. Med. Phys. 33, 3781–3795 (2006).
- Hu YH, Zhao B, Zhao W. Image artifacts in digital breast tomosynthesis: investigation of the effects of system geometry and reconstruction parameters using a linear system approach. Med. Phys. 35, 5242–5252 (2008).
- Wu T, Moore RH, Kopans DB. Voting strategy for artifact reduction in digital breast tomosynthesis. Med. Phys. 33, 2461–2471 (2006).
- Zhang Y, Chan HP, Sahiner B et al. Application of boundary detection information in breast tomosynthesis reconstruction. Med. Phys. 34, 3603–3613 (2007).
- Zhang Y, Chan HP, Sahiner B et al. Artifact reduction methods for truncated projections in iterative breast tomosynthesis reconstruction. J. Comput. Assist. Tomogr. 33, 426–435 (2009).
- Karellas A, Giger ML (Eds). Advances in Breast Imaging: Physics, Technology, and Clinical Applications. Radiological Society of North America, IL, USA, 149–163 (2004).
- Wallis JW, Miller TR, Lerner CA, Kleerup EC. Three-dimensional display in nuclear medicine. IEEE Trans. Med. Imaging 8, 297–303 (1989).
- D’Orsi CJ, Getty DJ, Pickett RM et al. Stereoscopic digital mammography: improved specificity and reduced rate of recall in a prospective clinical trial. Radiology 266, 81–88 (2013).
- Webb L, Samei E, Lo JY et al. Comparative performance of multiview stereoscopic and mammographic display modalities for breast lesion detection. Med. Phys. 38, 1972–1980 (2011).
- Poplack SP, Tosteson TD, Kogel CA, Nagy HM. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. Am. J. Roentgenol. 189, 616–623 (2007).
- Gur D. Tomosynthesis: potential clinical role in breast imaging. Am. J. Roentgenol. 189, 614–615 (2007).
- Good WF, Abrams GS, Catullo VJ et al. Digital breast tomosynthesis: a pilot observer study. Am. J. Roentgenol. 190, 865–869 (2008).
- Andersson I, Ikeda DM, Zackrisson S et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur. Radiol. 18, 2817–2825 (2008).
- Teertstra HJ, Loo CE, van den Bosch MA et al. Breast tomosynthesis in clinical practice: initial results. Eur. Radiol. 20(1), 16–24 (2010).
- Gennaro G, Toledano A, di Maggio C et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur. Radiol. 20(7), 1545–1553 (2010).
- Skaane P, Bandos AI, Gullien R et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 267, 47–56 (2013). & Recent clinical findings reporting an increase in detection rate for invasive and in situ carcinoma cancers and a decrease in false-positive rate when using mammography plus tomosynthesis.
- Ciatto S, Houssami N, Bernardi D et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 14(7), 583–589 (2013). & Recent clinical findings reporting that integrated mammography and tomosynthesis improves breast cancer detection and has the potential to reduce false-positive recalls.
- Rafferty EA. Digital mammography: novel applications. Radiol. Clin. North. Am. 45, 831–843 (2007).
- Spangler ML, Zuley ML, Sumkin JH et al. Detection and classification of calcifications on digital breast tomosynthesis and 2D digital mammography: a comparison. Am. J. Roentgenol. 196, 320–324 (2011).
- Kopans D, Gavenonis S, Halpern E, Moore R. Calcifications in the breast and digital breast tomosynthesis. Breast J. 17(6), 638–644 (2011).
- Gur D, Zuley ML, Anello MI et al. Dose reduction in digital breast tomosynthesis (DBT) screening using synthetically reconstructed projection images: an observer performance study. Acad. Radiol. 19, 166–171 (2012).
- Wallis MG, Moa E, Zanca F, Leifland K, Danielsson M. Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution x-ray imaging observer study. Radiology 262, 788–796 (2012).
- Smith AP, Rafferty EA, Niklason L. Clinical performance of breast tomosynthesis as a function of radiologist experience level. In: Digital Mammography. Krupinski EA (Ed.). Springer, Berlin, Germany, 61–66 (2008).
- Kuhl CK, Mielcareck P, Klaschik S et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 211, 101–110 (1999).
- Froeling V, Diekmann F, Renz DM et al. Correlation of contrast agent kinetics between iodinated contrast-enhanced spectral tomosynthesis and gadoliniumenhanced MRI of breast lesions. Eur. Radiol. 23(6), 1528–1536 (2013).
- Carton AK, Li J, Albert M, Chen S, Maidment AD. Quantification for contrast-enhanced digital breast tomosynthesis. Presented at: Proceedings of the International Society for Optical Engineering. San Diego, CA, USA, 2 March 2006.
- Chen SC, Carton AK, Albert M et al. Initial clinical experience with contrast-enhanced digital breast tomosynthesis. Acad. Radiol. 14, 229–238 (2007).
- Puong S, Patoureaux F, Iordache R, Bouchevreau X, Muller S. Dual-energy contrast enhanced digital breast tomosynthesis: concept, method, and evaluation on phantoms. Presented at: Proceedings of the International Society for Optical Engineering. San Diego, CA, USA, 15 March 2007.
- Carton AK, Currivan JA, Conant E, Maidment A. Temporal subtraction versus dual-energy contrast-enhanced digital breast tomosynthesis: a pilot study. In: Digital Mammography. Krupinski EA (Ed.). Springer Berlin Heidelberg, Germany, 166–173 (2008).
- Carton AK, Gavenonis SC, Currivan JA et al. Dual-energy contrast-enhanced digital breast tomosynthesis – a feasibility study. Br. J. Radiol. 83, 344–350 (2010).
- Samei E, Saunders RS. Dual-energy contrast-enhanced breast tomosynthesis: optimization of beam quality for dose and image quality. Phys. Med. Biol. 56, 6359–6378 (2011).
- Kiarashi N, Lin Y, Segars WP et al. Development of a dynamic 4D anthropomorphic breast phantom for contrast-based breast imaging. Presented at: Proceedings of the International Society for Optical Engineering. San Diego, CA, USA, 5–8 February 2012.
- Kiarashi N, Ghate SV, Lo JY, Nolte LW, Samei E. Application of a dynamic 4D anthropomorphic breast phantom in contrastbased imaging system optimization: dualenergy or temporal subtraction? In: Breast Imaging. Andrew DA, Bakic PR, Gavenonis S (Eds). Springer Berlin Heidelberg, Germany, 658–665 (2012). & Contrast-based breast x-ray imaging and its potential.
- Kao TJ, Boverman G, Kim BS et al. Regional admittivity spectra with tomosynthesis images for breast cancer detection: preliminary patient study. IEEE Trans. Med. Imaging 27, 1762–1768 (2008).
- Sinha SP, Roubidoux MA, Helvie MA et al. Multi-modality 3D breast imaging with x-ray tomosynthesis and automated ultrasound. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 1335–1338 (2007).
- Williams MB, Judy PG, Gunn S, Majewski S. Dual-modality breast tomosynthesis. Radiology 255, 191–198 (2010).
- Fang Q, Selb J, Carp SA et al. Combined optical and x-ray tomosynthesis breast imaging. Radiology 258, 89–97 (2011).
- Hammonds JC, Price RR, Donnelly EF, Pickens DR. Phase-contrast digital tomosynthesis. Med. Phys. 38, 2353–2358 (2011).
- Winey BA, Zygmanski P, Cormack RA, Lyatskaya Y. Balancing dose and image registration accuracy for cone beam tomosynthesis (CBTS) for breast patient setup. Med. Phys. 37, 4414–4423 (2010).
- Lin Y, Ghate SV, Lo JY, Samei E. 3D biopsy for tomosynthesis: simulation of prior information based reconstruction for dose and artifact reduction. Presented at: Proceedings of the International Society for Optical Engineering. San Diego, CA, USA, 23 Febuary 2012.
- Reiser I, Nishikawa RM, Giger ML et al. Computerized mass detection for digital breast tomosynthesis directly from the projection images. Med. Phys. 33, 482–491 (2006).
- Chan HP, Wei J, Zhang Y et al. Computer-aided detection of masses in digital tomosynthesis mammography: comparison of three approaches. Med. Phys. 35, 4087–4095 (2008).
- Singh S, Tourassi GD, Baker JA, Samei E, Lo JY. Automated breast mass detection in 3D reconstructed tomosynthesis volumes: a featureless approach. Med. Phys. 35, 3626–3636 (2008).
- Mazurowski MA, Lo JY, Harrawood BP, Tourassi GD. Mutual information-based template matching scheme for detection of breastmasses: from mammography to digital breast tomosynthesis. J. Biomed. Inf. 44, 815–823 (2011).
- Reiser I, Nishikawa RM, Edwards AV et al. Automated detection of microcalcification clusters for digital breast tomosynthesis using projection data only: a preliminary study. Med. Phys. 35, 1486–1493 (2008).
- Sahiner B, Chan HP, Hadjiiski LM et al. Computer-aided detection of clustered microcalcifications in digital breast tomosynthesis: a 3D approach. Med. Phys. 39, 28–39 (2012).
- Bakic PR, Kontos D, Carton AK, Maidment ADA. Breast percent density estimation from 3D reconstructed digital breast tomosynthesis images. Presented at: Proceedings of the International Society for Optical Engineering. San Diego, CA, USA, 18 March 2008.
- Tagliafico A, Tagliafico G, Astengo D et al. Mammographic density estimation: One-to-one comparison of digital mammography and digitalbreast tomosynthesis using fully automated software. Eur. Radiol. 22, 1265–1270 (2012).
Websites
- Hologic. Hologic introduces synthesized 2D image algorithm designed to eliminate the need for a 2D mammogram in a 2D plus 3D tomosynthesis exam. www.hologic.com/en/news-releases/ view/173-year.2011_173-id.234881907.html
- Hologic news release. http://investors.hologic.com/2013-05-21- Hologic-Receives-FDA-Approval-for-a-New- Low-dose-3D-Mammography-Breast- Tomosynthesis-Solution-for-Breast-Cancer- Screening
- National Institue for Health Research. TOMMY trial: a comparison of TOMosynthesis with digital MammographY in the UK NHS Breast Screening Programme, Study Protocol. www.hta.ac.uk/2296 &Clinical trial in progress comparing digital mammography and tomosynthesis in women with abnormal screening mammography or a family history.
- Malmö breast tomosynthesis screening trial. http://clinicaltrials.gov/ct2/show/ NCT01091545 &Clinical trial in progress comparing mammography and tomosynthesis as screening tools.