Review Article - Interventional Cardiology (2011) Volume 3, Issue 1
Coronary revascularization strategies in patients with chronic heart failure
- Corresponding Author:
- Salvatore Davide Tomasello
Department of Internal Medicine & Systemic Disease, Ferrarotto Hospital
University of Catania, Catania, Italy Department of Cardiology
Moriggia- Pelascini Hospital, Via Pelascini 3, 22015 Gravedona, Como, Italy
E-mail: davtomasello@gmail.com
Abstract
Keywords
coronary artery bypass grafting, chronic heart failure, PCI versus CABG, percutaneous coronary intervention, viability tests
Chronic heart failure (CHF) is a worldwide public health problem of major and ever-expanding proportions, affecting approximately 5 million patients in the USA alone [1]. Moreover, the cost of hospitalization was approximated at US$2.6 million during the last decade and may double in the next 40 years [2]. Coronary artery disease (CAD) is the predominant cause of CHF and left ventricular dysfunction (LVD). Most patients with CHF attributable to CAD have had a prior myocardial infarction (MI) and LVD can occur as consequence of this.
In Europe, where there are an estimated 15 million patients with CHF, its prevalence is between 2 and 3%, and increases sharply at 75 years of age with a prevalence in 70–80-year-olds of between 10 and 20%. The overall prevalence of CHF is increasing owing to an aging population, the success of prolonging survival in patients suffering coronary events and the spread of secondary prevention. Almost 60% of the patients in the Acute Decompensated Heart Failure National Registry (ADHERE) had a history of CAD [3]. Similarly, of patients enrolled in CHF clinical trials, the majority (68%) had ischemic heart disease recorded as their heart failure etiology [4]. Although coronary revascularization by coronary artery bypass grafting (CABG) and/or percutaneous coronary intervention (PCI) has been studied exhaustively and analyzed in many clinical contexts, there are a lack of studies specifically designed to address the role of revascularization in patients with CAD and CHF. This article reviews the existing data concerning revascularization as a treatment of CHF in patients with CAD, exploring noninvasive testing for the identification of patients who are most likely to benefit from revascularization therapy.
Rationale for the revascularization of CHF patients
Revascularization of patients with LVD may improve their clinical outcome by several mechanisms. Dysfunctional myocardium can be related to the presence of necrosis, and/or with two condition states, including stunning and hibernating myocardium.
Cell death is categorized pathologically as either coagulation, contraction band necrosis, or both, and usually evolves through oncosis, but it can result to a lesser degree from apoptosis [5]. Careful analysis of histological sections by an experienced observer is essential for distinguishing these entities. After the onset of myocardial ischemia, cell death does not occur immediately but takes a finite period to develop (as little as 15 min in some animal models, but even this may be an overestimate). It takes 6 h before myocardial necrosis can be identified by standard macroscopic or microscopic postmortem examination [6]. Complete necrosis of all myocardial cells at risk requires at least 4–6 h or longer, depending on the presence of collateral blood flow into the ischemic zone, persistent or intermittent coronary artery occlusion and the sensitivity of the myocytes. Infarcts are usually classified by size – microscopic (focal necrosis), small (<10% of the left ventricle), medium (10– 30% of the left ventricle) or large (>30% of the left ventricle) – as well as by location (anterior, lateral, inferior, posterior or septal, or a combination of locations) [7]. The pathologic identification of myocardial necrosis is made without reference to morphologic changes in the epicardial coronary artery tree or to the clinical history. The presence of mononuclear cells and fibroblasts and the absence of polymorphonuclear leukocytes characterize a healing infarction [8]. A healed infarction manifests as scar tissue without cellular infiltration. The entire process leading to a healed infarction usually requires 5–6 weeks or more. Furthermore, reperfusion alters the gross and microscopic appearance of the necrotic zone by producing myocytes with contraction bands and large quantities of extravasated erythrocytes [9]. Obviously, the extent of necrosis in the myocardium determines the loss of contractile function in the tissue, and although the necrosis may be confined to a low number of ventricular segments, it may promote an intra-ventricular remodeling phenomenon with a decrease of global left ventriclular function.
Hibernating myocardium is a chronically hypocontractile tissue, due to persistently low flow, with the potential to improve function after restoration of the blood supply [10]. Hibernating myocardium might represent a solution to both an impaired coronary flow reserve and a reduced resting coronary blood flow [11]. Biopsies of human hibernating myocardium demonstrate histological changes of cellular dedifferentiation and an embryonic phenotype [12]. The severity of ultrastructural changes correlates directly with the time course of functional recovery, but correction of cellular changes after the restoration of flow or flow reserve is likely to only be partial [11,12]. These observations and evidence demonstrating that apoptosis is important in hibernation, underscore the importance of early revascularization in this dynamic transition from reversible to irreversible contractile dysfunction [11]. An elegant study by Pitt et al. demonstrated that chronically dysfunctional ischemic myocardium can improve function following revascularization, but waiting for revascularization might cause a decline in viable function accompanied by pathophysiological changes indicative of disease progression [13]. Moreover, such a decline attenuates myocardial function recovery. Therefore, in these patients, the therapeutic decision should be made as promptly as possible following initial assessment.
‘Stunning’ is contractile dysfunction resulting from transient ischemia followed by restoration of perfusion [14]. Its pathogenesis likely involves oxyradicals and calcium and the associated dysfunction might persist for hours or days, but generally improves with time. An exception to this is ‘repetitive stunning’, defined as repeated episodes of ischemia producing prolonged postischemic contractile dysfunction [11], which is similar to hibernation in that revascularization has the potential to improve contractile function. Hibernation and stunning are often practically indistinct and they might coexist in varying degrees in the same patient or myocardial region and represent a continuum of the same process.
The timing of functional recovery after revascularization appears to differ between both stunned and hibernating myocardium. In an echocardiography and radionuclide imaging study, nearly two-thirds of stunned segments exhibited early contractile recovery 3 months after revascularization, and only one tenth showed late improvement at 14 months [12]. By contrast, only approximately a third of hibernating segments exhibited early improvement, but nearly two-thirds showed late recovery. These observations suggest that many published studies might have assessed contractile recovery too early and therefore underestimated the degree of recovery. Recently, Rahimtoola et al. suggested a unifying concept of hibernation and remodeling, with an emphasis on the importance of early revascularization [15].
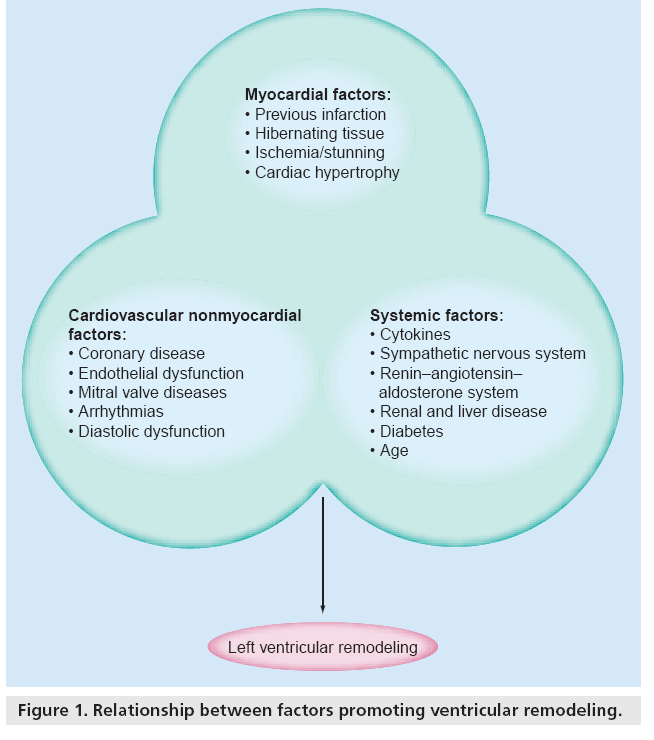
The interaction between this myocardial process, cardiovascular disease and several systemic conditions contributes to remodeling, progression of systolic dysfunction and CHF (Figure 1). Of note is post-infarction remodeling, which involves owing to the replacement of nonviable myocardium with scar tissue and is accompanied by secondary pathologic changes in the shape and size of the left ventricle.
The Open Artery trial (OAT) tested the ‘open artery strategy’ in stable post-MI patients with late occluded infarct-related arteries [16]. Analyzing the outcome of 2201 patients, late routine PCI of infarct-related artery (IRA) demonstrated no benefit in comparison with medical therapy in terms of the combined end point of death, MI and class IV heart failure [16]. Recently, a nuclear substudy, conducted in 27% of the total OAT population, demonstrated a lack of clinical benefit after late recanalization of IRA in patients with mild-to-moderate inducible ischemia [17]. However, this evidence does not allow a definite conclusion to be drawn because of the post hoc nature of the study and the absence of patients with severe inducible ischemia in the main analysis.
In CHF patients, mitral valve regurgitation is also a factor that might accelerate the remodeling process. Remodeling appears to progress over time and the ability to reverse the process may also be time sensitive.
A study by Samady et al. demonstrated an increased survival in patients with LVD after revascularization, independently of left ventricular functional improvement [18]. Several pathophysiological mechanisms may be responsible for this benefit. First, restoration of blood flow to ischemic myocardium adjacent to the endocardial scar relieves resting ischemia, enhances the reparative process of the myocyte contractile machinery and may protect against future infarction [19]. Second, revascularization may limit infarct expansion and ventricular dilation, by providing a scaffolding which supports the surrounding necrotic myocardium and reduces myocardial compliance [19]. These mechanisms may also improve diastolic function and may even reduce dynamic mitral regurgitation, culminating in further symptomatic improvement. Finally, by reducing left ventricular remodeling and the ischemic burden, revascularization of ischemic myocardium may reduce the incidence of ventricular arrhythmias related to the re-entry phenomenon.
Which strategy for revascularization should be followed: surgical or percutaneous?
Owing to the paucity of data from contemporary therapies, decision making in CHF patients requiring revascularization is largely based on surgical studies performed nearly 20 years ago. In the two largest retrospective series, the Coronary Artery Surgery Study registry (420 medical and 231 surgical patients) [20] and the Duke University Cardiovascular Database (409 medical and 301 surgical patients) [21], a significant long-term survival advantage was observed with CABG over medical therapy, but the surgical survival benefits were greatest for those patients with the most severe LVDs (LVEF < 25%), the most extensive CAD and the most intense angina. Although results from these and other smaller studies have favored surgery over medical therapy overall, important limitations have included selection bias for revascularization, inadequate medical therapy in both medical and surgical groups, the use of outdated surgical techniques and the small numbers of patients, particularly those whose symptoms are predominantly of heart failure. Recently, an update of more than 18,000 patients entered into the Duke Databank from 1986 to 2000 confirmed the survival benefit of revascularization in patients with CAD and reduced ejection fraction (EF) [22].
It is interesting to note that peri-operative mortality rates for CABG in patients with left ventricular systolic dysfunction vary widely, from approximately 5% among younger adults to more than 30% among older individuals, who have more severe left ventricular systolic dysfunction and comorbidities [23]. There are many limitations to consider when interpreting the data from this study, owing to their retrospective nature and the nonstandardized surgical strategy employed. For this reason, several trials have been specifically designed in order to address the real value of revascularization in CHF patients.
The aim of the Heart Failure Revascularization (HEART) trial was to evaluate whether revascularization could improve the prognosis in CHF patients [24]. Patients with LVEF under 35% requiring chronic diuretic therapy, with evidence of at least five segments being affected by ischemia and/or hibernation were included. The study aimed to enroll 800 patients, but owing to problems with recruitment and funding, the study was stopped early. Of the 139 patients enrolled, 69 were randomized to PCI and 45 underwent CABG. There were no differences in the incidence of all-cause mortality and in quality of life [25].
The Surgical Treatment for Ischemic Heart Failure (STICH) trial was designed to define the role of cardiac surgery in the treatment of patients with heart failure and CAD [26]. The study had two major hypotheses: the first was to compare the optimal medical therapy with surgical revascularization and the second was that surgical ventricular reconstruction, when added to CABG, would decrease the rate of death or hospitalization owing to a cardiac event when compared with CABG alone. After a randomization of 1000 patients (499 assigned to CABG and 501 to CABG plus surgical ventricular reconstruction) in the arm of the second hypothesis this study did not show any clinical benefit of adding surgical ventricular reconstruction to CABG [27]. However, no data have yet been reported concerning the first hypothesis. Therefore, the results of this randomized trial are underway to evaluate the effect of CABG on revascularization in CHF patients.
The Alberta Provincial Project for Outcomes Assessment in Coronary Artery Disease (APPROACH) trial used data obtained from a prospective data collection initiative [28]. In this study, a total of 4228 patients were enrolled; a total of 2538 patients received revascularization and a total of 1690 did not receive revascularization. Mortality rates at 1 year were 11.8% in those who underwent revascularization and 21.6% in those who did not (hazard ratio: 0.52; 95% CI: 0.47–0.58). The benefit of revascularization continued up to the 7-year follow-up (hazard ratio: 0.50; 95% CI: 0.44–0.57). After propensity score adjusting, patients undergoing CABG appeared to have slightly better survival than those who received PCI.
Much data supporting the benefit of PCI for treating acute heart failure, presumably related to myocardial stunning, have been reported. Percutaneous revascularization with stenting can be safely performed in patients with low EF with acceptable late major adverse cardiac event rates [29].
Gioia and colleagues demonstrated for the first time, that drug-eluting stent (DES) implantation in patients with severe LVD improves mortality compared with the use of a bare-metal stent alone [30]. The use of DESs in this study decreased cardiovascular mortality by 78% and was the only predictor of survival.
Conversely, a recent substudy of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial investigated the outcome of high-risk patients (those with low EF or stabilized acute coronary syndrome) [31]. Therefore, an initial strategy of optimal medical therapy alone for these highrisk patients did not result in increased death or MI at 4.6 years or worse angina at 1 year, but it was associated with a high rate of crossover to revascularization.
In a recent large registry of 55,709 patients, Wallace et al. demonstrated that the severity of LV systolic dysfunction was directly related to hospital mortality and major adverse cardiac events (MACE), in patients who underwent elective PCI [32]. On the basis of this study’s data, patients with EFs of 25% or less and 26–35% had fourfold and twofold increased risks, respectively, of hospital mortality, independent of multiple other clinical and procedural risk factors. These findings were consistent across multiple subgroups, including gender, heart failure, kidney disease, diabetes and multi-vessel CAD, and, therefore, we maintain that the extent of systolic dysfunction is independently important in risk assessment.
A single small randomized trial comparing PCI with CABG in a low-EF population concluded that outcomes were similar with the two revascularization methods [33].
By contrast, a large registry report from the State University of New York (USA) suggested that better outcomes were obtained for patients with impaired LV function who underwent CABG compared with those who underwent stenting. They compared a total of 37,212 patients with multivessel disease who underwent CABG (26% with EF <40%) and a total of 22,102 patients who underwent PCI (18.5% with EF <40%) from January 1997 to December 2000 [34]. For the patients with an EF less than 40% and with two- or three-vessel disease with involvement of the proximal left anterior descending coronary artery, the hazard ratios strongly favored CABG over stenting. The hazard ratios were not significantly different for other patients with two-vessel disease.
These results were confirmed by propensity analysis of long-term outcomes after surgical or percutaneous revascularization in patients with multivessel disease and a high-risk profile, in which CABG was associated with better survival than PCI after adjustment for risk profile [35].
However, another small retrospective registry did not show any clinical difference in terms of mortality and major adverse cardio- and cerebro-vascular events rate in a selected highrisk patients cohort with severe LVD treated with DES implantation or CABG [36].
The recent Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial demonstrated lower rates of MACE and/or major adverse cerebrovascular events at 1 year after CABG in comparison with PCI (12.4 vs 17.8%; p = 0.002) in patients affected by three-vessel or left main CAD, in large part guided by lower repeat revascularization (5.9 vs 13.5%; p < 0.001). Notably, the rates of death and MI were similar between the two groups and stroke was significantly more likely to occur with CABG (2.2 vs 0.6% with PCI; p = 0.003) [37].
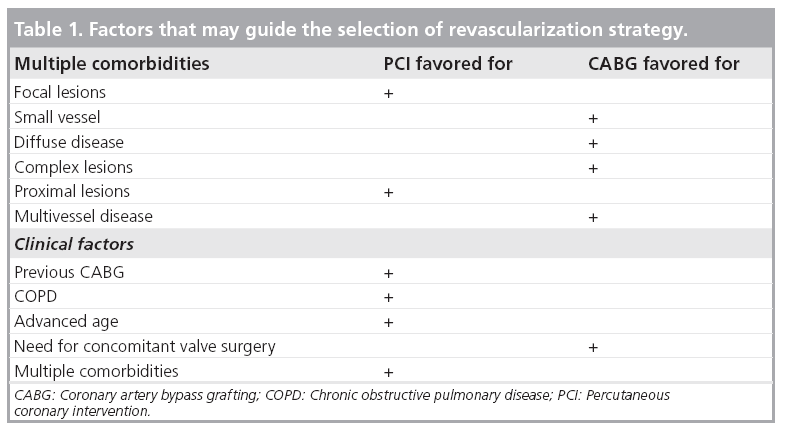
In practice, a careful revision of a patient’s clinical history is needed to choose the most appropriate revascularization approach. A history of previous bypass surgery is a common reason for prefering PCI over CABG, whereas the need for concomitant mitral valve repair makes a surgical approach more appealing. The ability to achieve complete revascularization is often an important topic when choosing between PCI and surgical revascularization, with some believing that late outcome is influenced more by completeness of revascularization than by method. It especially seems reasonable to try to achieve revascularization of all viable arterial segments. Finally, PCI is often the procedure of last resort for patients who have been denied surgery. Table 1 highlights the factors that might influence the selection of revascularization strategy.
Test for the assessment of viability
Viability testing may be useful to identify a subset of patients with ischemic LVD who are likely to benefit from cardiac revascularization procedures. Viability may be evaluated by an assortment of techniques, including single photon emission computed tomography (SPECT), PET, dobutamine echocardiography and, most recently, MRI.
▪ Nuclear imaging techniques
Single photon emission computed tomography and PET rely predominantly on the demonstration of cellular integrity (intact cell membrane and mitochondria) and metabolic functions (preserved glucose utilization), respectively, to identify viable myocardium. With SPECT, both stress-induced perfusion abnormalities and resting isotope uptake of approximately 50–60% predict functional recovery [38]. Compared with PET, SPECT underestimates viability [39,40]; SPECT is widely available, but its lower-energy tracers, lower spatial resolution and lack of builtin attenuation correction limit its diagnostic accuracy. The most established PET technique for assessing viability is the combined examination of myocardial perfusion (with either N-13 ammonia or rubidium-82) and myocardial glucose metabolism (with F-18 fluorodeoxyglucose [FDG]). The most specific pattern for functional recovery is the mismatch between reduced perfusion and preserved metabolism [41]. Hypocontractile regions with more than 50% FDG uptake (compared with normal or remote regions) might also recover contractile function after revascularization, but at a lower frequency, likely because of subendocardial scarring [38]. With its built-in attenuation correction, greater temporal and spatial resolution, and higherenergy tracers, PET has superior image quality and high diagnostic accuracy. Its disadvantages include limited availability, cost, complexity and dependence of FDG uptake on the patient’s metabolic state. Moreover, a recent randomized trial did not demonstrate a significant reduction in cardiac events in patients with LVD and suspected coronary disease for FDG PET-assisted management versus standard care [42].
▪ Dobutamine echocardiography
The most commonly used echocardiographic technique for assessing viability relies on demonstrating contractile reserve with dobutamine administration. Dobutamine increases heart rate, blood pressure and contractility, and, therefore, myocardial oxygen demand and coronary blood flow. Functional improvement in hypocontractile segments, with or without subsequent contractile deterioration (biphasic response), indicates viability and ischemia and predicts functional recovery [43]. When conventional echocardiographic imaging does not provide adequate images, both harmonic imaging [44] and intravenous blood pool contrast [45] can improve endocardial definition. Although widely available and less technically complex in comparison with other functional testes, echocardiography is limited by its qualitative assessment, with high interobserver and intercenter variation and inadequate acoustic windows in a substantial number of patients, even when combined with harmonic imaging and contrast.
▪ MRI
MRI has enormous potential thanks to its major attributes of high image quality and resolution combined with nonionizing radiation and versatility. It can provide high-quality diagnostic information about cardiac and valvular function, coronary anatomy, coronary flow reserve, myocardial perfusion, myocardial viability, contractile reserve and cardiac metabolism. It allows the assessment of even subtle wall motion disturbances resulting from the consistently high endocardial border definition, and the measurement of myocardial perfusion can be integrated into the same examination, with the high spatial resolution of the scans facilitating the determination of the transmural extent of a regional perfusion deficit.
Recently, the technique of late enhancement with gadolinium contrast agent has been described. The gadolinium concentrates in the necrotic area (in acute infarction) or scar tissue (in chronic infarction) because of an increased partition coefficient, and the infarct area becomes bright [46]. There is a very close correlation between the volume of signal enhancement and infarct size in animal experiments of acute infarction. The technique has high resolution and can define the transmural extent of necrosis and scar, for the first time, in vivo. Although the technique has been recently developed, it has obvious applications in defining whether infarction has actually occurred in borderline cases.
The technique of late enhancement also has a relevant clinical application in the assessment of viability and it is an excellent technique for the detection and quantification of MI, as reported by many studies [47,48]. First-pass perfusion is the most widely used MRI technique for the detection of reduced myocardial blood flow and yields superior results compared with those of SPECT [49]. Recently, the US FDA reported a warning on the use of gadolinium contrast in patients with impaired renal function (glomerular filtration rate <30 ml/min/1.73 m2) owing to an increased risk of nephrogenic system fibrosis [101].
In recent studies, the simple morphological measure of the percentage of transmural replacement of normal myocardium by scar tissue, has been demonstrated to be a powerful predictor of post-revascularization contraction recovery [50]. Segments with less than 50% transmural replacement had improved function, while those with higher grades of transmural replacement failed to improve [49]. This technique has many clinical advantages, including being morphologically based, making analysis straightforward and that there is no radiation burden. It is anticipated that the late-enhancement technique will make a substantial clinical impact in the management of infarction and its sequelae.
A recent meta-analysis has described the relative merits of dobutamine echocardiography, tallium-201 and technetium-99m scintigraphy, PET and MRI for the diagnosis of hibernating myocardium and the prediction of patients’ outcome. This study demonstrated a lower sensitivity of dobutamine echocardiography in comparison with other imaging techniques and a similar sensitivity compared with nuclear tests. On the other hand, no significant changes were observed in the specificity of these imaging techniques [51].
▪ Clinical implication
Nowadays, the decisions regarding revascularization in patients with ischemic severe LVD are a challenge for clinicians. The benefits of pharmacologic management are strongly evidence based and all patients should receive optimal medical management with recommended agents according to current CHF guidelines [52,102]. In addition, device therapy with defibrillators and possibly cardiac resynchronization therapy should be considered in many of these patients [53].
Routine coronary angiography in these patients is not suggested in the American College of Cardiology (ACC)/American Heart Association (AHA) and the European Society of Cardiology (ESC) guidelines, but in the case of angina symptoms, both guidelines recommend, as class I, the invasive assessment of CAD and revascularization. Moreover, the guidelines also recommend coronary angiography in patients without symptoms but with a high risk of CAD.
Nowadays, the viability tests do not play a pivotal role in clinical decision making regarding patients with CHF; instead, their use is more common to refine the therapeutical strategy in those patients suitable for revascularization. In a study similar to the OAT, but in which the decision to revascularize the infarct-related artery was made considering the presence of myocardium viability assessed by a nuclear stress test, PCI resulted in a better outcome in terms of a lower rate of MACEs in comparison with medical therapy. Moreover, LVEF remained preserved in PCI patients, which underlines the importance of viability for myocardial recovery recovery [54].
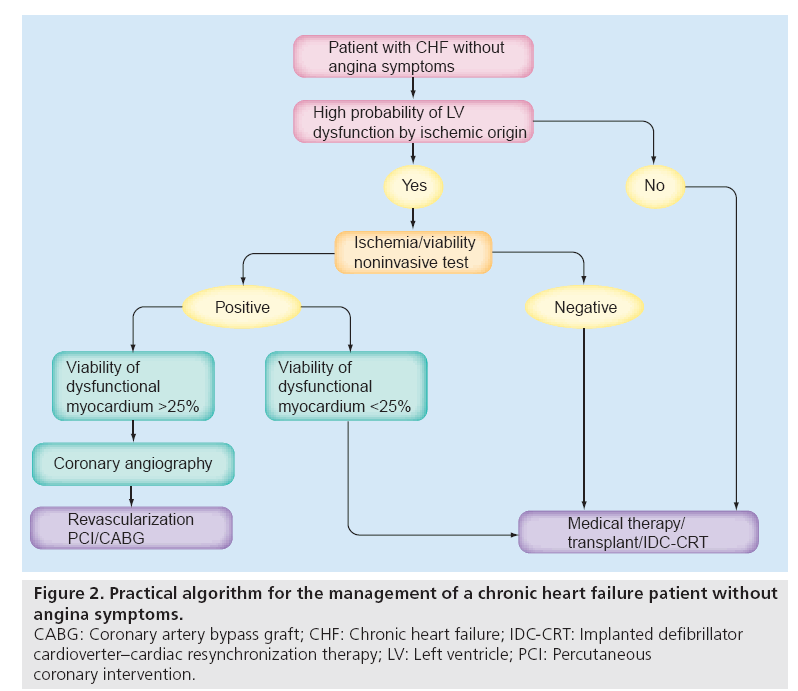
An updated guideline for CABG incorporated viability in its class IIa recommendation, stating that a revascularization decision might be undertaken in patients with poor LV function with significant viable noncontracting myocardium, and recognized that a subgroup of patients might experience benefit [55]. However, there are no strict criteria to determine which patients are too sick to be considered for revascularization. On the basis of the available literature, performing revascularization might be a reasonable strategy when noninvasive testing shows at least 25–30% LV viability of dysfunctional myocardium. However, it should be underlined that in patients with advanced age or who have compelling comorbidities, such as multiorgan failure, a conservative management is obviously required. Figure 2 shows a possible algorithm for the treatment of patients with CHF by ischemic origin in the absence of angina symptoms.
Figure 2: Practical algorithm for the management of a chronic heart failure patient without
angina symptoms.
CABG: Coronary artery bypass graft; CHF: Chronic heart failure; IDC-CRT: Implanted defibrillator
cardioverter–cardiac resynchronization therapy; LV: Left ventricle; PCI: Percutaneous
coronary intervention.
For these reasons, viability testing might have a potential role in patient selection and in predicting response to therapy [56]. The choice of viability techniques depends on many factors, including availability, expertise and cost. Although reported diagnostic accuracies differ among techniques for predicting functional recovery, there are no data to meaningfully compare individual techniques with respect to patient outcome.
Conclusion & future perspective
The outcomes of several multiple observational studies support the benefit of revascularization in patients with CHF. Viability testing can identify those patients who may have hibernating or stunned myocardium and may play a supportive role in clinical decision making. The choice of revascularization technique, either surgical or percutaneous, should be made on the basis of anatomical and clinical characteristics. The first hypothesis of the STICH randomized trial should better define the role of revascularization in this subset of patients, clarifying how the revascularization strategy should be chosen. However, other large randomized trials are needed to assess the role of viability testing, which will allow the development of more comprehensive clinical guidelines for the management of CHF patients.
Executive summary
Epidemiology
▪ Chronic heart failure (CHF) is a worldwide public health problem of major and ever-expanding proportions. In Europe, there are at least 15 million patients with CHF and its prevalence in 70–80-year-olds is between 10 and 20%.
Rationale for the revascularization of CHF patients
▪ Revascularization of patients with CHF may improve patient outcomes by several mechanisms:
- Chronically dysfunctional ischemic myocardium can improve function following revascularization.
- Revascularization reduces repeated episodes of ischemia, which determines prolonged post-ischemic contractile dysfunction.
- Restoration of blood flow to ischemic myocardium adjacent to endocardial scar tissue relieves resting ischemia and enhances the reparative process of the myocyte contractile machinery influencing the post-infarct ventricular remodeling.
- Revascularization may improve diastolic function and may even reduce dynamic mitral regurgitation, culminating in further symptomatic improvement.
- Revascularization can reduce the incidence of ventricular arrhythmias related to the re-entry phenomenon.
Selection of revascularization strategy
▪ Data reported in the literature showed that both surgical and percutaneous revascularization can reduce the mortality of CHF patients. Although there is a lack a conclusive evidence regarding the best revascularization modality, it is reasonable to select a surgical approach in case of multivessel or complex coronary disease, concomitant valve disease and the absence of co-morbidities. Conversely, percutaneous coronary intervention is preferably selected in case of single-vessel coronary disease and high-risk-profile patients.
Test for the assessment of myocardium viability
▪ Viability testing may be useful to identify a subset of patients with ischemic left ventricular dysfunction who are likely to benefit from cardiac revascularization procedures. Viability may be evaluated by an assortment of techniques including nuclear imaging, dobutamine echocardiography and, most recently, MRI.
Clinical implication
▪ On the basis of the available literature, performing revascularization might be a reasonable strategy when noninvasive testing reveals at least 25–30% left ventricular viability of a dysfunctional myocardium. However, it should be underlined that in patients with advanced age or who have compelling comorbidities; such as multiorgan failure, conservative management is obviously required.
Conclusion & future perspective
▪ The selection method of revascularization strategy in patients affected by CHF remains debatable. The first hypothesis of the Surgical Treatment for Ischemic Heart Failure randomized trial should better define the role of revascularization in patients affected by CHF clarifying how a revascularization strategy should be chosen.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪▪ of considerable interest
- Thom T, Haase N, Rosamond W et al.; forthe American Heart Association StatisticsCommittee and Stroke StatisticsSubcommittee: heart disease and strokestatistics – 2006 update: a report from theAmerican Heart Association StatisticsCommittee and Stroke StatisticsSubcommittee. Circulation 113, e85–e151(2006).
- Hunt SA, Baker DW, Chin MH et al.:ACC/AHA guidelines for the evaluation andmanagement of chronic heart failure in theadult: a report of the American College ofCardiology/American Heart AssociationTask Force on Practice Guidelines(Committee to Revise the 1995 Guidelinesfor the Evaluation and Management of HeartFailure). Circulation 104, 2996 –3007(2001).
- Adams KF Jr, Fonarow GC, Emerman CLet al.: Characteristics and outcomes ofpatients hospitalized for heart failure in theUnited States: rationale, design, andpreliminary observations from the first100,000 cases in the Acute DecompensatedHeart Failure National Registry(ADHERE). Am. Heart J. 149, 209–216(2005).
- Gheorghiade M, Bonow RO: Chronic heartfailure in the United States: a manifestationof coronary artery disease. Circulation 97,282–289 (1998).
- Myocardial Infarction Redefined. Aconsensus document of The Joint EuropeanSociety of Cardiology/American College ofCardiology Committee for the Redefinition ofMyocardial Infarction and The JointEuropean Society of Cardiology/AmericanCollege of Cardiology Committee. J. Am.Coll. Cardiol. 3, 959–969 (2000).
- Buja LM: Modulation of the myocardialresponse to injury. Lab. Invest. 78, 1345–1373(1998).
- Kajstura J, Cheng W, Reiss K et al.: Apoptoticand necrotic myocyte cell deaths areindependent contributing variables of infarctsize in rats. Lab. Invest. 74, 86–107 (1996).
- Fishbein MC, Maclean D, Maroko PR: Thehistopathologic evolution of myocardialinfarction. Chest 73, 743–749 (1978).
- Mallory GK, White PD, Salcedo-Salga J: Thespeed of healing of myocardial infarction: astudy of the pathologic anatomy in 72 cases.Am. Heart J. 18, 647–671 (1939).
- Rahimtoola SH: The hibernatingmyocardium. Am. Heart J. 117, 211–221(1989).
- Dispersyn GD, Borgers M, Flameng W:Apoptosis in chronic hibernatingmyocardium: sleeping to death? Cardiovasc.Res. 45, 696–703 (2000).
- Bax JJ, Visser FC, Poldermans D et al.: Timecourse of functional recovery of stunned andhibernating segments after surgicalrevascularization. Circulation 104(Suppl. 1),I314–I318 (2001).
- Pitt M, Dutka D, Pagano D, Camici P,Bonser R: The naural history of myocardiumawating revascularization in patients withimpaired left ventricular function. Eur. HeartJ. 25, 500–507 (2004).
- Kim SJ, Depre C, Vatner SF: Novelmechanisms mediating stunned myocardium.Heart Fail. Rev. 8, 143–153 (2003).
- Rahimtoola SH, La Canna G, Ferrari R et al.:Hibernating myocardium another piece of thepuzzle falls into place. J. Am. Coll. Cardiol.47, 978–980 (2006).
- Menon V, Pearte CA, Buller CE et al.: Lackof benefit from percutaneous intervention ofpersistently occluded infarct arteries after theacute phase of myocardial infarction is timeindependent: insights from Occluded ArteryTrial. Eur. Heart J. 30, 183–191 (2009).
- Cantor WJ, Baptista SB, Srinivas VS et al.:Impact of stress testing before percutaneouscoronary intervention or medicalmanagement on outcomes of patients withpersistent total occlusion after myocardialinfarction: analysis from the occluded arterytrial. Am. Heart J. 157, 666–672 (2009).
- Samady H, Elefteriades JA, Abbott BG et al.:Failure to improve left ventricular functionafter coronary revascularization for ischemiccardiomyopathy is not associated with worseoutcome. Circulation 100, 1298–1304(1999).
- Braunwald E: Myocardial reperfusion,limitations of infarct size, reduction of leftventricular dysfunction and improvedsurvival: should the paradigm be expanded?Circulation 79, 441–444 (1989).
- Alderman E, Fisher LD, Litwin P et al.:Results of coronary artery surgery in patientswith poor left ventricular dysfunction(CASS). Circulation 68, 785–795 (1983).
- Bounous EP, Mark DB, Pollock BG et al.:Surgical survival benefits for coronary diseasepatients with left ventricular dysfunction.Circulation 78, 1151–1157 (1988).
- Smith PK, Califf RM, Tuttle RH et al.:Selection of surgical or percutaneous coronaryintervention provides differential longevitybenefit. Ann. Thorac. Surg. 82, 1420–1428(2006).
- Baker DW, Jones R, Hodges J et al.:Management of heart failure. III. The role ofrevascularization in the treatment of patients with moderate or severe left ventricularsystolic dysfunction. JAMA 272, 1528–1534(1994).
- Cleland JG, Freemantle N, Ball SG et al. Theheart failure revascularization trial(HEART): rationale, design andmethodology. Eur. J. Heart Fail. 5, 295–303(2003).
- Coletta AP, Cleland JG, Cullington D,Clark AL: Clinical trials update from HeartRhytm 2008 and Heart Failure 2008:ATHENA, URGENT, INH study, HEARTand CK-1827452. Eur. J. Heart Fail. 10,917–920 (2008).
- Velazquez EJ, Lee KL, O’Connor CM et al.:The rationale and design of the SurgicalTreatment for Ischemic Heart Failure(STICH) trial. J. Thorac. Cardiovasc. Surg.134, 1540–1547 (2007).
- Jones RH, Valazquez EJ, Michler RE et al.:Coronary bypass surgery with or withoutsurgical ventricular reconstruction. N. Engl. J.Med. 17, 1705–1717 (2009).
- Tsuyuki RT, Shrive FM, Galbraith PD,Knudtson ML, Graham MM: for theAPPROACH Investigators: revascularizationin patients with heart failure. CMAJ 175,361–365 (2006).
- Di Sciascio G, Patti G, D’Ambrosio A et al.:Coronary stenting in patients with depressedleft ventricular function: acute and long-termresults in a selected population. CatheterCardiovasc. Interv. 59, 429–433 (2003).
- Gioia G, Matthai W, Benassi A, Rana H,Levite HA, Ewing LG: Improved survivalwith drug-eluting stent implantation incomparison with bare metal stent in patientswith severe left ventricular dysfunction.Catheter Cardiovasc. Interv. 68, 392–398(2006).
- Maron DJ, Spertus JA, Mancini GB et al.:Impact of an initial strategy of medicaltherapy without percutaneous coronaryintervention in high-risk patients from theClinical Outcomes UtilizingRevascularization and Aggressive DruGEvaluation (COURAGE) trial. Am. J.Cardiol. 104, 1055–1062 (2009).
- Wallace TW, Berger JS, Wang A,Velazquez EJ, Brown DL: Impact of leftventricular dysfunction on hospital mortalityamong patients undergoing electivepercutaneous coronary intervention. Am. J.Cardiol. 103, 355–360 (2009).
- Sedlis SP, Ramanathan KB, Morrison DAet al.: Outcome of percutaneous coronaryintervention versus coronary bypass graftingfor patients with low left ventricular ejectionfractions, unstable angina pectoris, and riskfactors for adverse outcome with bypass (theAWESOME randomized trial and registry).Am. J. Cardiol. 94, 118–120 (2004).
- Hannan EL, Racz MJ, Walford G et al.:Long-term outcomes of coronary-arterybypass grafting versus stent implantation.N. Engl. J. Med. 352, 2174–2183 (2005).
- Brener SJ, Lytle BW, Casserly IP et al.:Propensity analysis of long-term survival aftersurgical or percutaneous revascularization inpatients with multivessel coronary arterydisease and high-risk features. Circulation109, 2290–2295 (2004).
- Gioia G, Matthai W, Gillin K et al.:Revascularization in severe left ventriculardysfunction: outcome comparison ofdrug-eluting stent implantation versuscoronary artery by-pass grafting. CatheterCardiovasc. Interv. 70, 26–33 (2007).
- Serruys PW, Morice MC, Kappetein AP,Colombo A, Holmes DR et al.: Percutaneouscoronary intervention versus coronary arterybypass grafting for severe coronary arterydisease. N. Engl. J. Med. 360, 961–972(2009).
- Bax JJ, van der Wall EE, Harbinson M:Radionuclide techniques for the assessment ofmyocardial viability and hibernation. Heart90(Suppl. 5), V26–V33 (2004).
- Brunken RC, Mody FV, Hawkins RA,Nienaber C, Phelps ME, Schelbert HR:Positron emission tomography detectsmetabolic viability in myocardium withpersistent 24-hour single-photon emissioncomputed tomography 201Tl defects.Circulation 86, 1357–1369 (1992).
- Sawada SG, Allman KC, Muzik O et al.:Positron emission tomography detectsevidence of viability in rest technetium-99msestamibi defects. J. Am. Coll. Cardiol. 23,92–98 (1994).
- Knuuti J, Schelbert HR, Bax JJ: The need forstandardisation of cardiac FDG PET imagingin the evaluation of myocardial viability inpatients with chronic ischaemic leftventricular dysfunction. Eur. J. Nucl. Med.29, 1257–1266 (2002).
- Beanlands RS, Nichol G, Huszti E et al.:F-18-fluorodeoxyglucose positron emissiontomography imaging-assisted management ofpatients with severe left ventriculardysfunction and suspected coronary disease.A randomized, controlled trial (PARR-2).J. Am. Coll. Cardiol. 50, 2002–2012 (2007).
- Afridi I, Grayburn PA, Panza JA, Oh JK,Zoghbi WA, Marwick TH: Myocardialviability during dobutamineechocardiography predicts survival in patientswith coronary artery disease and severe leftventricular systolic dysfunction. J. Am. Coll.Cardiol. 32, 921–926 (1998).
- Monaghan MJ: Second harmonic imaging: anew tune for an old fiddle? Heart 83, 131–132(2000).
- Zoghbi WA: Evaluation of myocardialviability with contrast echocardiography. Am.J. Cardiol. 90, 65J–71J (2002).
- Kim RJ, Fieno DS, Parrish RB et al.:Relationship of MRI delayed contrastenhancement to irreversible injury, infarctage, and contractile function. Circulation 100,185–192 (1999).
- Kim RJ, Fieno DS, Parrish TB et al.:Relationship of MRI delayed contrastenhancement to irreversible injury, infarctage, and contractile function. Circulation 100,1992–2002 (1999).
- Rehwald WG, Fieno DS, Chen EL, Kim RJ,Judd RM: Myocardial magnetic resonanceimaging contrast agent concentrations afterreversible and irreversible ischemic injury.Circulation 105, 224–229 (2002).
- Schwitter J, Wacker C, van Rossum A et al.:MRIMPACT: comparison of perfusioncardiacmagnetic resonance with singlephotonemission computed tomography forthe detection of coronary artery disease in amulticentre, multivendor, randomized trial.Eur. Heart J. 29, 480–489 (2008).
- Kim RJ, Wu E, Rafael A et al.: The use ofcontrast-enhanced magnetic resonanceimaging to identify reversible myocardialdysfunction. N. Engl. J. Med. 343, 1445–1453(2000).
- Schinkel AF, Bax JJ, Poldermans D,Elhendy A, Ferrari R, Rahimtoola SH.Hibernating myocardium: diagnosis andpatient outcomes. Curr. Probl. Cardiol. 32,375–410 (2007).
- Dickstein K, Cohen-Solal A, Filippatos Get al: ESC guidelines for the diagnosis andtreatment of acute and chronic heart failure2008: the Task Force for the Diagnosis andTreatment of Acute and Chronic HeartFailure 2008 of the European Society ofCardiology. Developed in collaboration withthe Heart Failure Association of the ESC(HFA) and endorsed by the European Societyof Intensive Care Medicine (ESICM). Eur. J.Heart Fail. 10, 933–989 (2008).
- Shams OF, Ventura HO: Device therapy forheart failure: when and for whom? Am. J.Cardiovasc. Drugs 8, 147–153 (2008).
- Erne P, Schoenenberger AW, Burckhardt Det al.: Effects of percutaneous coronaryinterventions in silent ischemia aftermyocardial infarction: the SWISSI IIrandomized controlled trial. JAMA 297,1985–1991 (2007).
- Eagle KA, Guyton RA, Davidoff R et al.:ACC/AHA 2004 guideline update forcoronary artery bypass graft surgery:summary article: a report of the AmericanCollege of Cardiology/American HeartAssociation Task Force on Practice Guidelines(Committee to Update the 1999 Guidelinesfor Coronary Artery Bypass Graft Surgery).Circulation 110, 1168–1176 (2004).
- Pagano D, Townend JN, Littler WA,Horton R, Camici PG, Bonser RS: Coronaryartery bypass surgery as treatment forischemic heart failure: the predictive value ofviability assessment with quantitative positronemission tomography for symptomatic andfunctional outcome. J. Thorac. Cardiovasc.Surg. 115, 791–779 (1998).
▪▪ Design of the first randomized trial in patients with chronic heart failure (CHF) treated by medical therapy, coronary artery bypass grafting (CABG) or percutaneous coronary intervention.
▪▪ The largest randomized trial that analyzed the outcome of patients with CHF who underwent CABG with or without ventricular surgical reconstruction.
▪▪ Provided important information regarding the therapeutic strategy for ischemic non-high-risk patients.
▪▪ Large retrospective study on long-term outcomes on the basis of the revascularization strategy.
▪▪ First randomized trial that compared the long-term outcome of patients treated with percutaneous coronary intervention with drug-eluting stent versus CABG.
▪▪ European guidelines on CHF.
▪▪ American guideline update for CABG surgery.
▪ Websites
101 Information on gadolinium-containing contrast agents www.fda.gov/drugs/drugsafety/ postmarketdrugsafetyinformationforpatientsandproviders/ ucm142882.htm
102 Hunt SA, Abraham WT, Chin MH et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to update the 2001 guidelines for the evaluation and management of heart failure). American College of Cardiology Web Site 2005 www.acc.org/clinical/guidelines/failure// index.pdf.
▪▪ US guidelines on CHF.