Review Article - Imaging in Medicine (2012) Volume 4, Issue 1
Contrast-enhanced ultrasound assessment of focal liver lesions with Sonovue
Ioan Sporea* & Roxana SirliDepartment of Gastroenterology & Hepatology, University of Medicine & Pharmacy Timisoara, Romania
- Corresponding Author:
- Ioan Sporea
Department of Gastroenterology & Hepatology
University of Medicine & Pharmacy Timisoara, Romania
E-mail: isporea@umft.ro
Abstract
Focal liver lesions are frequently discovered in daily practice due to extensive use of imaging methods. Their nature must be elucidated for prognosis assessment and to decide upon therapeutic strategy. Contrast enhanced ultrasonography with perfluoro-containing contrast agents is an imaging technique developed in the last years, with similar performance to contrast computed tomography and contrast-MRI in focal liver lesions diagnosis, but less expensive and without the risk of ionizing radiation. It is a real-time method, thus allowing for rapid diagnosis, using low mechanical index ultrasound waves. The vascularity and enhancement pattern of the lesion (hypo-, hyper- or isoechoic) as compared with the adjacent liver parenchyma during the arterial, portal venous and late phases are evaluated for contrast enhanced ultrasonography characterization.
Keywords
contrast-enhanced ultrasound ▪ enhancement pattern ▪ focal liver lesions ▪ ultrasound contrast agents
Currently, worldwide, ultrasound (US) is the most used imaging method for the evaluation of the liver. Thousands of liver lesions are discovered by this method in healthy, cirrhotic or oncologic patients. Also, focal liver lesions (FLLs) are quite frequently discovered in daily practice, in relationship with the routine use of radiological methods CT or MRI. The characterization of these lesions is essential for the final diagnosis. On the other hand, the screening strategy in patients with liver cirrhosis leads to early discovery of FLLs, lesions that must be evaluated in order to decide upon a therapeutic strategy (which includes transplantation, resection surgery or percutaneous echo-guided procedures). All kinds of evaluation (including contrast imaging studies and/or liver biopsy) are time consuming, expensive and sometimes stressful for the patients. Thus, the main purpose when confronted with a new FLL is its accurate characterization in order to obtain a rapid, sensitive and not very expensive diagnosis.
In the recent years, contrast-enhanced US (CEUS) became a reliable new imaging method for the assessment of FLLs. Incidental lesions discovered using US must be evaluated by means of different radiological methods and, usually, there is a waiting time for a second-line contrast method of evaluation (preferably multidetector contrast CT or contrast-enhanced MRI). Both methods increase the medical costs for these patients (since both multidetector contrast CT and contrast-enhanced MRI in particular are expensive), in addition to which the use of CT exposes the subject to ionizing radiation. The use of CEUS for FLL assessment can be an advantage, owing to the reduced costs and because it can be done immediately after the abdominal US exam that discovered the FLL, and so, in approximately 5 min – the total time of the investigation – a confident diagnosis can be obtained.
CEUS: technique
CEUS was used for the first time in cardiology. In hepatology, US contrast agents (UCAs) from the first generation (Levovist®; Schering, Berlin, Germany) were used to improve the quality of Doppler signal through the destruction of microbubbles, using high mechanical index. Perfluoro-containing UCA (such as SonoVue® [Bracco, Italy], the approved UCA in Europe, Definity®, the approved UCA in USA and Sonazoid®, the approved UCA in Japan) work differently, using low mechanical index US that causes the microbubbles to reverberate (not to burst), and increase the vascular signal. For SonoVue, the microbubble life cycle is approximately 4–6 min, which is enough to obtain a confident diagnosis in hepatic or extrahepatic lesions.
From a technical point of view, CEUS evaluation is performed in grayscale, nonlinear imaging modes, using continuous real-time imaging techniques, following an intravenous bolus injection of SonoVue, a second generation UCA. SonoVue is provided as a sterile, lyophilized powder contained in a septum-sealed vial. A white, milky suspension of sulfur hexafluoride (SF6) microbubbles is obtained by adding 5 ml of physiological saline (0.9% sodium chloride) to the powder (25 mg), followed by hand agitation. Each patient receives an intravenous bolus injection of SonoVue for each FLL to be characterized (usually 2.4 ml) via a 20-gauge intravenous catheter placed preferably in the ante-cubital vein, and followed by a 10-ml saline flush. To characterize the FLL, the hemodynamic behavior of SonoVue enhancement during the arterial (15–30 s), portal-venous (30–120 s) and late vascular (120–300 s) phases is evaluated. All sonographic examinations are digitally recorded so that they can be reviewed at a later time. The location and size of the lesion is assessed on unenhanced and CEUS scans. In addition, the vascularity and SonoVue enhancement pattern of the lesion (hypo-, hyper- or iso-echoic), as compared with the adjacent liver parenchyma, during the arterial, portal venous and late phases are evaluated for the CEUS diagnosis. According to the enhancement pattern, the lesion can be classified following European Federation of Societies in Ultrasound in Medicine and Biology (EFSUMB) guidelines concerning the use of CEUS [1,2], thus obtaining a confident diagnosis.
The first EFSUMB guidelines concerning the use of CEUS were published in 2004 [1], followed by an update in 2008 [2]. The main indications and the specific CEUS (vascular) pattern of different FLLs are included. In August 2011, during the World Federation of Ultrasound in Medicine and Biology (WFUMB) Congress in Vienna (Austria), new guidelines for nonhepatic use of the CEUS were issued [3].
CEUS in the diagnosis algorithm of FLL
The place of CEUS in the diagnostic algorithm of FLLs is not very well established. The EFSUMB guidelines formulated indications regarding the use of this method [1,2], and several published papers demonstrated its practical value [4–8]. On the other hand, despite the effervescence of this method in Europe (and partially in Asia and Canada), there are some regions (such as the USA) in which perfluoro-containing UCA are not accepted yet.
The value of CEUS for FLL characterization was demonstrated in two multicenter studies, one German and the other one French, each study examined at least 1000 nodules.
The German study included 1349 patients in which the FLL discovered in standard US could not be characterized by standard US alone [4]. CEUS accuracy was compared with biopsy in more than 75% of the lesions, spiral contrast CT or contrast MRI in the rest of the cases. The study included 573 benign lesions (242 hemangiomas, 170 cases with focal nodular hyperplasia [FNH], 19 adenomas and 142 other types of lesions), and 755 malignant tumors (383 metastases, 279 cases with hepatocellular carcinoma [HCC], 93 other tumors). From the lesions that were evaluated, 62.3% were incidentally discovered; 17.3% of the lesions were discovered during the follow-up of patients with known liver cirrhosis and in 27.0% of cases an extrahepatic tumor was known.
In the German study, CEUS had 90.3% accuracy for the diagnosis of FLL [4]. CEUS correctly characterized 723 out of 755 of the malignant lesions and 476 out of 573 of the benign lesions, with high sensitivity, specificity, positive predictive value and negative predictive value (95.8, 83.1, 95.4 and 95.9%, respectively) for differentiating benign versus malignant lesions. Thus, CEUS proved to be a sensitive method for the diagnosis of liver metastases and HCCs, but less sensitive for the diagnosis of adenoma.
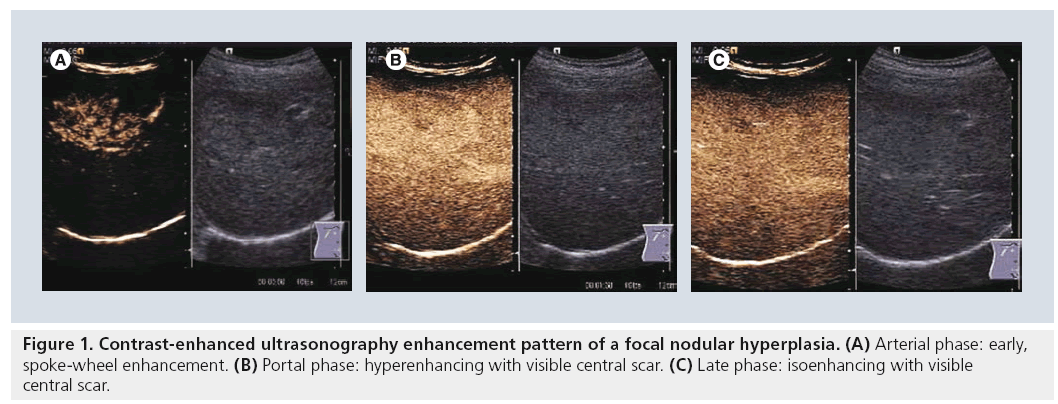
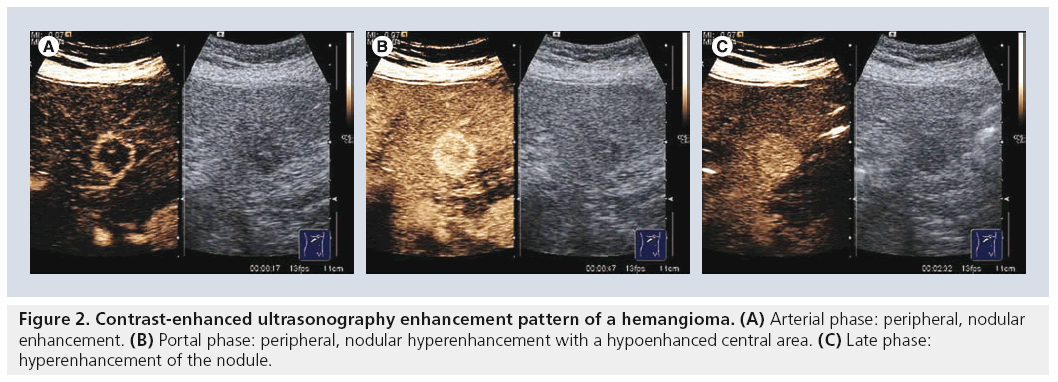
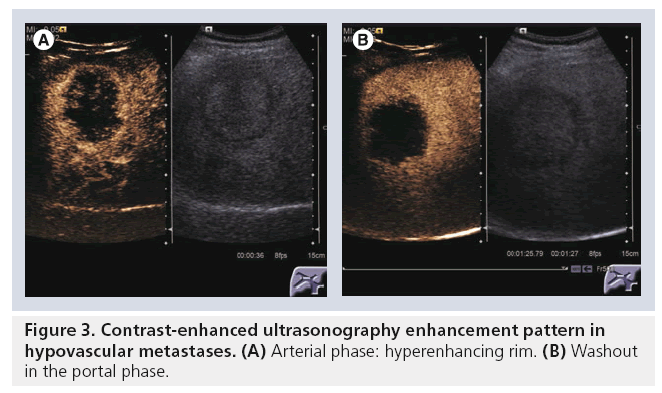
Another important aspect assessed in a study based on the DEGUM multicenter study was the tumor-specific vascularization pattern [9]. Typical patterns are: wheel-spoke pattern and arterial hyperenhancement followed by isoenhancement in the late phases in FNH (Figure 1); nodular peripheral enhancement and partial or complete fill-in pattern in hemangiomas (Figure 2); and late-phase hypoenhancement in metastases (Figure 3). In this study the tumor-specific vascularization pattern could not be assessed in all cases, therefore the diagnostic accuracy of CEUS was 83.1% for all benign lesions and 95.8% for all malignant lesions (91.4% for metastases and 84.9% for HCCs).
The multicenter French study (STIC) included 1034 FLLs found in 874 patients [5]. The gold standards to which CEUS was compared were contrast spiral CT, contrast MRI or liver biopsy. By using CEUS the diagnostic accuracy improved from 62.4% (standard US) to 86.1%. The diagnostic concordance between CEUS and the gold standard method was 73.5% (k = 0.66) in FLLs on noncirrhotic liver, which was better than in nodules on cirrhotic liver (71.8%, k = 0.42), overall 73% (k = 0.67).
CEUS had 79% sensitivity and 88% specificity for differentiating between benign versus malignant lesions. For the diagnosis of the most frequent FLLs (hemangioma, FNH, metastases and HCC), the sensitivities were 85.4, 82.5, 79.3
CEUS versus contrast CT & contrast MRI
In a study on a subgroup of patients from the DEGUM multicenter study, CEUS was compared with standardized spiral CT (SCT) [6]. From the 267 patients, histological findings were available in 158 subjects. In this subgroup, tumor differentiation assessment with CEUS and SCT was discordant in 30 cases and concordant in 124 cases (CEUS/SCT: accuracy 90.3 vs 87.8%, sensitivity 94.0 vs 90.7%, specificity 83.0 vs 81.5%, positive predictive value 91.6 vs 91.5%, negative predictive value 87.5 vs 80.0%). Tumor specification was different in 51 cases and matched in 103 cases (CEUS/SCT: accuracy 91.6 vs 87.7%, sensitivity 95.3 vs 90.6%, specificity 83.7 vs 81.6%, positive predictive value 92.7 vs 91.4%, negative predictive value 89.1 vs 80.0%). A statistically significant difference could not be established. CEUS performed a little better than SCT with regard to tumor differentiation in the case of hemangioma, FNH, HCC and metastases, but it was not determined to be statistically significant.
CEUS was compared with contrast MRI in a study on a subgroup of patients from the DEGUM multicenter study [10]. The definitive diagnosis of the 262 patients included was based on MRI as the diagnostic gold standard in typical liver hemangioma and FNH, on clinical evidence and additional follow-up in 180 patients, or on histology in 82 patients. The subgroup verified by means of histology comprised mainly malignant liver lesions (n = 55) with eight hemangiomas and five FNHs. Tumor differentiation was concordant in 56 cases (68.3%) and tumor entity in 44 cases (53.7%). There were no statistically proven differences between CEUS and MRI.
From this cohort of patients we can see that the evaluation with CEUS for some types of lesions, such as hemangiomas and FNHs, metastasis is very accurate. Published data showed very good accuracy of CEUS for the diagnosis of hemangioma and FNH: for atypical hemangioma the accuracy was 93 versus 43% in standard US [11]. In another cohort, the sensitivities and specificities of CEUS for the diagnosis of FNH and hemangioma were also very high: 100% and 87%, respectively, resulting in an accuracy of 94.5% [12].
For other types of lesions, such as HCC, cholangiocarcinoma or adenoma, the accuracy of CEUS is not so high. In patients with liver cirrhosis or advanced fibrosis, the correct CEUS diagnosis in some HCCs (usually small or undifferentiated ones), as well as in cases with cholangiocarcinoma, the positive diagnosis can be difficult, published data showing that the accuracy of CEUS in cholangiocarcinoma is only 57% [13].
Some US machines are able to perform realtime elastography (either acoustic radiation force impulse elastography, or freehand realtime elastography), thus being able to assess the severity of liver fibrosis. This could be useful for the differential diagnosis, because solid liver lesions in cirrhosis have a high probability for being HCCs and acoustic radiation force impulse elastography and real-time elastography have proven to be good noninvasive methods for predicting the presence of severe fibrosis and cirrhosis [14–16].
A limitation of CEUS, is that FLLs which are not clearly visible in standard US are not candidates to be examined with this method. We must emphasize that CEUS is used for the characterization of liver lesions in cirrhotic patients and not for the detection of such lesions.
Accuracy of CEUS for the diagnosis of HCC
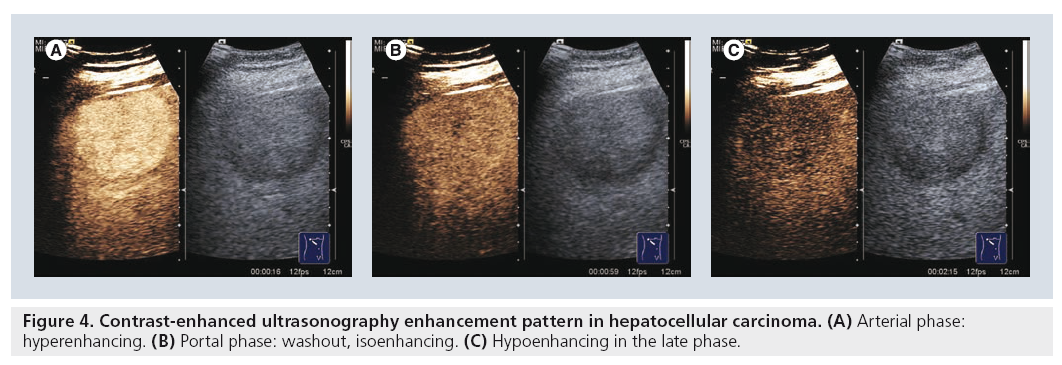
HCC shows a typical behavior in CEUS, characterized by early arterial enhancement upon administration of the UCA followed by ‘washout’ in portal and/or late phases (Figure 4). This typical behavior is due to the fact that the blood supply in malignant nodules is mainly arterial, as compared with the normal liver parenchyma that thrives on dual blood supply (i.e., the portal vein and the hepatic artery). The sensitivity of CEUS is equal to that of CT and MRI in finding the early arterial enhancement and is correlated with tumor differentiation [17]. The early arterial enhancement is observed by means of CEUS in 91–96% of the HCCs. In the study performed by Forner et al., in which the gold standard for the evaluation of nodules smaller than 2 cm was the histopathologic examination, the sensitivity of CEUS for suspected HCC was 78.3%, with 93.1% specificity [17].
Unlike the early arterial enhancement that is present in the majority of HCCs, the washout in the portal phase occurs less frequently, in approximately 43% of cases (up to 90 s after SonoVue bolus). In later stages of the examination (90–180 s after bolus), 26% of the HCCs displayed wash-out, while 22% of carcinomas presented only a delayed wash-out, between 181 and 300 s [18].
CEUS sensitivity for the diagnosis of HCC is related to the tumor size. In small nodules (2 cm or less), Giorgio et al. [19] and Gaiani et al. [20] observed 53.6 and 83.3% sensitivities, respectively, while in larger nodules (>2 cm), they were 91.3 and 94.5%, respectively. Another published study, showed that in well-differentiated (G1) HCCs early arterial enhancement is more frequent in tumors over 3 cm in diameter as compared with tumors smaller than 3 cm, but this feature was not observed in moderate or less differentiated tumors [21]. Thus, early arterial enhancement was found in 95% of HCCs larger than 3 cm in diameter and only in 43% of well-differentiated HCCs less than 3 cm in diameter (p < 0.001).
CEUS for the diagnosis of other types of FLL
Many studies published in recent years have attempted to assess the real value of CEUS for characterization of different FLLs.
In a published multinational study that included 134 patients with one FLL detected in baseline US, second-line imaging methods included CEUS in 134 patients, contrastenhanced CT in 115 patients and/or dynamic contrast-enhanced MRI in 70 patients [12]. The lesions were firstly classified as malignant, benign or indeterminate after which the type of lesion was assessed. The final diagnosis was based on clinical information, combined information from all imaging examinations and histology (n = 32). Comparisons were made to see if the use of CEUS led to the improvement of characterization in doubtful FLL. In comparison with unenhanced US, CEUS markedly improved the sensitivity and specificity for the malignant/benign characterization of FLL. In comparison with CT and/or dynamic MRI, CEUS applied for FLL characterization was 30.2% more sensitive in the recognition of malignancy and 16.1% more specific in the exclusion of malignancy and overall 22.9% more accurate. In the subgroup with confirmative histology available (n = 32), the sensitivities were 95.5 (CEUS), 72.2 (CT) and 81.8% (MRI), and the specificities were 75.0 (CEUS), 37.5 (CT) and 42.9% (MRI). The sensitivities and specificities of CEUS for the identification of FNH and hemangioma were 100 and 87%, resulting in an accuracy of 94.5%. The conclusion of this recent study is that CEUS emerges as the most sensitive, most specific and most accurate imaging modality for the characterization of FLL.
In a study performed in 11 centers from China on 250 patients with 306 FLLs a subgroup of 148 patients with 164 lesions were evaluated [11]. The final diagnosis in malignant lesions was based on the gold standard (liver biopsy) in 129 of 164 of cases. With unenhanced US, 43% of the lesions were characterized: 12 of 48 (25%) as benign and 56 of 116 (48%) as malignant. After CEUS, 94% of the lesions were characterized; 36 of 48 (75%) were correctly characterized as benign and 108 of 116 (93%) as malignant. As compared with the gold standard, CEUS accuracy (88%) was markedly higher than that of standard US (41%; p < 0.01), also with higher specificity and sensitivity (p < 0.01). The number of undetermined lesions with CEUS was markedly lower than in baseline US (6 vs 57%).
For hemangiomas, the concordance with the gold standard increased after CEUS from 43% (standard US) to 93%, for malignant lesions from 48 to 95%, while for metastases increased from 50 to 91%.
Quaia et al. reported in a large study (452 undetermined lesions by standard US) that the diagnostic accuracy for FLL characterization increased from 49% at baseline US examination to 85% after CEUS [22]. Also, following contrast enhancement, the sensitivity and specificity increased from 53 and 41% to 83 and 95%, respectively.
In another study on 126 lesions CEUS improved, the sensitivity of standard US from 78 to 100% and the specificity from 23 to 92% [23].
Very recently, in a large cohort of patients, we evaluated the value of CEUS in daily practice [24]. We performed a multicenter retrospective study, including 1244 FLLs, evaluated by means of CEUS in four Romanian centers with extensive experience in US, during a 15-month period. This study included 1244 FLLs, both de novo (1056 cases) and pre-existing (such as HCCs evaluated after percutaneous treatment by means of PEIT and RFA, to assess the treatment results). In 1046 of 1244 of cases (84.1%) CEUS showed a typical enhancement pattern (according to the EFSUMB Guidelines 2008), thus being sufficient for a correct and final diagnosis, while in 198 of 1244 of cases (15.9%) other methods of diagnosis were required, such as contrast CT/MRI or biopsy. In our study, CEUS established the benign or malignant nature of lesions in 1139 out of 1244 cases (91.5%). The conclusion of this study was that CEUS can be used as the first-line imaging method for the diagnosis of uncharacteristic FLLs detected by standard US, providing a correct classification in 84.1% of cases and a correct differentiation between benign/malignant lesions in 91.5% of cases.
All of these studies demonstrated that CEUS is better than standard US for the characterization of FLLs, with better sensitivity and specificity. However, unfortunately, CEUS has some limitations: similar to standard US the acoustic window for the liver must adequate; also, the FLLs must be well seen in standard US for CEUS to be possible. Additionally, if several lesions are present in the liver, repeated injections of contrast agent are needed for their characterization in every vascular phase (especially in cirrhotic patients).
When confronted by a new lesion discovered in standard US, we must decide the next step for the final diagnosis by considering several aspects: accessibility, risk of irradiation, center expertise and last but not least, the cost of the proposed method (cost–efficiency).
CEUS: cost–efficiency
Regarding the financial analysis of the use of CEUS as the first step for the evaluation of a new FLL discovered by US, there are published data demonstrating that CEUS is also a cost-effective method. In the French multicenter study, the diagnosis costs of 149 nodules were evaluated [25]. By using CEUS versus liver biopsy as the gold standard, and considering that the mean CEUS cost is €155.20, of multislice contrast CT is €191.65, of contrast MRI is €322.3, the total savings were €128.5/nodule. In an Italian multicenter study that included 575 lesions (485 patients), the costs of a classic patient work-up (baseline US followed by contrast CT or MRI) were compared with a new scheme in which CEUS examination was performed following the baseline US [26]. The total costs with CEUS were €55,674 as compared with €134,576 in the classic workup. Thus, the total savings were €78,902, or €162/patient. A study published by Giesel who compared CEUS to multiphase CT as the diagnostic standard for diagnosing incidentally found FLLs, concluded that CEUS was the more cost-effective method provided that CEUS examinations were performed at specialized centers (€122.18–186.53) as compared with multiphase CT (€223.19) [27].
In a study performed in our center, in which the costs of CEUS as a first-line imaging method used for the diagnosis of 316 FLLs were compared with those of contrast CT or contrast MRI as first-line methods, the total savings were approximately €4000 as compared with contrast CT and approximately €24,900 as compared with contrast MRI for the 316 FLLs included [28].
CEUS: safety profile
Regarding the safety profile of SonoVue, a study published in 2006 that retrospectively analyzed 23,188 abdominal CEUS studies reported 29 adverse events, of which only two were graded as serious. The overall reporting rate of serious adverse events was 0.0086% with no fatal events [29]. A Dutch study published in 2009, that reviewed 352 consecutive cardiac SonoVue studies, revealed that 2.0% of the patients experienced adverse events, mild allergic reactions in 1.1% of the cases and severe allergic reaction resulting in nonfatal shock in 0.9% of the cases, a much higher incidence than that reported in the postmarketing surveillance studies, in which 19 nonfatal severe (0.01%) and three fatal (0.002%) complications were reported after the use of SonoVue in 157,838 patients [30]. On the other hand, we must emphasize that CT contrast is an iodine substance that can produce allergic reactions and cannot be used in patients with renal failure (SonoVue can be used without any restrictions in this type of patients because the microbubble shell is metabolized by the liver while the gas released is exhaled).
Conclusion
CEUS is an accurate imaging method for FLL characterization. It is also a safe method (with rare allergic side effects, no ionizing radiation) well tolerated by the patient. As compared with contrast CT and MRI CEUS is less expensive and, sometimes, available at the time of the initial detection of FLLs.
In published studies, CEUS was conclusive in approximately 85–90% of FLLs (irrespective of cirrhotic or normal liver), and was able to differentiate between benign or malignant lesions in approximately 90–95% of the cases. Therefore, CEUS can be the first-line investigation in such cases (thus avoiding other expensive examinations). For nonconclusive CEUS evaluations further imaging or morphological evaluation are needed for the final diagnosis. This type of strategy is adopted by many clinical centers from Europe and Japan, decreasing the evaluation costs of patients with FLLs.
Future perspective
Considering the high performance of CEUS for FLL assessment, comparable to that of contrast CT and MRI, it’s relatively low- ost and lack of serious adverse effects, in the next 10–15 years this method could become the first-line imaging method used for the evaluation of FLL. Only if CEUS assessment is inconclusive should secondline contrast imaging methods, such as multidetector CT or MRI, and/or liver biopsy be used.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Albrecht T, Blomley M, Bolondi L et al.; EFSUMB study group. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 25(4), 249–256 (2004).
- Claudon M, Cosgrove D, Albrecht T et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS). Update 2008. Ultraschall Med. 29, 28–44 (2008).
- Piscaglia F, Nolsoe C, Dietrich CF et al. The EFSUMB guidelines and recommendations of the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 32, 1–27 (2011).
- Strobel D, Seitz K, Blank W, Schuler A, Dietrich C, von Herbay A. Contrast-enhanced ultrasound for the characterization of focal liver lesionsdiagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med. 29(5), 499–505 (2008).
- Tranquart F, Le Gouge A, Correas JM et al. Role of contrast-enhanced ultrasound in the blinded assessment of focal lesions in comparison with MDCT and CEMRI: results from a multicentre clinical trial. EJC 6(Suppl.), S9–S15 (2008).
- Seitz K, Strobel D, Bernatik T et al. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions – prospective comparison in clinical practice: CEUS vs CT (DEGUM multicenter trial). Ultraschall Med. 30(4), 383–389 (2009).
- Braun B. Focal liver processes: ‘better is the enemy of good’: CEUS in the fast lane. Ultraschall Med. 30(4), 329–332 (2009).
- Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? Am. J. Roentgenol. 193(1), 55–60 (2009).
- Strobel D, Seitz K, Blank W et al. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med. 30(4), 376–378 (2009).
- Seitz K, Bernatik T, Strobel D et al. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions in clinical practice (DEGUM multicenter trial): CEUS vs MRI – a prospective comparison in 269 patients. Ultraschall Med. 31(5), 492–499 (2010).
- Wang WP, Wu Y, Luo Y et al. Clinical value of contrast-enhanced ultrasonography in the characterization of focal liver lesions: a prospective multicenter trial. Hepatobiliary Pancreat. Dis. Int. 4, 370–376 (2009).
- Trillaud H, Bruel JM, Valette PJ et al. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J. Gastroenterol. 15(30), 3748–3756 (2009).
- Xu HX, Liu GJ, Lu MD et al. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J. Clin. Ultrasound 34, 261–272 (2006).
- Sporea I, Sirli R, Deleanu A et al. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 32(Suppl. 1), S46–S52 (2011).
- Sporea I, Badea R, Sirli R et al. Usefulness of real-time-elastography (Siemens) for the evaluation of liver stiffness. Gut 58(Suppl. 2), A503 (2009).
- Friedrich-Rust M, Wunder K, Kriener S et al. Liver fibrosis in viral hepatitis: non-invasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 252(2), 595–604 (2009).
- Forner A, Vilan R, Ayuso C et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 47, 97–104 (2008).
- Jang HJ, Kim TK, Burns PN, Wilson DR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histological differentiation. Eur. J. Radiol. 72(3), 418–424 (2009).
- Giorgio A, Ferraioli G, Tarantino L et al. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced HELICAL CT appearance. Am. J. Roentgenol. 183(5), 1319–1326 (2004).
- Gaiani S, Celli N, Piscaglia F et al. Usefulness of contrast-enhanced perfusion sonography in assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. J. Hepatol. 41, 421–426 (2004).
- von Herbay A, Vogt C, Westendorff J, Häussinger D, Gregor M. Correlation between SonoVue enhancement in CEUS, HCC differentiation and HCC diameter: analysis of 130 patients with hepatocellular carcinoma (HCC). Ultraschall Med. 30(6), 544–550 (2009).
- Quaia E, Calliada F, Bertolotto et al. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology 232, 420–430 (2004).
- von Herbay A, Vogt C, Willers R, Häussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J. Ultrasound Med. 23, 1557–1568 (2004).
- Sporea I, Badea R, Socaciu M et al. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions: a multicentric retrospective study. Ultrasound Med. Biol. 37(Suppl. 8), S19–S20 (2011).
- Tranquart F, Correas JM, Ladam Marcus V et al. [Echographie de contraste temps reel dans la prise en charge diagnostique des lesions nodulaires hepatiques: evaluation des performances diagnostiques et de l’impact economique sur une etude multicentrique francaise]. J. Radiol. 90, 109–122 (2009).
- Romanini L, Passamonti M, Aiani L et al. Economic assessment of contrast-enhanced ultrasonography for evaluation of focal liver lesions: a multicentre Italian experience. Eur. Radiol. 17(Suppl. 6), F99–F106 (2007).
- Giesel FL, Delorme S, Sibbel R, Kauczor HU, Krix M. Contrast-enhanced ultrasound for the characterization of incidental liver lesions – an economical evaluation in comparison with multi-phase computed tomography. Ultraschall Med. 30(3), 259–268 (2009).
- Sirli R, Sporea I, Martie A, Popescu A, Danila M. Contrast enhanced ultrasound in focal liver lesions – a cost–efficiency study. Med. Ultrason. 12(4), 280–285 (2010).
- Sirli R, Sporea I, Martie A, Popescu A, Danila M. Contrast enhanced ultrasound in focal liver lesions – a cost–efficiency study. Med. Ultrason. 12(4), 280–285 (2010).
- Piscaglia F, Bolondi L. Italian society for ultrasound in medicine and biology (SIUMB) study group on ultrasound contrast agents. The safety of SonoVue in abdominal applications: retrospective analysis of 23,188 investigations. Ultrasound Med. Biol. 32(9), 1369–1375 (2006).
- Geleijnse ML, Nemes A, Vletter WB et al. Adverse reactions after the use of sulphur hexafluoride (SonoVue) echo contrast agent. J. Cardiovasc. Med. 10, 75–77 (2009).
• First European guidelines for the use of contrast-enhanced ultrasonography (CEUS) in clinical practice.
• • Current European guidelines for the use of CEUS in clinical practice, including CEUS assessment of focal liver lesions.
• Current European guidelines for the use of CEUS in clinical practice regarding nonhepatic use of CEUS.
• • German DEGUM multicenter trial regarding CEUS for focal liver lesion assessment.
• • French multicenter trial regarding CEUS for focal liver lesion assessment.