Contrast Agent Evaluation - Imaging in Medicine (2012) Volume 4, Issue 4
[123I]FP-CIT SPECT in atypical degenerative parkinsonism
Ioannis U Isaias1,2,3*, Giorgio Marotta4, Gianni Pezzoli2, Osama Sabri5 and Swen Hesse5,6
1Università degli Studi di Milano, Dipartimento di Fisiologia Umana, Milano, Italy
2Centro per la Malattia di Parkinson e i Disturbi del Movimento, Istituti Clinici di Perfezionamento, Milano I-2016, Italy
3Universitätsklinik Würzburg, Neurologische Klinik und Poliklinik, Würzburg, Germany
4Dipartimento di Medicina Nucleare, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy
5Department of Nuclear Medicine, University of Leipzig, Germany
6IFB Adiposity Diseases, Leipzig University Medical Centre, Leipzig, Germany
Abstract
One of the most widely used techniques to support the clinical diagnosis of Parkinson’s disease is the SPECT scan with [123I]FP-CIT. This tracer binds reversibly and visualizes the striatal presynaptic dopamine transporters. Several uncertainties remain on the value of [123I]FP-CIT and SPECT in atypical degenerative parkinsonian syndromes. In this concise review, we discuss the contribution of SPECT and [123I]FP-CIT in supporting the clinical diagnosis of Parkinson’s disease and their role in the differential diagnosis of Parkinson’s disease and atypical degenerative parkinsonism. The chemistry, pharmacodynamics and pharmacokinetics of [123I]FP-CIT are also discussed.
Keywords
atypical degenerative parkinsonism; FP-CIT; ioflupane; SPECT
Parkinson’s disease (PD) is the second most common neurodegenerative disorder [1], yet early accurate diagnosis remains challenging. The estimated prevalence of PD is 0.5–1% in those aged 65–69 years and 1–3% in those aged ≥80 years [1]. Although the clinical diagnosis of PD may be straightforward in cases with a classic presentation [2], accurate distinction between PD and atypical degenerative parkinsonism (ADP) may be difficult, particularly in the early or mild stages of disease [3]. In autopsy series, ADP (multiple system atrophy [MSA], progressive supranuclear palsy [PSP] and corticobasal syndrome [CBS]) accounted for half of PD misdiagnoses at specialized centers [4,5], while in the community Alzheimer’s disease and vascular parkinsonism were most common [6,7]. Assessment of the clinical features suggests that an accuracy of 90% for PD may be the highest that can be expected using the currently available clinical diagnostic criteria. Accurate diagnosis of patients with ADP is important to predict the disease course and avoid unnecessary medical examinations and therapies and their associated side effects, safety risks and financial costs. Correct diagnosis is also critically important when patients are being recruited into clinical trials.
Post-mortem studies demonstrate severe reductions in dopamine concentration in the striatum of patients with PD, with greater reductions in the putamen [8,9]. SPECT with [123I]FP-CIT specifically identifies presynaptic dopaminergic deficits within the striatum [10–13]. Accordingly, an abnormal dopamine transporter SPECT image should be regarded as exclusion criteria for essential tremor [14], dystonic tremor [15] and psychogenic parkinsonism [16,17]. In this concise review, we will discuss the role of SPECT and [123I]FP-CIT in supporting the clinical diagnosis of PD and its differential diagnosis with ADP.
[123I]FP-CIT SPECT scans in atypical degenerative parkinsonism
Clinically, MSA is a sporadic, progressive neurodegenerative disease characterized by varying severity of parkinsonian features, cerebellar ataxia, autonomic failure and corticospinal disorders [18].
PSP presents with early onset postural instability associated with supranuclear vertical gaze impairment, symmetrical akinetic-rigid syndrome together with prominent bulbar dysfunction, dementia and axial rigidity [19,20].
CBS is characterized by asymmetric akineticrigid parkinsonism and limb dystonia, variably associated with cortical signs [21].
The parkinsonian types of MSA (MSA-P) and PSP (PSP-P) can be very difficult to distinguish from PD before disease-specific signs and symptoms occur. This also applies to CBS because of its marked asymmetrical akinetic-rigid syndrome before apraxia, myoclonus and cognitive problems become evident. Correct differentiation is important as PD has a better prognosis than ADP syndromes and responds better to a symptomatic treatment [22].
In PD the decrease in [123I]FP-CIT binding usually occurs in the dorsal putamen contralateral to the side of the neurological symptoms, in time progressing anteriorly and ipsilaterally (Figure 1) [12,14,23].
Several studies investigated PD and ADP by means of [123I]FP-CIT and SPECT scans. However, only few specifically established the value of [123I]FP-CIT in setting a differential diagnosis (Table 1).
The amount and pattern of reduced striatal DAT binding in MSA have been demonstrated to be within the range of PD. However, asymmetry of DAT binding loss tends to be more pronounced in PD and the progression of dopaminergic innervation loss is faster in MSA compared with PD [24,25]. In a recent study that compares the accuracy of dual-tracer DAT and perfusion SPECT imaging in the differential diagnosis of parkinsonism using template-based discriminant analysis [26], no reduction of MSA versus PD was noted. In contrast to what was reported by Scherfler and colleagues, demonstrating differential [123I]FP-CIT binding capacity between PD and MSA [27], the aforementioned study could not detect differences even at the least stringent threshold (p = 0.05 uncorrected). The reverse contrast (PD < MSA) demonstrated a decreased binding in the left posterior putamen. However, El Fakhry and colleagues reported lower striatal binding values in PD (55%) and MSA (23%) compared with normal controls (p < 0.01) and lower values in PD compared with MSA (p < 0.05) [28]. Asymmetry index was greater for PD than for MSA and controls in both the caudate nucleus and the putamen (p < 0.05). In addition, there was a significantly decreased perfusion in the left and right nucleus lentiformis in MSA compared with PD and controls (p < 0.05).
A reduction of [123I]b-CIT uptake in the midbrain appears to separate patients with clinically fully developed MSA-P or PSP from patients with PD [23,27,29]. Indeed, a reduced midbrain [123I]b-CIT uptake was found in patients with MSA-P and allowed a correct classification of 95% of MSA-P and PD patients [23,30]. Finally, clinically pure forms of MSA-C (cerebellar variant) may show a DAT binding loss but less compared with MSA-P or PD [31].
Antonini and colleagues reported a greater DAT reduction in patients with PSP (0.51 ± 0.39; p < 0.01) compared with MSA-P patients (0.70 ± 0.33) or PD patients (0.95 ± 0.38) [32]. No difference was found between patients with MSA and PD. Putamen/caudate ratios were greater in PSP (0.83 ± 0.12; p < 0.01) than in PD patients (0.51 ± 0.11), suggesting a moreuniform involvement of dopamine nerve terminals in both caudate nucleus and putamen. Van Laere and colleagues also demonstrated a greater involvement of the caudate heads in PSP patients, although when directly contrasted with PD patients, a difference was found in the left striatum only at a p = 0.05 uncorrected level [26].
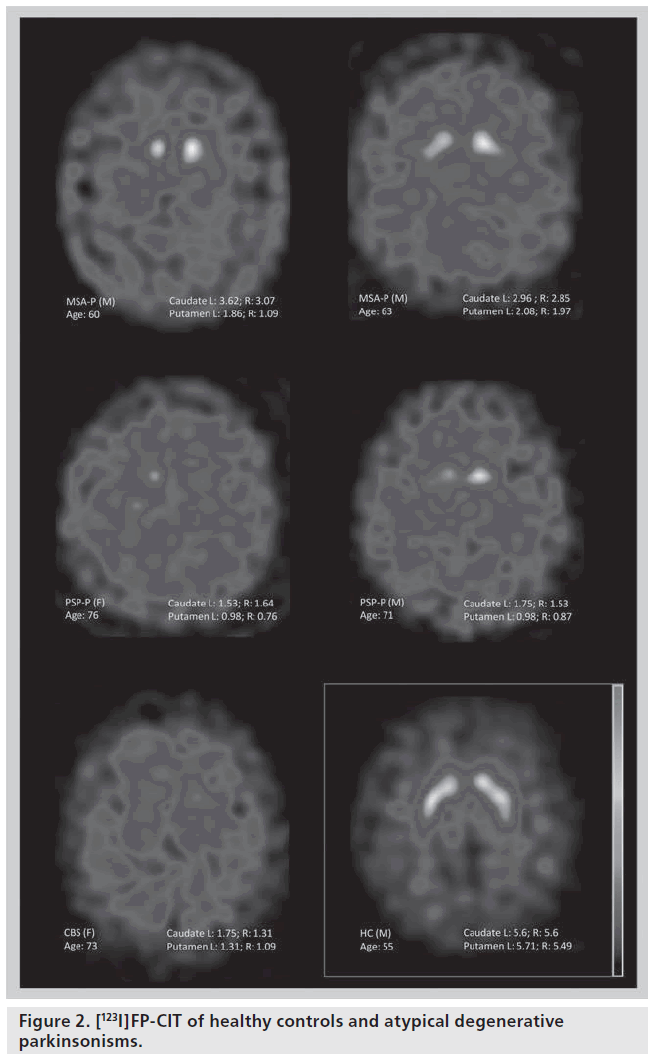
DAT loss in CBS is in the same range as that in PD patients [33–35]; but more asymmetrical and less pronounced than in MSA and PSP patients [34]. In CBS, unlike PSP or PD, unilateral balanced (caudate/putamen) reduction in tracer uptake has been observed [33]. DAT binding may result occasionally within the normal range in patients with CBS [35–37]. The low sensitivity and specificity of [123I]FP-CIT for the diagnosis of CBS also relies on the pathological and clinical heterogeneity of this syndrome (Figure 2) [37,38].
Overall, a meta-analysis of diagnostic accuracy on SPECT in parkinsonian syndromes [39] revealed, for presynaptic tracers in general, a moderate to high sensitivity but a low specificity in differentiating PD from MSA and PSP (pooled odds ratio with 95% CI was 2). Similarly, presynaptic tracers showed a very high sensitivity (78–100%) but a low specificity (0–33%) in discriminating between MSA and PSP (pooled odds ratio with 95% CI was 2).
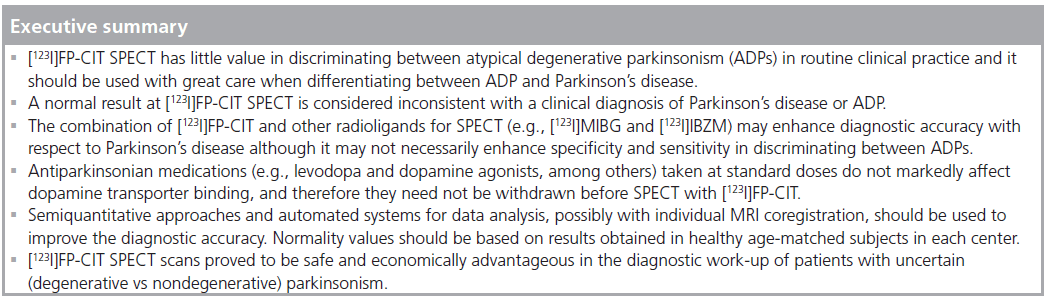
In conclusion, [123I]FP-CIT and SPECT have little value in discriminating between ADPs in routine clinical practice and they should be used with great care when differentiating ADP from PD, as only hints towards a more accurate clinical diagnosis can be obtained. On the other hand, a normal result is considered inconsistent with a clinical diagnosis of PD or ADP [39,40].
Postsynaptic dopamine receptor imaging, in addition to presynaptic dopamine transporter imaging, may be necessary, together with clinical reassessment and follow-up imaging, to improve diagnostic accuracy in PD and ADP [41]. The most widely applied radiotracer for imaging D2 receptors with SPECT is [123I]iodobenzamide ([123I]IBZM) [42,43]. Postsynaptic receptor density is normal or upregulated in early PD but invariably decreased in ADP [44–47]. Along with PD progression, binding values of PD patients are still in the range of control subjects, possibly due to a decline in the presynaptic dopaminergic drive, which results in dopamine receptor upregulation [48–51]. The preserved D2/D3 receptor availability is a prerequisite for the response to l-Dopa therapy [52]. The combination of pre- ([123I]FP-CIT) and post-synaptic ([123I]IBZM) dopamine SPECT can serve as an indicator for excluding ADP with a reasonably high accuracy of 85%, especially in early diagnosed drug-naive PD patients [23,53]. Compared with [123I]FP-CIT [30], [123I]IBZM has a distinctly lower ability to detect alterations of the dopaminergic system, and therefore should only be used as an additional examination to further corroborate a potential sufferer of ADP. [123I] IBZM binding is variably decreased in ADPs and cannot discriminate between them [40]. As a cautious suggestion, [123I]IBZM SPECT may more often result in a normal range in CBS than in MSA or PSP [34]. The diagnostic performance might be substantially improved with the use of a D2/D3 receptor radioligand and PET [54].
Metabolic and perfusion studies using PET or SPECT have also shown some value in the differential diagnosis of ADP. In particular, disease-related spatial covariance patterns identified a marked bilateral reduction in the lentiform nuclei and the cerebellum in MSA. By contrast, PSP is characterized by the presence of metabolic decrements in midline frontal regions and in the brainstem. The distinguishing feature of the pattern of glucose metabolism in CBS is the asymmetrical distribution of radiotracer uptake with a relative metabolic reduction in many cortical areas, the insula and in the basal ganglia contralateral to the most affected side [55,56].
However, the overall diagnostic accuracy of metabolic and perfusion studies seems rather poor in a single patient. When comparing [123I] IBZM SPECT and [18F]FDG PET in neurodegenerative parkinsonism, it is clear that interrater agreement of visual analysis is substantial in both methods [57]. However, findings of either are discordant in a significant number of cases.
It is an open issue which nuclear medicine examination best relates to clinical course. Clinical tests and follow-up examinations, as well as morphologic information (e.g., MRI), are still necessary as additional diagnostic tools to discriminate within ADP [46,58,59].
As an alternative, cardiac imaging with ([123I] MIBG) has demonstrated changes consistent with heart denervation in patients with PD that are not present in patients with MSA or PSP [60]. However, the use of this tracer is limited because of insufficient sensitivity in patients with short disease duration [61]. Furthermore, by applying a PET radioligand, cardiac sympathetic denervation was found to occur not only in PD but also in other movement disorders, such as MSA and PSP [62]. This finding implies that scintigraphic detection of cardiac sympathetic denervation cannot be used independently to discriminate idiopathic PD from other movement disorders, such as MSA and PSP. Furthermore, cardiac sympathetic denervation was not correlated with striatal denervation (measured with a PET-compound for vesicular monoamine transporter, which is supposed to be of at least similar diagnostic values as the DAT radiotracers). This suggests that the pathophysiologic processes underlying cardiac denervation and striatal denervation occur independently in patients with parkinsonian syndromes [62].
The combined use of [123I]MIBG and [123I]FP-CIT or [123I]IBZM signif icantly improved diagnostic accuracy in PD versus ADP reaching a sensitivity of 94%, specificity of 94%, positive predictive value of 89% and negative predictive value of 97%. However, even the combination of these tracers was not able to discriminate between PSP and MSA with more success than clinical follow-up at 2 years [63].
Although disease-related differences in the pattern of nigrostriatal degeneration of PD and ADP are present [64], DAT imaging does not significantly improve diagnostic accuracy in all cases and it is of little help in the differential diagnosis between ADP [25]. The best imaging approach to clarify whether the cause of presynaptic dopaminergic loss is PD or ADP is a combined radiotracer or multimodal approach including dopamine D2 receptor imaging [30,65], MRI techniques [66,67] and eventually cardiac imaging of the sympathetic nervous system [62].
Finally, in the absence of histopathological material nearly all imaging studies have used clinical diagnoses as the gold standards so far, which may not always be accurate. Given the high rate of clinical misdiagnosis [4,5] this should be regarded as the main bias of all such studies.
Introduction to the compound
Chemistry
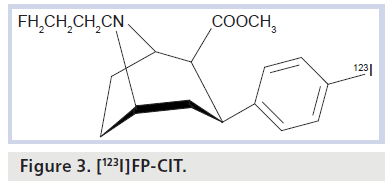
[123I]FP-CIT (ioflupane) is commercially available as DaTSCAN in many European countries (e.g., in Germany since 2000) and also in the USA since 2011. It is delivered as a pyrogen-free radiopharmaceutical for intravenous injection in single-use vials. It is a cocaine analog substance and tropane derivative (Figure 3).
Iodine-123 is a cyclotron-produced, g-emitting radionuclide with a main energy level of 159 keV and a physical half-life of 13.2 h. The active drug compound is N-w-fluoropropyl- 2b-carbomethoxy-3b-(4-[123I]iodophenyl)nortropane. In vitro, ioflupane binds reversibly to the human recombinant DAT with IC50 ranging from 0.71 to 1.67 nM as examined using rat striatal tissue homogenates by displacement of the radiotracer [3H]-WIN 35,428 [68–70]. Potency of ioflupane was much greater than for cocaine (IC50: 89.1 nM) and similar to b-CIT (IC50: 1.24 nM). High binding affinity of ioflupane in human striatum has been shown autoradiographically using a C-11 labeled compound [71]. Binding was low in the cortex and other brain regions, and in the thalamus it was 10% of binding in the putamen. Approximately 80–90% of the striatal binding was blocked by the DAT inhibitor GBR 12,909; the inhibition constant was Ki = 0.62 nM. High specificity to the presynaptic DAT was demonstrated by competition studies with GBR 12,909, the serotonin reuptake inhibitor citalopram and the norepinephrine reuptake inhibitor desipramine in post-mortem human brain slices exposed to radiolabeled ioflupane. Autoradiographically, binding was at high concentrations in the DAT-rich striatum; (in other words, the caudate nucleus and the putamen) and this binding to the striatum was abolished in the presence of high concentrations of GBR 12,909.
The recommended dosage of [123I]FP-CIT is 111–185 MBq (3–5 mCi), typically 185 MBq (5 Ci). In our own experience, no doses less than 150 MBq (4 mCi) should be applied, since lower amounts of activity may have an impact on scan quality and hence, diagnostic performance.
Pharmacodynamics
Overall, imaging agents contain only a small quantity of active compound so that no pharmacologic effects are expected. In general, the DAT-binding tropanes are good markers for the integrity of the nigrostriatal systems. Ex vivo studies in an animal lesion model using both an analog of levodopa and a DAT-radiotracer revealed that the uptake of [18F]FDOPA and [18F]CFT correlated well with the density of dopaminergic fibers [72]. This indicated a high sensitivity of both radiotracers in PD. However, [18F]FDOPA demonstrated a higher unspecific uptake that was probably due to extensive compensatory metabolism. So it seems that this tracer was less sensitive than the DAT-tracer [18F]CFT in detecting nigrostriatal degeneration [72]. Since the uptake of the latter was heavily reduced in degeneration stage two, a downregulation of DAT was hypothesized. Another lesion study in rats using [125I]b-CIT and [14C] L-DOPA provides similar results, indicating that the marker of the decarboxylase underestimated the decrease of dopaminergic neurons and that DAT levels more precisely reflected the decrease [73]. Such different detection sensitivities of radiotracers for DAT, the vesicular monoamine transporters (VMAT2) and [18F] FDOPA in (early) PD [74] were also predicted in humans. Other head-to-head comparisons in humans did not reveal any significant differences in the diagnostic utilities of radiotracers for DAT, the vesicular monoamine transporters (VMAT2) or [18F]FDOPA [13].
In addition, there are no differences among tropane derivatives in detecting early dopaminergic degeneration (FP-CIT; b-CIT; IPT; TRODAT-1 [75–78]). A direct comparison of [123I]FP-CIT versus [123I]b-CIT revealed similar capabilities for the detection of dopaminergic degeneration [79].
Pharmacokinetics and metabolism
The pharmacokinetics of [123I]FP-CIT were studied by monitoring radioactivity following intravenous injection and whole-body scintigraphy. Such biodistribution studies revealed that 5% of the administered radioactivity remains in whole blood 5 min after the injection and 7% of injected radioactivity enters the brain 10 min after the injection. Radioactivity in the brain decreases to 3% after 5 h. The striatum-to-background ratio is relatively constant between 3 and 6 h after the injection, meaning that clinical imaging is feasible with comparative results during this time window. This is a major advantage of [123I]FP-CIT over other tropane-based radiotracers, in particular [123I]b-CIT, which reaches an ‘equilibrium’ after 24 h for DAT-rich regions. For [123I] FP-CIT, approximately 30% of the whole brain radioactivity was attributed to striatal uptake. The biodistribution, metabolism and dosimetry of ioflupane in nonhuman primates and in humans are further described in several papers [71,80–83].
Drug interactions
Based on published data, it is likely that several drugs of abuse, including cocaine, amphetamines, modafinil, certain antidepressants (e.g., mazindol, bupropion and radafaxine, among others), adrenergic agents (e.g., phenylephrine and norepinephrine) and the anticholinergic agent benzatropine, influence the visual interpretation and quantification of [123I]FP-CIT SPECT scans in routine clinical studies.
Ideally, such medications should be withdrawn, before the administration of the radiotracer, at a time five-times greater that the drug’s biological half-life [84]. The decision to withdraw any medication must always be made by the specialist in charge of the patient’s care, balancing the potential risks of such a withdrawal.
Antiparkinsonian medications (i.e., levodopa, dopamine agonists, N-methyl-d-aspartate receptor blockers, monoamine oxidase-B inhibitors and catechol-O-methyltransferase inhibitors) taken at standard doses do not markedly affect dopamine transporter binding, and therefore they need not be withdrawn before dopaminergic imaging [84,85].
Interestingly, one study showed a significant higher binding in patients with ADP without antiparkinsonian medication in comparison to subjects in drugs-on state [23].
Data acquisition & analysis
A prerequisite for fully utilizing the diagnostic potential of DaTSCAN imaging is, however, good quality control and standardization of the entire procedure, from patient preparation through to positioning, g camera specifications, acquisition, reconstruction parameters and quality control of the acquired data. In addition data should be analyzed and reported according to guidelines (i.e., European Association of Nuclear Medicine [84] and the guidelines of the Society of Nuclear Medicine). Technical issues with regard to data acquisition comprise correct field of view, rotational radius, energy window set at the photopeak, additional scatter windows (if applicable), matrix size and zoom factor, among others. Strict standardizations of acquisition time after radiotracer injection and collection of sufficient numbers of total counts within the acquisition time also have to be considered. After reviewing the projection data (i.e., for motion artifacts), images are processed with distinct reconstruction methods (iterative reconstruction or filtered back-projection) and filtering (e.g., with a low pass filter). Employing the correct filter is mandatory for either visual or quantitative readings. Attenuation correction is recommended, either with simultaneously or sequentially acquired transmission scans, or calculated, as with a correction matrix, according to Chang [86].
For display, images are reformatted into slices in three planes (axial, coronal and sagittal). Reorientation makes visual interpretation easier and is essential when semi-quantification is used (not least to ensure the right placement of the reference region). The anterior commissure-posterior commissure line represents a good anatomical standard here as it is used for brain MRI. A simultaneously acquired CT scan or, alternatively, coregistration with (individual) MRI by commercial available software (e.g., HERMES MultiModality, Hermes Medical Solutions, Stockholm, Sweden) will allow precise re-alignment of the head.
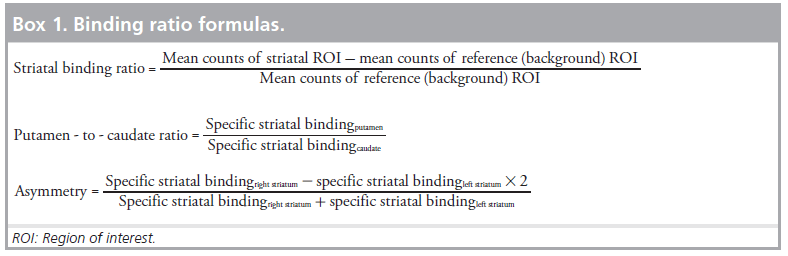
Visual assessment is robust in detecting presynaptic DAT binding. However, in visually uncertain cases and for intergroup, as well as for interinstitutional comparisons, semiquantitative approaches using regions of interest (ROIs) might help and have been recommended by nuclear medicine associations to be incorporated in the routine work-up of DAT-SPECT. With semi-quantification, striatal binding ratios (specific striatal binding) are calculated by comparing the activity in the target region with the activity in a reference region (with a very low DAT-density) according to listed formula (Box 1).
Reference region should conventionally refer to the occipital cortex for [123I]FP-CIT. Other parameters are described in Box 1.
ROIs are manually drawn on to one or more slices (usually three or four adjacent slides) with the highest striatal activity. This method is simple and provides a quantitative measure to allow comparisons of healthy reference data (where an age-dependent decline in healthy volunteers always has to be considered) but interobserver variability is considerable (due to variability in ROI placement). Therefore, it is important to standardize realignment (using predefined ROIs). Here, coregistration with individual MRI for delineation of striatal and reference volumes of interest offers the most accurate manual results [87].
Besides these observer-dependent approaches, fully-automated image analysis techniques are under validation in the clinical setting and have the potential as tools to improve the diagnostic accuracy and confidence in DAT-SPECT in patients who have parkinsonian features. Examples of these more advanced automated systems using volumes of interest, and voxel-based mathematical systems are DATQUANT™ (GE Healthcare, WI, USA), EXINI dat™ (EXINI Diagnostics AB, Lund, Sweden), and a modified version of the Brain Analysis Software (BRASS, BRASS-DaT, Hermes Medical Solutions, Stockholm, Sweden). They are all capable of producing more objective, observer-independent results and are faster compared with individual ROI/volume-of-interest-based methods. The voxel-based systems often use statistical parametric mapping (Wellcome Department of Cognitive Neurology, University College London, UK) that runs on a MATLAB® platform (The MathWorks Inc., MA, USA); however, this is for scientific purposes and not in routine clinical practice.
Safety & tolerability
No adverse event has been directly correlated with the tracer itself. However, several symptoms (e.g., headache, flu-like symptoms, injection site bleeding, vertigo and parasthesia) were described to be possibly or probably due to [123I]FP-CIT injection [10].
Cost–effectiveness
[123I]FP-CIT SPECT scans proved to be economically advantageous in the diagnostic workup of patients with uncertain parkinsonism (including essential tremor), especially when total indirect treatment costs over time were calculated [88,89]. No studies specifically addressed cost–effectiveness of [123I]FP-CIT SPECT for ADP. Cost–effectiveness may also derive from the incorrect screening of suitable candidates for drug trials or complex surgical procedures (e.g., deep brain stimulation) that may not be effective for ADP.
Conclusion & future perspective
Differentiating PD from ADP solely on [123I] FP-CIT imaging is still a challenge. Some suggestions may derive from asymmetry and diffusion of dopaminergic deficit or from targeting the brainstem. No additional value to the clinical experience is added instead in discriminating between ADPs. This result derives also in part from the limited number of patients with ADP investigated and from the uncertainties of clinical diagnosis still used as the reference in the absence of anatomopathological findings. The combination of several available SPECT tracers may not necessarily enhance specificity and sensitivity in discriminating between ADPs. Nowadays, imaging should not be considered as a replacement for a thorough clinical investigation and patients should be referred to movement disorder specialists. However, [123I] FP-CIT and SPECT imaging has been proven to be a safe and effective tool to investigate dopaminergic innervation and help exclude diseases without such an innervation loss.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: * of interest ** of considerable interes
- Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 348, 1356–1364 (2003).
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 56, 33–39 (1999).
- Litvan I, Bhatia KP, Burn DJ et al. Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov. Disord. 18, 467–486 (2003).
- Hughes AJ, Daniel SE, Kilford L et al. The accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study. J. Neurol. Neurosurg. Psychiatry 55, 181–184 (1992).
- Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 57, 1497–1499 (2001). & A detailed clinical–pathological analysis showing the relatively low diagnostic accuracy of a clinical evaluation in Parkinson’s disease (PD) and atypical parkinsonism.
- Meara J, Bhowmick BK, Hobson P. Accuracy of diagnosis in patients with presumed Parkinson’s disease. Age Ageing 28, 99–102 (1999).
- Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson’s disease in the community? J. Neurol. Neurosurg. Psychiatry 73, 529–534 (2002).
- Kish SJ, Shannak K, Hirnykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiological and clinical implications. N. Engl. J. Med. 318(14), 876–880 (1988).
- Piggott MA, Marshall EF, Thomas N et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain 122, 1449–1468 (1999).
- Benamer TS, Patterson J, Grosset DG et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov. Disord. 15, 503–510 (2000). & The first multicenter study using a blind consensus reading and showing that a simple visual assessment of [123I]FP-CIT SPECT images is an easily applied diagnostic test for a reliable differentiation of tremor disorders and in confirming a clinical diagnosis of a hypokinetic-rigid syndrome.
- Isaias IU, Benti R, Goldwurm S et al. Striatal dopamine transporter binding in Parkinson’s disease associated with the LRRK2 Gly2019Ser mutation. Mov. Disord. 21, 1144–1147 (2006).
- Isaias IU, Benti R, Cilia R et al. [123I]FP-CIT striatal binding in early Parkinson’s disease patients with tremor vs. akinetic-rigid onset. Neuroreport 18, 1499–1502 (2007).
- Eshuis SA, Jager PL, Maguire RP, Jonkman S, Dierckx RA, Leenders KL. Direct comparison of FP-CIT SPECT and F DOPA PET in patients with Parkinson’s disease and healthy controls. Eur. J. Nucl. Med. Mol. Imaging 36, 454–462 (2009).
- Isaias IU, Marotta G, Hirano S et al. Imaging essential tremor. Mov. Disord. 25, 679–686 (2010).
- Schneider SA, Edwards MJ, Mir P et al. Patients with adult-onset dystonic tremor resembling Parkinsonism tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov. Disord. 22, 2210–2215 (2007).
- Benaderette S, Zanotti Fregonara P, Apartis E et al. Psychogenic parkinsonism: a combination of clinical, electrophysiological, and [123I]-FP-CIT SPECT scan explorations improves diagnostic accuracy. Mov. Disord. 21, 310–317 (2006).
- Gaig C, Martí MJ, Tolosa E et al. 123I-Ioflupane SPECT in the diagnosis of suspected psychogenic parkinsonism. Mov. Disord. 21, 1994–1998 (2006).
- Gilman S, Wenning GK, Low PA et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676 (2008).
- Brooks DJ. Diagnosis and management of atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 72, 10–16 (2002).
- Lubarsky M, Juncos JL. Progressive supranuclear palsy: a current review. Neurologist 14, 79–88 (2008).
- Mahapatra RK, Edwards MJ, Schott JM, Bhatia KP. Corticobasal degeneration. Lancet Neurol. 3, 736–743 (2004).
- Adbo WF, Borm GF, Munneke M, Verbeek MM, Esselink RA, Bloem BR. Ten steps to identify atypical parkinsonism. J. Neurol. Neurosurg. Psychiatry 77, 1367–1369 (2006).
- Hesse S, Oehlwein C, Barthel H et al. Possible impact of dopamine SPECT on decision-making for drug treatment in Parkinsonian syndrome. J. Neural Transm. 113, 1177–1190 (2006).
- Pirker W, Djamshidian S, Asenbaum S et al. Progression of dopaminergic degeneration in Parkinson’s disease and atypical parkinsonism: a longitudinal b-CIT SPECT study. Mov. Disord. 17, 45–53 (2002).
- Varrone A, Marek KL, Jennings D et al. [123I] b-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov. Disord. 16, 1023–1032 (2001).
- Van Laere K, Casteels C, De Ceuninck L et al. Dual-tracer dopamine transporter and perfusion SPECT in differential diagnosis of parkinsonism using template-based discriminant analysis. J. Nucl. Med. 47, 384–392 (2006).
- Scherfler C, Seppi K, Donnemiller E et al. Voxel-wise analysis of [123I]-CIT SPECT differentiates the Parkinson variant of multiple system atrophy from idiopathic Parkinson’s disease. Brain 128, 1605–1612 (2005). & An excellent approach to distinguishing between multiple system atrophy and PD at the voxel level.
- El Fakhri G, Habert MO, Maksud P et al. Quantitative simultaneous 99mTc-ECD/123IFP- CIT SPECT in Parkinson’s disease and multiple system atrophy. Eur. J. Nucl. Med. Mol. Imaging 33, 87–92 (2006).
- Seppi K, Scherfler C, Donnemiller E et al. Topography of dopamine transporter availability in progressive supranuclear palsy: a voxel wise [123I] bCIT SPECT analysis. Arch Neurol. 63, 1154–1160 (2006).
- Plotkin M, Amthauer H, Klaffke S et al. Combined [123I]-FP- CIT and [123I]-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J. Neural Transm. 112, 677–692 (2005). & Evaluates the accuracy of combined SPECT imaging versus clinical follow-up in a large series of patients with PD.
- Rinne JO, Burn DJ, Mathias CJ et al. Positron emission tomography studies on the dopaminergic system and striatal opioid binding in the olivopontocerebellar atrophy variant of multiple system atrophy. Ann. Neurol. 37, 568–573 (1995).
- Antonini A, Benti R, De Notaris R et al.123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol. Sci. 24, 149–150 (2003). & One of the first attempts to investigate and compare dopamine transporter binding values, as measured by [123I]FP-CIT SPECT, in atypical degenerative parkinsonism.
- Sawle GV, Brooks DJ, Marsden CD et al. Corticobasal degeneration: a unique pattern of regional cortical oxygen hypometabolism and striatal fluorodopa uptake demonstrated by positron emission tomography. Brain 114, 541–556 (1991).
- Pirker W, Asenbaum S, Bencsits G et al. [123I] b-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov. Disord. 15, 1158–1167 (2000).
- Klaffke S, Kuhn AA, Plotkin M et al. Dopamine transporters, D2 receptors, and glucose metabolism in corticobasal degeneration. Mov. Disord. 21, 1724–1727 (2006). & Addresses distinct SPECT features of corticobasal syndrome. However, without any neuropathological reference the results are preliminary.
- O’Sullivan SS, Burn DJ, Holton JL, Lees AJ. Normal dopamine transporter single photon emission CT scan in corticobasal degeneration. Mov. Disord. 23, 2424–2426 (2008).
- Cilia R, Rossi C, Frosini D et al. Dopamine transporter SPECT imaging in corticobasal syndrome. PLoS ONE 6(5) e18301 (2011).
- Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS. Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 48, 959–969 (1997).
- Vlaar AM, van Kroonenburgh MJ, Kessels AG, Weber WE. Meta-analysis of the literature on diagnostic accuracy of SPECT in parkinsonian syndromes. BMC Neurol. 7, 27 (2007). & Meta-analysis of the literature describing the diagnostic accuracy of SPECT in parkinsonian syndromes.
- Vlaar AM, de Nijs T, Kessel AG et al. Diagnostic value of123I-ioflupane and 123I-iodobenzamide SPECT scans in 248 patients with parkinsonian syndromes. Eur. Neurol. 59, 258–266 (2008).
- Felicio AC, Shih MC, Godeiro-Junior C, Andrade LA, Bressan RA, Ferraz HB. Molecular imaging studies in Parkinson disease: reducing diagnostic uncertainty. Neurologist 15, 6–16 (2009).
- Tissingh G, Booij J, Winogrodzka A, van Royen EA, Wolters EC. IBZM- and CIT- SPECT of the dopaminergic system in parkinsonism. J. Neural Transm. Suppl. 50, 31–37 (1997).
- Halldin C, Gulyas B, Langer O, Farde L. Brain radioligands state of the art and new trends. Q. J. Nucl. Med. 45, 139–152 (2001).
- Antonini A, Leenders KL, Vontobel P et al. Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson’s disease. Brain 120, 2187–2195 (1997).
- Kim YJ, Ichise M, Ballinger JR et al. Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov. Disord. 17, 303–312 (2002).
- Ghaemi M, Hilker R, Rudolf J, Sobesky J, Heiss WD. Differentiating multiple system atrophy from Parkinson’s disease: contribution of striatal and midbrain MRI volumetry and multi-tracer PET imaging. J. Neurol. Neurosurg. Psychiatry 73, 517–523 (2002).
- Knudsen GM, Karlsborg M, Thomsen G et al. Imaging of dopamine transporters and D2 receptors in patients with Parkinson’s disease and multiple system atrophy. Eur. J. Nucl. Med. Mol. Imaging 31, 1631–1638 (2004).
- Schwarz J, Tatsch K, Arnold G et al. 123I-iodobenzamide-SPECT predicts dopaminergic responsiveness in patients with de novo parkinsonism. Neurology 42, 556–561 (1992).
- Hierholzer J, Cordes M, Schelosky L et al. The differential diagnosis of Parkinson diseases123I-IBZM-SPECT vs. the apomorphine test. Rofo 159, 86–90 (1993).
- Hierholzer J, Cordes M, Venz S et al. Loss of dopamine-D2 receptor binding sites in Parkinsonian plus syndromes. J. Nucl. Med. 39, 954–960 (1998).
- Pizzolato G, Cagnin A, Rossato A et al. Striatal dopamine D2 receptor alterations and response to L-DOPA in Parkinson’s disease. A [123I]IBZM SPET study. Adv. Neurol. 69, 467–473 (1996).
- Schwarz J, Tatsch K, Gasser T, Arnold G, Oertel WH [123].IBZM binding predicts dopaminergic responsiveness in patients with parkinsonism and previous dopaminomimetic therapy. Mov. Disord. 12, 898–902 (1997).
- Mo SJ, Linder J, Forsgren L, Larsson A, Johansson L, Riklund K. Pre- and postsynaptic dopamine SPECT in the early phase of idiopathic parkinsonism: a population-based study. Eur. J. Nucl. Med. Mol. Imaging 37, 2154–2164 (2010).
- la Fougère C, Pöpperl G, Levin J et al. The value of the dopamine D2/3 receptor ligand 18F-desmethoxyfallypride for the differentiation of idiopathic and nonidiopathic parkinsonian syndromes. J. Nucl. Med. 51, 581–587 (2010). & Interesting paper showing the higher accuracy of dopamine D2/3 receptor PET when compared with D2/3 receptor SPECT. 55 Eckert T, Barnes A, Dhawan V et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage 26, 912–921 (2005). & A milestone publication demonstrating different patterns of brain glucose metabolism in idiopathic and atypical PD.
- Eckert T, Van Laere K, Tang C et al. Quantification of Parkinson’s disease-related network expression with ECD SPECT. Eur. J. Nucl. Med. Mol. Imaging 34, 496–501 (2007).
- Derlin T, Afzal W, Wilke F et al. IBZM SPECT and FDG PET in the differential diagnosis of Parkinsonian syndromes: comparison with respect to inter-rater agreement. Nuklearmedizin 49, 139–147 (2010).
- Morelli M, Arabia G, Novellino F et al. MRI measurements predict PSP in unclassifiable parkinsonisms: a cohort study. Neurology 77, 1042–1047 (2011).
- Köllensperger M, Wenning GK. Assessing disease progression with MRI in atypical parkinsonian disorders. Mov. Disord. 24, S699–S702 (2009).
- Spiegel J, Möllers MO, Jost WH et al. FP-CIT and MIBG scintigraphy in early Parkinson’s disease. Mov. Disord. 20, 552–561 (2005).
- Sawada H, Oeda T, Yamamoto K et al. Diagnostic accuracy of cardiac metaiodobenzylguanidine scintigraphy in Parkinson disease. Eur. J. Neurol. 16, 174–182 (2009).
- Raffel DM, Koeppe RA, Little R et al. PET measurement of cardiac and nigrostriatal denervation in Parkinsonian syndromes. J. Nucl. Med. 47, 1769–1777 (2006). nn Excellent study that questions the accuracy of dedicated heart SPECT.
- Südmeyer M, Antke C, Zizek T et al. Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J. Nucl. Med. 52, 733–740 (2011).
- Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain 134, 3146–3166 (2011).
- Koch W, Hamann C, Radau PE, Tatsch K. Does combined imaging of the pre- and postsynaptic dopaminergic system increase the diagnostic accuracy in the differential diagnosis of parkinsonism? Eur. J. Nucl. Med. Mol. Imaging 34, 1265–1273 (2007).
- Stoessl AJ, Martin WW, McKeown MJ, Sossi V. Advances in imaging in Parkinson’s disease. Lancet Neurol. 10, 987–1001 (2011).
- Brooks DJ. Imaging approaches to Parkinson disease. J. Nucl. Med. 51, 596–609 (2010).
- Scheffel U, Lever JR, Abraham P et al. N-substituted phenyltropanes as in vivo binding ligands for rapid imaging studies of the dopamine transporter. Synapse 25, 345–349 (1997).
- Neumeyer JL, Wang S, Gao Y et al. N-w-fluoroalkyl analogs of (1R)- 2b- carbomethoxy-3b-(4-iodophenyl)-tropane (b-CIT): radiotracers for positron emission tomography and single photon emission computed tomography imaging of dopamine transporters. J. Med. Chem. 37, 1558–1561 (1994).
- Neumeyer JL, Tamagnan G, Wang S et al. N-substituted analogs of 2b-carbomethoxy- 3b- (4´-iodophenyl) tropane (b-CIT) with selective affinity to dopamine or serotonin transporters in rat forebrain. J. Med. Chem. 39, 543–548 (1996).
- Lundkvist C, Halldin C, Ginovart N, Swahn CG, Farde L. [18F] b-CIT-FP is superior to [11C] b-CIT-FP for quantitation of the dopamine transporter. Nucl. Med. Biol. 24, 621–627 (1997).
- Forsback S, Niemi R, Marjamäki P et al. Uptake of 6-[18F]fluoro-l-dopa and [18F]CFT reflect nigral neuronal loss in a rat model of Parkinson’s disease. Synapse 51, 119–127 (2004).
- Ito Y, Fujita M, Shimada S et al. Comparison between the decrease of dopamine transporter and that of L-DOPA uptake for detection of early to advanced stage of Parkinson’s disease in animal models. Synapse 31, 178–185 (1999).
- Fischman AJ. Role of [18F]-dopa-PET imaging in assessing movement disorders. Radiol. Clin. North Am. 43, 93–106 (2005).
- Tissingh G, Booij J, Bergmans P et al. Iodine-123-N-W-fluoropropyl- 2b- carbomethoxy-3b-(4-iodophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. J. Nucl. Med. 39, 1143–1148 (1998).
- Marek KL, Seibyl JP, Zoghbi SS et al. [123I] b-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology 46, 231–237 (1996).
- Schwarz J, Linke R, Kerner M et al. Striatal dopamine transporter binding assessed by [I-123]IPT and single photon emission computed tomography in patients with early Parkinson’s disease: implications for a preclinical diagnosis. Arch. Neurol. 57, 205–208 (2000). & Demonstrates that an early (preclinical) PD diagnosis is feasible with dopamine transporter SPECT in cases of hemiparkinsonism.
- Huang WS, Lin SZ, Lin JC, Wey SP, Ting G, Liu RS. Evaluation of early-stage Parkinson’s disease with 99mTc-TRODAT-1 imaging. J. Nucl. Med. 42, 1303–1308 (2001).
- Seibyl JP, Marek K, Sheff K et al. Iodine-123- b-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson’s patients. J. Nucl. Med. 39, 1500–1508 (1998).
- Booij J, Andringa G, Rijks LJ et al. [123I] FP-CIT binds to the dopamine transporter as assessed by biodistribution studies in rats and SPECT studies in MPTP-lesioned monkeys. Synapse 27, 183–190 (1997).
- Booij J, Busemann Sokole E, Stabin MG, Janssen AG, de Bruin K, van Royen EA. Human biodistribution and dosimetry of [123I]FP-CIT: a potent radioligand for imaging of dopamine transporters. Eur. J. Nucl. Med. 25, 24–30 (1998).
- Lundkvist C, Halldin C, Swahn CG et al. O-methyl-11C] b-CIT-FP, a potential radioligand for quantitation of the dopamine transporter: preparation, autoradiography, metabolite studies, and positron emission tomography examinations. Nucl. Med. Biol. 22, 905–913 (1995).
- Chaly T, Dhawan V, Kazumata K et al. Radiosynthesis of [18F] N-3-fluoropropyl-2-bcarbomethoxy- 3-b-(4-iodophenyl) nortropane and the first human study with positron emission tomography. Nucl. Med. Biol. 23, 999–1004 (1996).
- Darcourt J, Booij J, Tatsch K et al. EANM procedure guidelines for brain neurotransmission SPECT using 123I-labelled dopamine transporter ligands, version 2. Eur. J. Nucl. Med. Mol. Imaging 37, 443–450 (2010).
- Schillaci O, Pierantozzi M, Filippi L et al. The effect of levodopa therapy on dopamine transporter SPECT imaging with 123I-FP-CIT in patients with Parkinson’s disease. Eur. J. Nucl. Med. Mol. Imaging 32, 1452–1456 (2005).
- Chang CC, Liu JS, Chang YY, Chang WN, Chen SS, Lee CH. 99mTc-ethyl cysteinate dimer brain SPECT findings in early stage of dementia with Lewy bodies and Parkinson’s disease patients: a correlation with neuropsychological tests. Eur. J. Neurol. 15, 61–65 (2008).
- Barthel H, Müller U, Wächter T et al. Multimodal SPECT and MRT imaging data analysis for an improvement in the diagnosis of idiopathic Parkinson’s syndrome. Radiologe 40, 863–869 (2000).
- Van Laere K, Everaert L, Annemans L, Gonce M, Vandenberghe W, Vander Borght T. The cost effectiveness of 123I-FP-CIT SPECT imaging in patients with an uncertain clinical diagnosis of parkinsonism. Eur. J. Nucl. Med. Mol. Imaging 35, 1367–1376 (2008).
- Antonini A, Berto P, Lopatriello S et al. Cost-effectiveness of 123I-FP-CIT in the differential diagnosis of essential tremor and Parkinson’s disease in Italy. Mov. Disord. 23(15), 2202-2209 (2008).