Study Protocol - Diabetes Management (2017) Volume 7, Issue 4
DESTINE: A practice-based intervention to increase empowerment in patients with type 2 diabetes: a study protocol of a randomized controlled trial
- *Corresponding Author:
- du Pon E
Utrecht University of Applied Sciences
Utrecht, Netherlands
E-mail: esther.dupon@hu.nl
Abstract
Trial design: Self-management plays a central role in diabetes management. However, not all patients are able to translate the health care providers’ recommendations for effective self-management in daily life. Diabetes Education and Self-management to Increase Empowerment (DESTINE) primarily investigates the effects of group education program Proactive Interdisciplinary Self-Management (PRISMA) in primary care treated people with Type 2 Diabetes Mellitus (T2DM) on the use of an online care platform. Methods: The DESTINE study has a randomized controlled design (1:1). 200 patients with T2DM using an online care platform called e-Vita will receive either PRISMA in addition to usual care or usual care only. The primary endpoint of this study is usage of the e-Vita platform. The secondary endpoints are participation in the consultation with the care provider, adherence to oral diabetes medications, and a selection of self-reported and clinical measures. After six months, both groups will receive PRISMA in a 6 month extension phase. Discussion: PRISMA focuses on aligning treatment goals from different health care providers while the individual patient remains in the lead. The goal is to shift patients from being an information receiver towards applying self-management and becoming empowered health care participants. Though recognized as important; theoretically based group education is still not routinely offered in the Netherlands. In the future, depending on the study results, e-Vita and PRISMA could be implemented in regular diabetes care. Trial registration: Current Controlled Trials NTR4693.
Keywords
self-management, diabetes type 2, eHealth, study protocol, patient empowerment, group education, adherence
Abbreviations
NAD: National Action Program Diabetes (in Dutch: Nationaal Actieprogramma Diabetes), NHG: Dutch GP Society (in Dutch: Nederlandse Huisartsen Genootschap), KCK: Diabetes Centre Zwolle (in Dutch: KennisCentrum voor Ketenzorg), GP: General Practitioner, T2DM: Type 2 Diabetes Mellitus, PN: Practice Nurse, HCP: Health Care Provider, NIVEL: Netherlands institute for health services research, PRISMA: Proactive Interdisciplinary Self- Management, DESTINE: Diabetes Education and Self-management to Increase Empowerment, DESMOND: Diabetes Education and Self- Management for Ongoing and Newly Diagnosed, RIAS: Roter Interaction Analysis System, METC: Medical Ethics Committee, WMO: Dutch Law on Medical Scientific Research in Humans
Introduction
Worldwide, the prevalence of diabetes mellitus is increasing dramatically. The number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014 [1]. The prevalence in the Netherlands is expected to rise from 830,000 in 2011 to over 1,300,000 in 2025 [2]. Over 90% of this population is estimated to have type 2 diabetes mellitus (T2DM) [3]. In the Netherlands, T2DM is mainly treated in primary care, where the general practitioners (GP) usually see patients once a year to manage their diabetes. In addition to these GP visits, every three to six months, the primary care Practice Nurse (PN) checks the patients’ body weight, blood pressure and (fasting) blood glucose levels. The PNs also inquire about their patients’ well-being, hypo- or hyperglycemia, nutrition, exercise and medication, when indicated.
Self-management plays a central role in diabetes management [4]. Influential self-management behaviors are using a healthy diet, being active, acting upon self-measured blood glucose levels when needed, taking medication according to prescription, and problem solving [5]. Unfortunately, not all patients are able to translate the GPs’ and PNs’ recommendations for effective self-management into appropriate action in daily life [6]. For many people with T2DM, self-management is challenging, since they do not possess adequate knowledge, skills or motivation to initiate and maintain behaviors that can help them control their condition [7]. Furthermore, although good communication between a PN and a patient can help to properly manage their condition [8,9], many patients have not yet evolved into active health care consumers when it comes to medical consultations [9].
In addition, the number of PNs will not keep up with the projected growth in patient numbers. Therefore, diabetes care will be in need of a decrease in the workload per patient for PNs, which eventually will result in a decrease in face-to- face time per patient. The general growth of health care costs will further restrict the possibilities to spend adequate time per patient [10]. Consequently, this implies the necessity of alternative forms of support, treatment, and patient self-management [3]. Improving patient empowerment could not only lead to more/ increasingly empowered patients, but could also decrease the workload of PNs. Improved empowerment may be achieved by promoting patient knowledge regarding T2DM and thus giving patients more insights into their own situation. Patients may also obtain these insights using web based self-management programs [11]. In 2012, an online care platform (e-Vita) was developed in the Netherlands. This platform was designed to offer people with T2DM insight into their diabetes-related health data as well as to educate and inform them on their condition and their data [12]. Users of this platform had lower HbA1c levels compared to non-users [13]. Furthermore, users reported higher quality of life and improved medication adherence, and suffered less from diabetes-related distress. However, offering patients this platform alone did not yield the usage results that were expected; of 633 patients registered for platform use 57% never logged on, 29% logged on once and only 14% logged on for at least two sessions [13]. Even though this platform was designed to be suitable and available for all people with T2DM, the interested patients were primarily men, younger, more often higher educated and had shorter diabetes duration [14].
In the current study, called Diabetes Education and Self-management To Increase Empowerment (DESTINE), the primary objective is to test the effect of the Proactive Interdisciplinary Self-Management (PRISMA: a program that aims to specifically increase self-management skills in people with T2DM) group education program on the use of the e-Vita care platform. The secondary objectives are to test the effect of the PRISMA group education program on participation in the consultation with the PN, adherence to oral diabetes medications and a selection of self-reported and clinical measures.
Patients and methods
▪ Study design
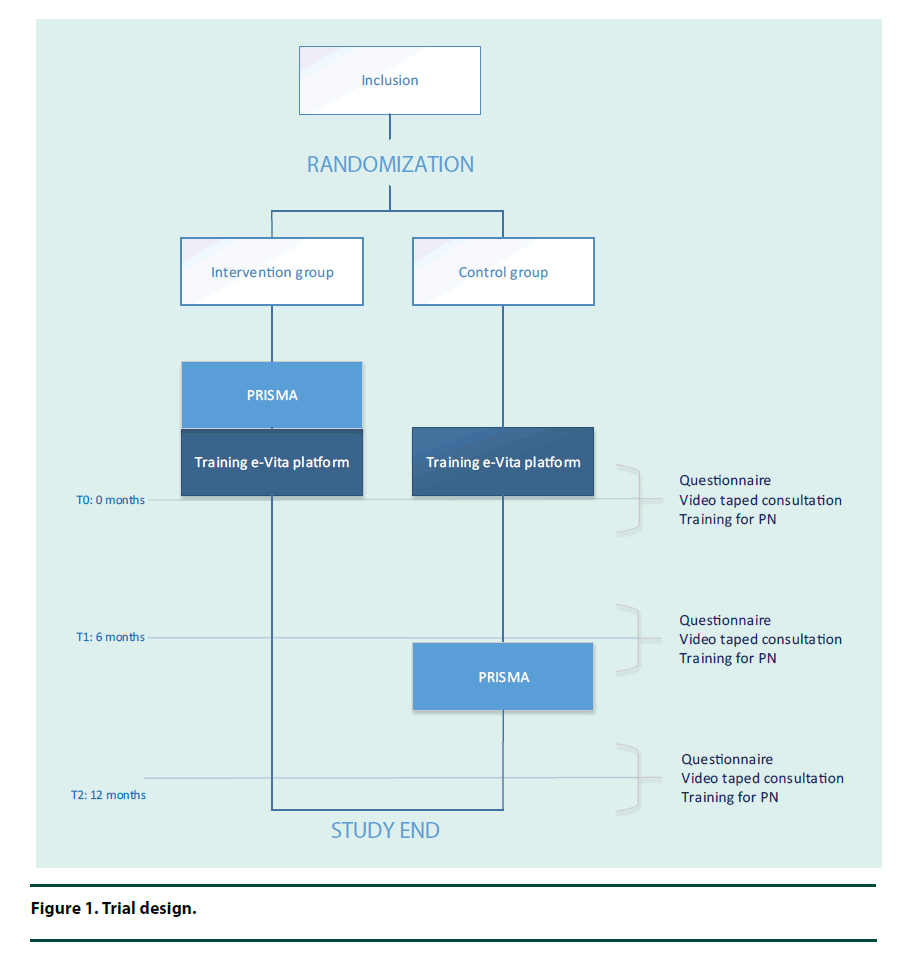
The study will be a Randomized Controlled Trial (RCT), as shown in Figure 1.
▪ Recruitment
We will use a small-scaled implementation strategy. General practices in the region of Zwolle, a city in the east of the Netherlands, will be included. The PNs will all be trained by the author (EdP) in the month prior to the first study activity.
Next, the GPs will make a selection of their patients with T2DM and exclude the patients not matching the inclusion criteria. The selected patients will be recruited by the primary investigator (EdP). This process will be mediated by the GPs. These patients will receive information about the study, including an informed consent form. The patient is encouraged to ask his or her treating PN and/ or GP or the investigator any question about the study. After giving informed consent, patients will be included in the study.
▪ Study population
People of 18 years and older, diagnosed with T2DM (ongoing and newly) and treated in one of the participating general practices with the GP defined as their main caregiver will be included.
The following exclusion criteria will apply:
(1) insufficient knowledge of the Dutch language to understand the requirements of the study and/or the questions posed in the questionnaires, (2) mental retardation, psychiatric treatment for schizophrenia, (3) mental disorder or bipolar disorder, (4) life expectancy less than one year due to malignancies, (5) and any other condition that according to the investigators may interfere with trial participation or evaluation of results, for example multiple sclerosis or dementia.
▪ Description of the intervention
The PRISMA group education program will be offered in addition to usual care. PRISMA aims to improve self-management skills in patients with T2DM [15,16], and is based on the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) program developed in the UK. The DESMOND programs’ curriculum has been described in detail elsewhere [16]. The philosophy of PRISMA is based on patient empowerment. This program is adapted by the VU University Medical Center in Amsterdam specifically for patients [17] and grounded in the following four psychological models: the self-regulation theory [18], the dual process theory [19], the self-determination theory [20], and the social learning theory [21]. In short, the PRISMA program consists of two meetings of 3.5 hours with a group size of maximum 12 patients plus possible partners. Groups are guided by two diabetes professionals, for example a dietician and a PN. These trainers have followed a standardized training program to ensure the quality of information delivery. PRISMA aims to empower patients by using a non-didactic learning approach. Patients are stimulated to consider their own personal risk factors and to choose a specific goal of behavior change.
▪ Usual care
Visits at the GP or PN
In line with clinical guidelines at that time, patients of the participating general practices should have 2 to 4 visits a year with their GP or PN, one of which is an annual check-up.
The e-Vita platform
The current study elaborates on the study performed by Roelofsen et al. (2014). For the present study, an improved version of the e-Vita care platform will be developed. Technical and visual adjustments will be conducted to increase usability. e-Vita offers patients the following four functions: firstly, view and track their lab results of the last three visits at their GP or PN; secondly, formulating and monitoring their health related actions and goals; thirdly, conversing with their PN; and fourthly, taking part in educational modules about T2DM tailored to their actual knowledge.
Training for PNs
We will use the following strategies to involve the PNs of the participating practices in our study. First, the PNs will be trained in using e-Vita as well, in order to be able respond to their patients’ messages and questions about the platform and to follow their patients’ activity in educational modules. Second, the PNs will be stimulated to take part in one of the PRISMA courses along with their patients to become familiar with the non-didactic learning approach of PRISMA, which they can implement in their consultations afterwards. Last, the PNs will be invited to participate in trainings to improve their communication skills (motivational interviewing) in interactions with their patients. These trainings will not be linked to the randomization order of the patients and will be organized by EdP in cooperation with a care group.
Outcomes
Baseline and outcome parameters will be measured at the start of the study (T0), and after 6 months (T1) and 12 months (T2), respectively. Table 1 shows a time schedule of the data-collection. The patient profile includes the following parameters: surname, family name, gender, birth date, home address, and level of education.
| T0 | T1 | T2 | |||
|---|---|---|---|---|---|
| Months | 0 | 6 | 12 | ||
| - | - | - | - | ||
| Primary outcome | Patient profile | - | X | - | - |
| Use of platform | - | - | X | X | |
| Secondary outcomes | Self-reported data (questionnaires) | - | X | X | X |
| Participation in consultation with PN (videos) | - | X | X | X | |
| Adherence to diabetes medication | - | X | X | X | |
| Clinical measures | - | X | X | X | |
Table 1. Time schedule of data-collection.
▪ Primary outcome
The primary endpoint of this study is usage (number of log-ons and time spent per session) of the e-Vita platform. Log-data will be used to track individual use of the platform over time.
▪ Secondary outcomes
Self-reported data (questionnaires)
The following parameters will be examined through questionnaires (APPENDIX 1): Well Being (WHO-5 scale) [22], Health Related Quality of Life (EQ-5D) [23], Self- Reliance (PAM) [24], Quality of Received Care (HowRwe), eHealth Literacy (eHEALS) [25], Diabetes Self-management Behavior (SDSCA) [26], Self-Reported Adherence to Medication Prescriptions (MARS-5) [27], and Self-Efficacy in Patient-Physician Interactions (PEPPI-5) [28].
Participation in consultation with PN (videos)
After providing PRISMA to the intervention group, the participants’ consultations (intervention and control group) about their condition with their PN will be recorded by an unmanned camera at T0, T1 and T2 in order to analyze verbal as well as non-verbal behavior.
• Verbal behavior: the videotaped consultations will be reviewed using relevant communication categories from the Roter Interaction Analysis System (RIAS) to analyse the level of information exchange and the topics discussed (condition, treatment, lifestyle, psychological issues).
• Non-verbal behavior: coding of eye gaze direction, which is a sign of participation, will be done by coding ‘patient–PN’ directed gaze, ‘PN–patient’ directed gaze and ‘PN–computer’ directed gaze.
Adherence to diabetes medication
Information with regards to the amount of prescribed diabetes medication and rates and times of prescriptions will be derived from the pharmacies connected to the participating GPs. We will extract the medication status and medication history out of the pharmacy’s information system. Next, we determine adherence using the Medication Possession Ratio, specifically suitable for oral diabetes medication [29].
Clinical measures
Clinical measures described in the national medical guidelines of diabetes parameters (APPENDIX 2) will be included in the study to objectify a person’s health status. These clinical measures are already being collected by the participants’ PN (and GP) during their routine check-ups, and will be sent anonymously to KCK for research purposes.
▪ Participant flow
In the intervention group, participants will start with receiving PRISMA on top of usual care including platform usage (Figure 1). The control group will continue to receive usual care including platform usage for the duration of 6 months and will be offered PRISMA after 6 months.
In both groups participants will get the option to voluntarily use the platform and its educational content in order to get more control over their health process. Therefore, participants will be registered on e-Vita and will be invited for training about this platform. They will be introduced to e-Vita and receive login data during the training. After this training participants are able to start using the platform immediately.
Consultations between the participants and their PN will be video-recorded and participants are asked to complete a questionnaire at three points during the study (0, 6 and 12 months).
The clinical measures collected by the KCK will be combined with the results of the collected questionnaires to assemble a complete dataset on each included subject.
▪ Randomization
From the start of the study, participants will be included based on the availability of PRISMA training capacity. The PRISMA groups will consist of 10 patients. Participants will be randomized in 10 blocks of each 20 participants at patient level in a general practice (Figure 1) using a randomization list. The inclusion period will last 12 months. There will be no blinding for the participant, investigator and PN after assignment to either intervention.
▪ Data
The reliability of the registered data in the ITsystem of the GP warrants their use for research purposes.
Using a research ID for every patient, all data will be collected and analyzed anonymously. Personal data gathered in this study will be stored encrypted in our database using the Rijndael cryptographic algorithm [30].
The video recordings will be processed and encrypted anonymously, are never used for public display (not for lectures or presentations) and will be stored securely at the Netherlands institute for health services research (NIVEL). The video recordings will be stored for a period of up to fifteen years.
▪ Statistical analysis
All analyses will be conducted by using IBM SPSS Statistics version 22. Normally distributed data will be presented as means and standard deviation, abnormally distributed data as median and interquartile range. Dichotomous/categorical data will be presented as numbers and percentage of total. To evaluate differences in target variables (use of the online platform: number of log-ons and time spent per session) over time and between arms, a T-test or Mann-Whitney test will be used. In addition, we will use (generalized) linear mixed model, depending on the distribution of the target variables, where time will be used as within-subjects variable. Immediate start with the platform or one year later will be used as a between-subjects factor. Relevant variables will be added as a covariate in the analyses. To provide unbiased comparisons among the groups and to avoid the effects of dropouts, an intention to treat analysis will be conducted. Also, per-protocol analysis will be conducted to compare groups including only patients who attended PRISMA.
▪ Sample size calculation
To show a difference on the primary outcome measure ‘usage of the e-Vita platform’ of 0.15 in the control group versus 0.35 in the intervention group with a two-sided risk alpha of 5% and a power of 80%, in both groups 81 individuals are needed using the unpooled Z-test. With an expected drop-out rate of 20%, we will include 200 patients in our RCT, 100 patients in each group.
Discussion
▪ Strengths/limitations
Our study design attempts to eliminate the following aspects that could affect the results. First, participant’s experiences with health information seeking behavior on the Internet could vary before the start of the study. Therefore, we will measure eHealth literacy using 4 items of the eHEALS questionnaire [25]. Second, it could be necessary to make adjustments to the e-Vita platform during the study, for example when technical problems occur. These adjustments will be monitored accurately by EdP. Third, experiences with communication techniques and motivational interviewing could vary between the participating PNs. This will depend on whether or not the PN works as PRISMA trainer.
Because of the nature of the treatments, the study will not be blinded. To limit bias, the randomisation will be conducted under blinded conditions using a randomization list; also analyses will be performed blinded by labelling the groups with non-identifying terms.
▪ Further implementation
Improving patient empowerment could not only lead to better empowered patients, but may also decrease the workload of diabetes HCPs. The e-Vita platform and the PRISMA program are additional to routine care and focus on aligning treatment goals from different HCPs while the individual patient remains in the lead. The goal is to shift patients from being an information receiver towards applying self-management and becoming empowered. The current study investigates whether our practice-based intervention (e-Vita plus PRISMA) will improve patient empowerment by involving patients in their own treatment, thus resulting in increased quality of life and a reduction of medical care utilization.
Offering patients e-Vita alone did not yield the usage results that were expected [13]. The participation rate and the continuity of e-Vita use by patients appeared to be low. Training patients and HCPs in the use of the online platform seems essential, as well as improving the usability of the platform. In the current study, we will focus on these aspects.
Theoretically based group education, such as PRISMA, can influence health, psychological and lifestyle outcomes [30-32]. In the UK, everyone diagnosed with T2DM is offered theoretically based group education. Though recognized as important, such programs are still not offered routinely in the Netherlands. Nowadays, the most frequently offered program is PRISMA, which is mainly offered in primary care. In the future, depending on the study results, e-Vita and PRISMA could be implemented in regular diabetes care.
Statement of funding sources
This work was supported by the University of Applied Sciences Utrecht and the Foundation Care Within Reach (In Dutch: Stichting Zorg Binnen Bereik).
Declarations
This study was reviewed by the Medical Ethics Committee of the Isala Clinics, Zwolle, the Netherlands. This kind of study does not fall under the scope of the Dutch Medical Scientific Research in Human Act (WMO), therefore, it did not require formal medical ethics approval. The burden to the patients was regarded to be minimal.
Acknowledgements
We are grateful to VitalHealth Software for making their e-Vita platform accessible to the patients who will participate in this study; Cas Kruitwagen for his advices concerning the statistics and Utrecht University of Applied Sciences and the Foundation Care Within Reach (In Dutch: Stichting Zorg Binnen Bereik) for their financial support.
References
- NCD-RisC. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4*4 million participants. Lancat. 387(10027), 1513–1530 (2016).
- https://www.volksgezondheidenzorg.info/onderwerp/diabetes-mellitus/cijfers-context/huidige-situatie
- Bilo HJ, Houweling ST. Toename van het aantal mensen met diabetes mellitus: noodzaak van een deltaplan (in Dutch). Ned Tijdschr Geneeskd. 153, A629 (2009).
- Norris SL, Lau J, Smith SJ et al. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect of glycemic control. Diabetes Care. 25(7), 1159–1171 (2002).
- Funnell M, Brown TL, Childs BP et al. National Standards for Diabetes Self-Management Education. Diabetes Care. 32(Suppl 1), S87–S94 (2009).
- Menon ST. Toward a model of psychological health empowerment: implications for health care in multicultural communities. Nurse Educ. Today. 22(1), 28–39 (2002).
- Thoolen B, de Ridder D, Bensing J et al. Beyond Good Intentions: the development and evaluation of a proactive self-management course for patients recently diagnosed with type 2 diabetes. Health Educ. Res. 23(1), 53–61 (2008).
- Zolnierek KB, DiMatteo MR. Physician Communication and Patient Adherence to Treatment: A Meta-Analysis. Med. Care. 47(8), 826–834 (2009).
- Dulmen S van, Bijnen E van. What makes them (not) talk about proper medication use with their patients? An analysis of the determinants of GP communication using reflective practice. Int. J. Person. Centered Med. 1, 27–34 (2011).
- Orchard M, Green E, Sullivan T et al. Chronic disease prevention and management: implications for health human resources in 2020. Healthc. Q. 11(1), 38–43 (2008).
- Tang PC, Ash JS, Bates DW et al. Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J. Am. Med. Inform. Assoc. 13(2), 121-126 (2006).
- Roelofsen Y, Hendriks SH, Sieverink F et al. Design of the e-vita diabetes mellitus study: effects and use of an interactive online care platform in patients with type 2 diabetes (e-VitaDM-1/ZODIAC-40). BMC. Endocr. Disord. 14, 22 (2014).
- Roelofsen Y, van Vugt M, Hendriks SH et al. Demographical, Clinical, and Psychological Characteristics of Users and Nonusers of an Online Platform for T2DM Patients (e-VitaDM-3/ZODIAC-44). J Diabetes Res. 2016(2016), 1–16 (2016).
- Roelofsen Y, Hendriks SH, Sieverink F et al. Differences Between Patients With Type 2 Diabetes Mellitus Interested and Uninterested in the Use of a Patient Platform (e-VitaDM-2/ZODIAC-41). J. Diabetes Sci. Technol. 8(2), 230–237 (2014).
- Leibbrandt AJ, Kiefte-de Jong JC, Hogenelst MHE et al. Effects of the PRo-active Interdisciplinary Self-MAnagement (PRISMA, Dutch DESMOND) program on dietary intake in type 2 diabetes outpatients: A pilot study. Clin. Nutr.29(2), 199–205 (2010).
- Skinner TC, Carey ME, Cradock S et al. Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND): process modelling of pilot study. Patient Educ. Couns. 64, 369–377 (2006).
- Anderson RM, Funnell MM, Barr PA et al. Learning to empower patients: Results of professional education program for diabetes educators. Diabetes Care. 14(7), 584–590 (1991).
- Leventhal H, Nerenz DR, Steele DJ et al. Illness representation and coping with health threats. Handbook of Psychology andHealth. 219–252 (1984).
- Chaiken S, Wood W, Eagly A. Principles of persuasion. Social Psychology. 702–744 (1996).
- http://selfdeterminationtheory.org/authors/edward-deci/
- http://garfield.library.upenn.edu/classics1991/A1991GD62000001.pdf
- Bech P, Raabaaek Olsen L, Kjoller M et al. Measuring well-being rather than the absence of distress symptoms: a comparison of the SF-36 Mental Health subscale and the WHO-Five Well-Being Scale. Int. J. Methods Psychiatr. Res. 2, 85–91 (2006).
- Herdman M, Gudex C, Lloyd A et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life. Res. 20(10), 1727–1736 (2011).
- Rademakers J, Nijman J, Hoek van der L. Measuring patient activation in the Netherlands: translation and validation of the American short form Patient Activation Measure (PAM13). BMC. Public. Health.12(1), 577 (2012).
- Norman CD, Skinner HA. eHEALS: The eHealth Literacy Scale. J. Med. Internet Res. 8(4), e27 (2006).
- Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care.23(7), 943–50 (2000).
- Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new medication adherence rating scale MARS for the psychoses. Schizophr. Res. 42(3), 241–247 (2000).
- Maly RC, Frank JC, Marshall GN et al. Perceived Efficacy in Patient–Physician Interactions (PEPPI): Validation of an instrument in older persons. J. Am. Geriatr. Soc.7(1), 889–894 (1998).
- https://www.pw.nl/vaste-rubrieken/sfk/2014/2014pw46p11.pdf
- https://autonome-antifa.org/IMG/pdf/Rijndael.pdf
- Davies M, Heller S, Skinner T et al. Effectiveness of the diabetes education and self-management for on-going and newly diagnosed (DESMOND) programme for people with newly diagnosed type-2 diabetes: cluster randomised controlled trial. BMJ. 336(7642), 491–495 (2008).
- Deakin TA, Cade JE, Williams R et al. Structured patient education: the Diabetes X-PERT programme makes a difference. Diabetes Med. 23(9), 944–954 (2006).