Research Article - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 5
Treatment patterns and costs of patients with ankylosing spondylitis initiating biologic therapy in taiwan - a population-based analysis
- *Corresponding Author:
- Chia-Fang Lee
Eli Lilly and Company (Taiwan), Inc. Taipei, Taiwan
E-mail: lee_maggie@lilly.com
Abstract
Objective: To evaluate the real-world treatment patterns and costs of patients initiating their first biologic therapy for the treatment of ankylosing spondylitis (AS) in Taiwan.
Methods: Taiwan’s National Health Insurance claims data between 1/1/2014 and 12/31/2017 was used to identify and follow patients with AS initiating their first biologic therapy in 2015. Patients >= 18 years of age, with AS (ICD-9-CM: 720.0), a claim for a biologic therapy in 2015, continuous enrollment for at least one year following index, and no claims for biologics in the previous year were indexed into the study. A matched cohort of non-biologic patients with AS receiving anti-inflammatory drugs was derived. Patients were followed from their index date (first biologic claim) through the end of the study period, death, or they were lost to follow-up.
Results: There were 430 as patients included in the biologic-initiators cohort (adalimumab = 191; etanercept = 122; golimumab = 177, etc.). Pre-Index utilization rates were significantly higher for biologic-initiators compared to the matched cohort for corticosteroids, opioids, and csDMARDs (all p<0.0001). Utilization rates of NSAIDs and csDMARDs were lower for biologic initiators and higher for the matched cohort during the follow-up period compared to the pre-index period. Mean total healthcare costs were higher and average non-medication costs were lower for biologic-initiators compared to the matched cohort during the two years post-index.
Conclusions: Patients initiating their first biologic therapy for AS had high rates of as-related medication utilization during the pre-index period followed by a decrease in utilization rates after biologic initiation.
Keywords
asia-pacific • economics • epidemiology
Introduction
Ankylosing Spondylitis (AS), a type of spondylarthritis, is a chronic inflammatory disease mainly affecting axial skeleton [1]. Patients with AS experience inflammatory back pain that can be associated with other musculoskeletal and non-musculoskeletal manifestations, which commonly include enthesitis, dactylitis, psoriasis, inflammatory bowel disease, and uveitis [2]. Patients with AS also have a higher prevalence and incidence after diagnosis of comorbidities such as depression, cardiovascular disease, and osteoporosis compared to their non-AS counterparts [3].
The global prevalence of AS has been estimated to range between 0.1% and 1.4% with significant regional variations. A systematic literature review of prevalence studies reported prevalence per 10,000 persons of 31.9 in North America, 23.8 in Europe, and 16.7 in Asia. A study of the prevalence of AS in Taiwan reported 54,857 patients in December of 2010, representing a prevalence rate of 23.7 per 10,000 persons [4, 5].
Patients with AS also experience significant detriments in quality of life [6]. A survey of 265 AS patients in Taiwan reported poor or very poor health in 19.6% of patients, and a significant association between higher AS disease activity and poorer physical function, emotional well-being, and social participation [7]. A real-world study in the United States reported that AS significantly impacted their lifestyle with 59.5% of patients reporting at least some level of impact on career choice and 77.7% reported on impact on the amount of time they were able to work, and 69.7% reporting it impacted time with friends and family [8].
Patients with AS have higher healthcare costs and resource utilization compared to their non-AS counterparts [9, 10]. A claims database analysis in the United States reported AS patients had ten times higher all-cause median total healthcare costs compared to a matched cohort of non-AS patients [9]. Indirect costs are a significant portion of the economic burden of AS [6, 11, 12]. A literature review of the economic burden of AS in Europe reported indirect costs were 53.4% to 62% of total costs [13]. A systematic literature review and meta-analysis estimated an annual indirect cost per patient of $6,455 (range: $661 - $45,954) [14]. In China, AS was estimated to cause a 17% decrease in productivity [12].
As there is currently no cure available for AS, the goals of treatment are generally to alleviate symptoms, improve functioning, maintain the ability to work, decrease disease complications, and forestall skeletal damage as much as possible [15]. The 2019 American College of Rheumatology (ACR) guidelines recommend physical therapy and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) as first-line therapy in patients with active AS [15]. The European League Against Rheumatism (EULAR) and ACR guidelines both agree that the initiation of a biologic in the second-line is recommended for patients remaining uncontrolled despite the use of non-pharmacological management and NSAIDs [15, 16]. Within patients initiating a biologic, a tumour necrosis factor inhibitor (TNFi) is recommended as the first biologic, patients remaining uncontrolled with initial TNFi therapy may then move to a non-TNFi biologic such as tofacitinib, secukinumab, or ixekizumab [15, 16].
Currently approved biologics for AS by The Taiwan Food and Drug Administration (TFDA) include adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, secukinumab, and ixekizumab. The Taiwan Rheumatology Association (TRA) has a similar recommendation to ACR and EULAR as biologics should be considered in patients failing conventional therapies including NSAIDs and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) [17]. However, recommended first-line biologics are not limited to TNFis as is the case for ACR and EULAR. The TRA notes that biologics with alternative mechanisms of action (MOAs) such as secukinumab, an IL-17 inhibitor, in patients with a concern for tuberculosis or a hepatitis B reactivation with TNFis [17].
The objective of this study was to conduct an analysis of Taiwan’s National Health Insurance Research Database (NHIRD) to understand the differences in patient profile, treatment patterns, and cost of ankylosing spondylitis patients initiating biologic therapy compared to patients receiving NSAIDs in Taiwan.
Methods
Data source
This study utilized Taiwan’s National Health Insurance Research Database (NHIRD), a population-based claims database covering 99.9% of Taiwan’s 23 million residents [18]. The NHIRD contains longitudinal patient records tied to claims for reimbursement beginning in 2000. The latest year of data is released with a lag of two years, at the time of this analysis the latest data available was for 2017. This analysis utilized a subset of the full database including all patients in Taiwan with AS.
The NHIRD includes health insurance claims for all services and materials that are reimbursed. Basic demographic information such as age, gender, date of enrollment or withdrawal, income, and level of urbanization were available. Each of the claims in the dataset is accompanied by an ICD-9 (through 2015) or ICD-10 (2016 – present) diagnostic code, the amount (NT$) reimbursed, and the date of the inpatient visit, outpatient visit, or hospital admission/discharge. Pharmacy orders were also tracked and included the drug name, strength, dose, quantity, and date of dispensing.
Study design
The primary objective of this study was to evaluate the treatment patterns and cost of AS patients initiating their first biologic (biologic initiators) and compare with non-biologic AS patients matched to the biologic initiators. To accomplish this, a longitudinal retrospective study with propensity score matching was conducted.
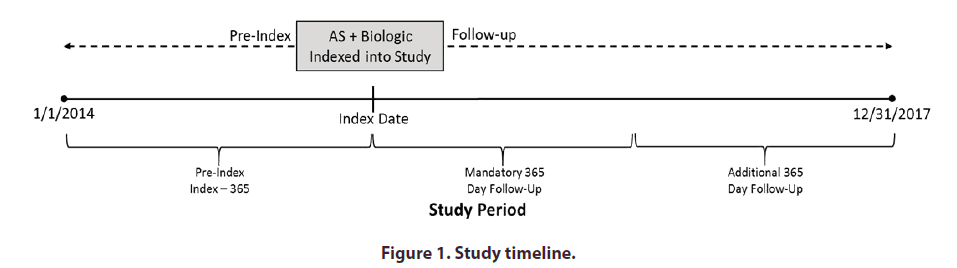
The study period ran from January 1, 2014, through December 31, 2017. A one-year index period from January 1, 2015, through December 31, 2015, was used to identify the cohorts included in the analysis (Figure 1). Patients in the biologic-initiators cohort were indexed into the study upon their first claim for a biologic during the index period. The index dates for patients in the matched cohort were the same as the patient they were matched with. The one-year pre-index period immediately prior to index date was used to evaluate inclusion/exclusion criteria and collect baseline characteristics. Patients were followed for a minimum of one year and up to a maximum of three years from index date through the end of the study period (December 31, 2017).
Population
There were two patient cohorts identified during the index period of the study: 1) AS patients initiating their first biologic (Biologic Initiators), and 2) a matched cohort of AS patients receiving NSAID therapy and no biologics during the study period matched to the biologic initiator cohort.
Biologic Initiators
Patients were indexed into the Biologic Initiators cohort on the day of their first biologic claim if the following conditions were met:
• (> & <) 2 primary or secondary healthcare claims for ankylosing spondylitis (ICD-9: 720.0 or ICD- 10: M45, M46.8, M46.9) in any setting, with the second claim coming within 90 days of the first claim during the index period
• (> & <) 1 claim for adalimumab (ATC=L04AB04), etanercept (ATC=L04AB01), or golimumab (ATC=L04AB06) during the index period
• No claim(s) for adalimumab (ATC=L04AB04), etanercept (ATC=L04AB01), or golimumab (ATC=L04AB06) during the one-year pre-index period
• (> & <) 18 years of age at index
• Continuous enrollment for one year of follow-up after index
Biologics reimbursed for ankylosing spondylitis in Taiwan during the index period (2015) included adalimumab, etanercept, and golimumab. Infliximab, secukinumab, and certolizumab were reimbursed during the follow-up period and were included in the analysis for evaluation of second-line biologics.
Matched cohort
The matched cohort was developed using a cohort of patients meeting the following criteria:
• ≥ 2 primary or secondary healthcare claims for ankylosing spondylitis (ICD-9: 720.0 or ICD- 10: M45, M46.8, M46.9) in any setting, with the second claim coming within 90 days of the first claim during the index period
• ≥ 1 claim for an NSAID (See Supplementary Table 1)
• No claim(s) for a biologic during the study period (January 1, 2014, and December 31, 2017)
• ≥ 18 years of age at index
• Continuous enrollment for one year of follow-up after index
Patients were matched using propensity scores generated by logistic regression modeling. The covariates included age, gender, and Charlson Comorbidity Index (CCI) scores. A greedy algorithm was used to match the cases to controls with no reconsideration for matching after a match is made. Cases were first matched to controls on eight digits of the propensity score. For cases without a match seven digits were used and so on until the lowest digit (one) of the propensity score was used.
As CCI was calculated at index and patients in the matched cohort assumed the same index date (date of first biologic claim for biologic-initiators) as the biologic-initiator they were indexed to, matching was conducted in two steps. First, patients were matched 1:16 (1 biologic-initiator to 16 controls). The CCI was then calculated for the matched patients using the index date of the biologic initiator they were matched to. Lastly, patients were matched 1:4 (1 biologic-initiator to 4 controls) based on CCI. In the event 1:4 matching cannot be accomplished after the initial 1:16 matching the highest number of possible controls will be used.
Measurement
Demographics including age, gender, and level of urbanization were included. Comorbidities measured in the analysis included those in the CCI [19]. Comorbidities included in a previously published database analysis for AS [3], and comorbidities of interest according to expert opinion in Taiwan. Diagnostic codes used to identify each of the included comorbidities can be found in Supplementary Table 2.
Medication classes measured included corticosteroids, muscle relaxants, opioids, NSAIDs, antidepressants, csDMARDs, topical analgesics, and sleeping aids. The medications examined within each class and the associated ATC codes can be found in Supplementary Table 1.
Biologic selection at index was measured for the Biologic Initiators cohort. During the index period adalimumab, golimumab, and etanercept were the only biologics approved and reimbursed in Taiwan for AS. However, infliximab, secukinumab, and certolizumab all received reimbursement during the follow-up period and were included in the analysis of switching patterns.
Healthcare costs included all medical costs reimbursed by the National Health Insurance Administration. Costs were categorized as medication and non-medication (all other healthcare costs), and further broken down into outpatient, inpatient, and emergency department (ED) costs.
Statistical analysis
Baseline demographics and clinical characteristics were calculated for both cohorts of all patients at index. Clinical characteristics including comorbidities were measured over the one-year pre-index period. Patients were classified as having a comorbidity if they had ≥ 1 in patient or ≥ 3 ambulatory claim(s) associated with a diagnostic code for the comorbidity during the pre-index period. ANOVA and Chi-square tests were used to test for significance in the prevalence of comorbidities between the Biologic Initiators and Matched cohorts.
Treatment utilization of AS-related medications was measured during the pre- and post-index periods for both the Biologic Initiators and Matched Cohorts. Patients with one or more claims during the measured time period were classified as having received the medication. The Chi-square test was used to test for statistical significance in the utilization of the included medications between the Biologic Initiators and Matched Cohorts in the pre- and post-index period. The relative change in utilization for each cohort between the pre- and post-index periods was also calculated.
In the biologic analysis, a descriptive analysis of switching patterns was performed. Patients were classified as switching to a second-line biologic if they had a claim for a biologic other than their index biologic during the follow-up period.
Healthcare costs were measured for the Biologic Initiators and Matched Cohorts for years one and two of follow-up, and a descriptive analysis was performed. Only patients with at least two years of continuous enrollment were included in the year two analysis. Allcause costs were measured and segmented by category (inpatient, outpatient, and emergency department) and type (medication and non-medication).
Results
Patient characteristics
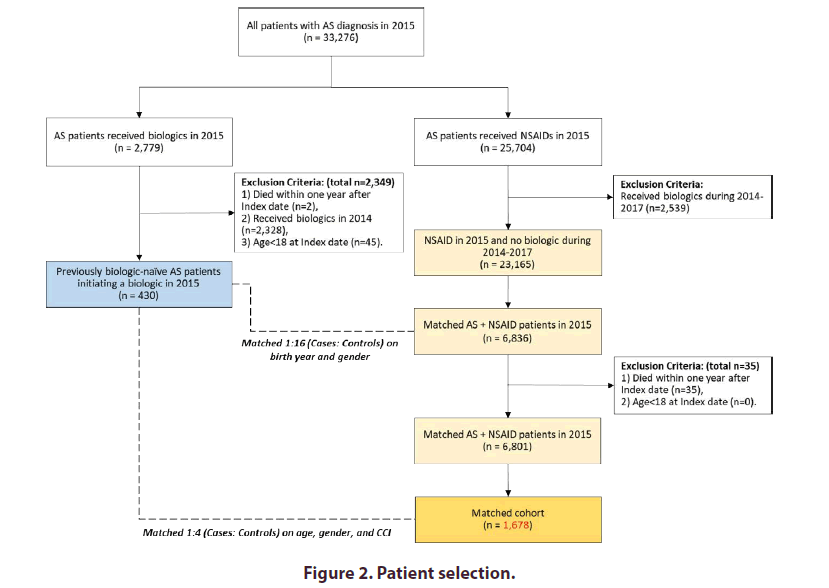
A total of 33,276 patients had an AS diagnosis in 2015. Of these patients 2,779 (8.4%) had a claim for an included biologic and 430 (15.5%) of these patients were initiating their first biologic for AS. Biologics at initiation included adalimumab (n=191; 44.4%), etanercept (n=122; 28.4%), and golimumab (n=117; 27.2%). A matched cohort of 1,678 patients was derived from a cohort of AS patients with a claim for an NSAID in 2015, and no claims for a biologic between 2014 and 2017 (Figure 2).
Patients demographics and clinical characteristics for the biologic initiators and the matched cohort at index are shown in Table 1. The two cohorts were well balanced with no significant differences between age, gender, and CCI. There were statistically significant differences in the prevalence of spondyloarthritis clinical manifestations including inflammatory bowel disease (IBD) (p<0.0001), uveitis (p<0.0001), and psoriasis (p<0.0001) as well as comorbidities including coronary artery disease (p=0.0193), hypertension (p<0.0001), peripheral vascular disease (p=0.0208), asthma (p=0.0209), and rheumatoid arthritis (p<0.0001) (Table 1).
| Characteristics | Biologic Initiators | Matched Cohort1 | P-Value | ||
|---|---|---|---|---|---|
| (n=430) | (n=1,678) | ||||
| N | % | N | % | ||
| Age | 0.6861 | ||||
| Mean (SD) | 41.9 | 14.1 | 41.6 | 13.9 | |
| Median (IQR) | 41 | 29.8-53.7 | 41.5 | 30.3-53.3 | |
| 18.0 - 20.5 | 21 | 4.9 | 84 | 5 | 0.9938 |
| 20.6 - 24.5 | 26 | 6 | 104 | 6.2 | |
| 24.6 - 30.5 | 63 | 14.7 | 252 | 15 | |
| 30.6 - 41.5 | 111 | 25.8 | 432 | 25.7 | |
| 41.6 - 53.5 | 100 | 23.3 | 395 | 23.5 | |
| 53.6 - 61.5 | 64 | 14.9 | 253 | 15.1 | |
| 61.6 - 64.5 | 23 | 5.3 | 89 | 5.3 | |
| 64.6 - 71.5 | 19 | 4.4 | 64 | 3.8 | |
| >71.6 | 3 | 0.7 | 5 | 0.3 | |
| Gender, Male | 310 | 72.1 | 1216 | 72.5 | 0.8769 |
| CCI (mean, SD) | 0.8 | 1.3 | 0.7 | 1.1 | 0.2706 |
| Cardiovascular | 108 | 25.1 | 278 | 16.6 | <0.0001 |
| Angina | 10 | 2.3 | 25 | 1.5 | 0.2263 |
| Atherosclerosis | 14 | 3.3 | 35 | 2.1 | 0.1508 |
| Cerebrovascular disease/Stroke | 7 | 1.6 | 48 | 2.9 | 0.1525 |
| Coronary Artery Disease | 21 | 4.9 | 45 | 2.7 | 0.0193 |
| Hypertension | 97 | 22.6 | 242 | 14.4 | <0.0001 |
| Myocardial Infarction | 3 | 0.7 | 4 | 0.2 | 0.1546 |
| Peripheral Vascular Disease (PVD) | 6 | 1.4 | 7 | 0.4 | 0.0208 |
| Venous Thromboembolism (VTE) | 3 | 0.7 | 4 | 0.2 | 0.1546 |
| GI Disorder | 97 | 22.6 | 313 | 18.7 | 0.068 |
| Inflammatory Bowel Disease (IBD) | 10 | 2.3 | 7 | 0.4 | <0.0001 |
| Peptic Ulcer Disease | 94 | 21.9 | 310 | 18.5 | 0.1115 |
| Malignancies | 7 | 1.6 | 32 | 1.9 | 0.7016 |
| Metabolic Syndrome | 73 | 17 | 244 | 14.5 | 0.2074 |
| Diabetes | 33 | 7.7 | 142 | 8.5 | 0.5972 |
| Dyslipidemia | 52 | 12.1 | 191 | 11.4 | 0.6807 |
| Neurologic / Psychologic Conditions | 2 | 0.5 | 1 | 0.1 | 0.1077 |
| Multiple Sclerosis | 0 | 0 | 0 | 0 | ----- |
| Parkinson Disease | 2 | 0.5 | 1 | 0.1 | 0.1077 |
| Respiratory Diseases | 11 | 2.6 | 81 | 4.8 | 0.0399 |
| Asthma | 8 | 1.9 | 71 | 4.2 | 0.0209 |
| Sleep Apnea | 3 | 0.7 | 12 | 0.7 | 0.9693 |
| Other Diseases | |||||
| Rheumatoid Arthritis | 45 | 10.5 | 88 | 5.2 | <0.0001 |
| Osteoporosis | 13 | 3 | 27 | 1.6 | 0.0552 |
| Uveitis | 59 | 13.7 | 69 | 4.1 | < 0.0001 |
| Psoriasis | 30 | 7 | 12 | 0.7 | <0.0001 |
Notes: CCI = Charlson Comorbidity Index
1Patients were matched on age, gender, and CCI score
Table 1. Baseline patient demographics and clinical characteristics.
Treatment utilization
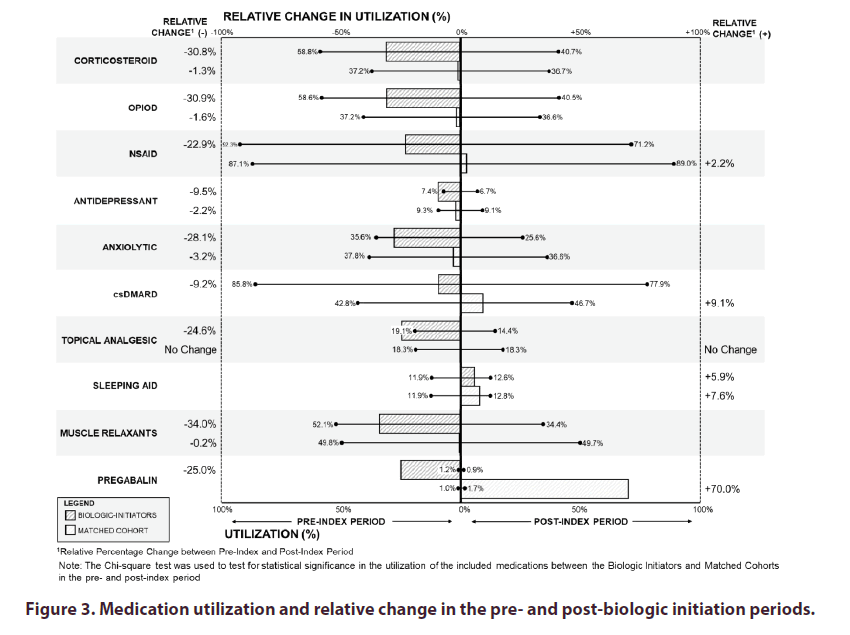
During the pre-index period biologic initiators had significantly higher utilization rates of corticosteroids (58.8% vs. 37.2%; p<0.0001), opioids (58.6% vs. 37.2%; p<0.0001), and csDMARDs (85.8% vs. 42.8%; p<0.0001) compared to the matched cohort (Figure 3). Biologic initiators also had non-significantly higher absolute utilization rates compared to the matched cohort for all medication classes including NSAIDs (92.3% vs. 87.1%), topical analgesics (19.1% vs. 18.3%), muscle relaxants (52.1% vs. 49.8%), and pregabalin (1.2% vs. 1.0%).
Relative reductions in medication usage between the pre- and post-index periods was generally higher for the biologic-initiators cohort than the matched cohort. The only increase in medication utilization for biologic-initiators was for sleep aids (+5.9%), compared to the matched cohort, which saw relative rates of utilization increase in NSAIDs (+2.2%), csDMARDs (+9.1%), sleep aids (+7.6%), and pregabalin (+70.0%). Relative reductions in medication utilization for biologic-initiators were found in muscle relaxants (-34.0%), opioids (-30.9%), corticosteroids (-30.8%), anxiolytics (-28.1%), pregabalin (-25.0%), topical analgesics (-24.6%), NSAIDs (-22.9%), csDMARDs (-9.2%), and antidepressants (-9.5%).
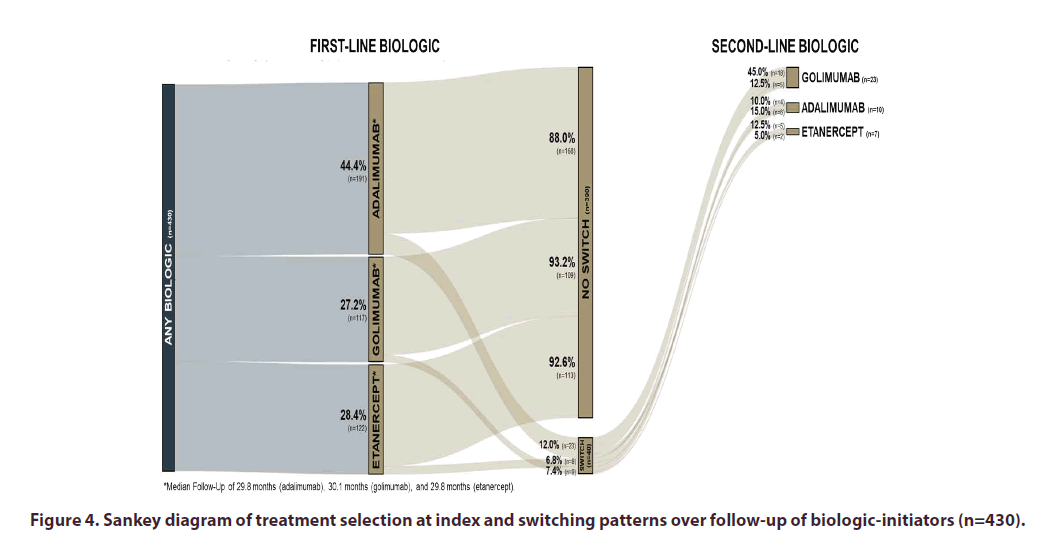
Biologic Switching
The median follow-up time of the biologic initiators was 29.9 months. The median length of follow-up did not significantly vary between first-line biologics with 29.8 months for adalimumab and etanercept, and 30.1 months for golimumab. Over the follow-up period, there were 40 (9.3%) patients switching to a second-line biologic. Switching patterns between biologics are described in the Sankey diagram (Figure 4). Patients initiating adalimumab were most likely to switch (12.0%; 23/191), followed by etanercept (7.4%; 9/122), and golimumab (6.8%; 8/117) during the follow-up period. The most utilized second-line therapy after switching was golimumab (57.5%; 23/40), followed by adalimumab (25.0%; 10/40), and etanercept (17.5%; 7/40).
Cost
Average mean total healthcare costs were higher for the biologic-initiators cohort (NT$579,444 and NT$571,244) compared to the matched cohort (NT$75,327 and NT$71,619) in both the first and second years of follow-up (Table 2). The incremental cost between the cohorts was driven by additional drug costs for the biologic-initiators cohort. Average mean non-medication costs were lower for the biologic-initiators cohort in both years of follow-up. Non-drug costs decreased by 15.2% (NT$8,179), from NT$53,885 to NT$45,707 in the second year of follow-up compared to a 4.3% (NT$2,446) decrease from NT$56,893 to NT$54.447 for the matched cohort.
| Cost (Nt$) | Biologic-Naive | Matched Cohort | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YEAR 1 | YEAR 2 | YEAR 1 | YEAR 2 | ||||||||||||||||
| (n=430) | (n=427) | (n=1,678) | (n=1,671) | ||||||||||||||||
| MEAN | SD | MEDIAN | IQR | MEAN | SD | MEDIAN | IQR | MEAN | SD | MEDIAN | IQR | MEAN | SD | MEDIAN | IQR | ||||
| Inpatient Costs | |||||||||||||||||||
| Medication | 3,993.80 | 31,749.40 | - | 0-0 | 2,953.80 | 24,332.70 | - | 0 - 0 | 2,800.30 | 29,310.10 | - | 0 - 0 | 1,729.20 | 11,694.10 | - | 0 - 0 | |||
| Non-Medication | 20,134.10 | 113,039.60 | - | 0-0 | 15,200.20 | 65,393.50 | - | 0 - 0 | 23,230.50 | 109,211.90 | - | 0 - 0 | 20,806.30 | 78,434.80 | - | 0 - 0 | |||
| Total Inpatient Costs | 24,128.00 | 138,208.00 | - | 0-0 | 18,153.90 | 80,088.30 | - | 0 - 0 | 26,030.70 | 133,812.70 | - | 0 - 0 | 22,535.50 | 87,316.00 | - | 0 - 0 | |||
| Outpatient Costs | |||||||||||||||||||
| Medication | 521,475.90 | 172,750.00 | 529,447.50 | 415,929–645,511 | 522,513.80 | 172,380.20 | 536,250.00 | 415,929 – 648,506 | 15,523.50 | 39,020.90 | 6,614.00 | 2,271 – 14,594 | 15,336.70 | 38,555.50 | 6,538.00 | 2,265 – 14,536 | |||
| Non-Medication | 32,594.70 | 48,518.20 | 23,151.50 | 16,932–36,189 | 29,664.70 | 21,338.30 | 23,018.00 | 16,926 – 36,189 | 31,781.70 | 55,414.40 | 20,584.00 | 11,961 – 35,542 | 31,783.30 | 55,488.80 | 20,593.00 | 11,961 – 35,575 | |||
| Total Outpatient Costs | 554,070.60 | 180,811.80 | 570,649.00 | 446,083–679,173 | 552,178.50 | 177,513.30 | 570,418.00 | 443,177 – 678,699 | 47,305.20 | 72,367.10 | 29,583.00 | 16,407 – 52,943 | 47,120.00 | 72,204.50 | 29,549.00 | 16,404 – 52,833 | |||
| ER Costs | |||||||||||||||||||
| Medication | 88.7 | 426.6 | - | 0-0 | 69.7 | 332.9 | - | 0 - 0 | 110.2 | 603.4 | - | 0 – 1,499 | 105.5 | 575.3 | - | 0 - 72 | |||
| Non-Medication | 1,156.50 | 5,176.80 | - | 0-0 | 841.8 | 2,458.80 | - | 0 - 0 | 1,881.10 | 5,716.70 | - | 0 - 73 | 1,857.80 | 5,704.20 | - | 0 – 1,403 | |||
| Total ER Costs | 1,245.20 | 5,495.10 | - | 0-0 | 911.5 | 2,657.30 | - | 0 - 0 | 1,991.30 | 6,017.80 | - | 0 – 1,412 | 1,963.40 | 5,991.40 | - | 0 – 1,489 | |||
| Total Costs | |||||||||||||||||||
| Medication | 525,558.50 | 172,269.40 | 543,485.50 | 419,344–649,572 | 525,537.30 | 172,795.30 | 543,313.00 | 415,929 – 650,620 | 18,434.00 | 50,619.40 | 7,012.50 | 2,536 – 16,312 | 17,171.40 | 41,107.20 | 6,978.00 | 2,506 – 16,277 | |||
| Non-Medication | 53,885.30 | 148,702.20 | 25,149.50 | 17,324–43,915 | 45,706.60 | 75,296.60 | 24,797.00 | 17,234 – 43,346 | 56,893.30 | 136,371.40 | 24,930.50 | 13,479 – 53,172 | 54,447.40 | 111,897.60 | 24,868.00 | 13,457 – 53,051 | |||
| Total Costs | 579,443.80 | 233,906.70 | 576,872.50 | 450,579–70,5261 | 571,243.90 | 194,007.10 | 576,741.00 | 448,664 – 704,487 | 75,327.20 | 167,513.60 | 35,260.00 | 18,920 – 71,446 | 71,618.80 | 130,881.30 | 35,117.00 | 18,829 – 71,228 | |||
Notes: Costs in NT$
ER: Emergency Room; IQR: Interquartile Range; SD: Standard Deviation
Table 2. Healthcare Costs (NT$) during the follow-up period.
Discussion
We evaluated the treatment patterns and costs of AS patients in Taiwan initiating their first biologic and a matched cohort of AS patients receiving NSAIDs without a biologic for a minimum of one year. There were significantly higher rates of IBD (p<0.0001), uveitis (p<0.0001), and psoriasis (p<0.0001) in the cohort of patients initiating their first biologic compared to the matched cohort, which reflect the heterogeneity of axial spondyloarthritis conditions. Baseline utilization rates for corticosteroids, opioids, NSAIDs, muscle relaxants, and csDMARDs were all numerically higher for biologic-initiators than the matched cohort during the pre-index period. The heightened presence of baseline axial spondyloarthritis burden and pre-index medication utilization may have been contributing factors to the selection of a biologic for AS treatment.
This study showed a higher rate of rheumatoid arthritis diagnosis (10.5%) within the cohort of AS patients initiating their first biologic compared to the matched cohort (5.2%). The high rate of rheumatoid arthritis with AS patients observed in our study is consistent with other published analyses of claims-based datasets in the Asia-Pacific region including another study in Taiwan (4.98% among all AS patients) and Japan (53.9% among biologic DMARD patients) [19, 20]. However, the coexistence of rheumatoid arthritis and AS in clinical practice is a relatively rare occurrence [21]. Rationales for the high RA prevalence rates in these claims analyses may include the use of rheumatoid arthritis diagnostic codes during the diagnostic process of AS or the presence of peripheral arthritis that is being misclassified.
An analysis of the hospital-based Medical Data Vision database in Japan also reported medication utilization during a 12-month follow-up period for AS biologic-initiators [22]. Utilization rates during the 12-month follow-up period for the biologic-initiators of the measured classes of medication higher for opioids (40.5% vs. 5.2%) and csDMARDs (77.9% vs. 66.1%) but lower for antidepressants (6.7% vs. 11.3%), anxiolytics (25.6% vs. 30.4%), and corticosteroids (40.7% vs. 52.2%) in our study compared to the recent study in Japan [21]. However, it should be noted that the study in Japan utilized a hospital-based database and may not be representative of the general AS population, which is observed in our analysis.
Of the patients initiating their first biologic for AS, 9.3% (n=40) switched to another TNFi during the follow-up period (median of 29.9 months) in this study. This rate of switching is consistent with other realworld studies evaluating treatment patterns of first-time biologic initiators in AS [23, 24]. A study by Lindstrom et al. of 2,590 AS patients starting their first TNFi in Sweden between 2006 – 2015 reported 13% of patients were on their second TNFi at the end of their first year on therapy [25]. Palmer et al. reported 8.9% of TNFi initiators switched to another biologic within a 3-year follow-up period [26]. A claims-based analysis in the United States reported a slightly higher percentage (26.1%) of switching for first-time TNFi users for AS to a second TNFi during the two-year follow-up period [27].
Patients receiving biologic therapy in this study had higher average total costs compared to the matched cohort of non-biologic patients. However, non-medication costs were similar between the two cohorts with average costs of NT$53,885 and NT$56,893 in the biologic and matched non-biologic cohorts, respectively. Furthermore, non-medication costs were reduced by 15.2% (NT$8,179) in the biologic cohort compared to 4.3% (NT$2,446) in the matched non-biologic cohort when comparing year one and year two of the follow-up period. The reduction in non-medication costs could be attributed to the effectiveness of biologics [28]. Comparison of costs across studies in different settings is difficult due to different health systems and pricing. However, most economic analyses of AS patients receiving biologics have reported that biologics are a high percentage of total healthcare costs [29].
The results of this study can be considered by decision-makers and stakeholders in Taiwan to understand the treatment patterns, cost, and unmet needs associated with TNFi biologics in Taiwan. Compared to the matched cohort, patients initiating a biologic experienced a greater decline in medication (corticosteroid, opioid, NSAID, antidepressant, anxiolytic, csDMARD, topical analgesic, muscle relaxants, and pregabalin) utilization after index, and a greater reduction in non-medication costs between year one and two of follow-up. These changes show the value of biologic therapy in lowering the medication utilization and non-medication cost burden. However, 9% of patients initiating their first biologic had to switch to another biologic during the follow-up period. Furthermore, during the time of this study patients were limited in their selection of biologics during the index date to only TNFis, and the only alternative-MOA biologic, secukinumab, only became available towards the tail-end of the follow-up period. As the TRA notes in their recommendations if there is intolerance and/or toxicity to the initial TNFi it is recommended for patients to try an alternative MOA [24]. As there was no alternative MOA available for the majority of this study’s timeline there may have been an even higher percentage of patients that would have benefited from switching to an alternative MOA. This suggests an unmet need for biologics with new mechanisms of action and/or better optimization of the first choice of biologic [24].
This study has several limitations. This was a non-randomized retrospective analysis that relied on propensity score matching of the limited clinical information available in the claims database and was subject to the potential of unmeasured confounding. The propensity score matching included age, gender, and CCI, but did not include the musculoskeletal and non-musculoskeletal manifestations and comorbidities included in this analysis, which could have accounted for differences between the cohorts. AS-related comorbidities were not controlled for to allow for differences in disease severity between the two cohorts. As it is a claims-based database analysis only limited clinical information such as diagnostic codes are available, clinical information that could have been used to evaluate the effectiveness of biologic and non-biologic treatment was not included. Thus, no inference as to the reason for treatment switching, discontinuation, or augmentation can be made. The database also only contains data for direct healthcare costs (reimbursed materials and services) and does not include indirect costs such as those related to absenteeism and presenteeism, which are important factors for consideration in ankylosing spondylitis. Second, ankylosing spondylitis is often subject to misdiagnosis and without clinical information validation of the ICD-code algorithm used to identify AS in this analysis cannot be trusted as fully accurate [28]. Lastly, the current treatment paradigm for biologic therapy of AS patients in Taiwan is likely different than in 2015 due to the reimbursement of new biologics with different mechanisms of action [29, 30].
Conclusions
Patients initiating their first biologic therapy experienced large declines in the utilization of other AS-related medications and non-medication costs during their first year of therapy compared to a matched cohort of AS patients receiving NSAID therapy without biologic use. The rate of switching to a second-line biologic may suggest the need for additional biologic options at initiation and improvements in initial treatment selection.
Funding
This study was funded by Eli Lilly & Co.
Disclosures
Bruce CM Wang and Wesley Furnback are paid consultants to Eli Lilly & Co. Ching-Yun Wei and Chia-Fang Lee are employed by Eli Lilly & Co. Masayo Sato was employed by Eli Lilly & Co. at the time of this study.
References
- Taurog Joel D, Avneesh Chhabra, Robert A. Colbert. Ankylosing spondylitis and axial spondyloarthritis. N. Engl. J. Med. 374.26 (2016): 2563–2574.
- Janneke J de Winter, Leonieke J van Mens, Désirée van der Heijde et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis. Res. Ther. 18(1), 1–11 (2016).
- Jessica A Walsh, Xue Song, Gilwan Kim et al. Evaluation of the comorbidity burden in patients with ankylosing spondylitis using a large US administrative claims data set. Clin. Rheumatol. 37(7), 1869–1878 (2018a).
- Hsieh M-Y, C-F Kuo. FRI0428 Epidemiology of Ankylosing Spondylitis in Taiwan: A Nationwide Population Study. 590–591 (2016).
- Department of Household Registration Affairs, MOI.
- T Y Zhu, L-S Tam, V W-Y Lee et al. Costs and quality of life of patients with ankylosing spondylitis in Hong Kong. Rheumatology. 47(9), 1422–1425 (2008).
- Ming-Chi Lu, Kuang-Yung Huang, Chien-Hsueh Tung et al. Factors associated with disease-specific quality of life in Taiwanese patients with ankylosing spondylitis: a crosssectional study. BMJ. open. 9(6), e028966 (2019).
- James T Rosenbaum, Lisa Pisenti, Yujin Park et al. Insight into the quality of life of patients with ankylosing spondylitis: real-world data from a US-based life impact survey. Rheumatol. Ther. 6(3), 353–367 (2019).
- Jessica A Walsh, Xue Song, Gilwan Kim et al. Healthcare utilization and direct costs in patients with ankylosing spondylitis using a large US administrative claims database. Rheumatol. Ther. 5(2), 463–474 (2018b).
- Klaus Krüger, Ulrich von Hinüber, Florian Meier et al. Ankylosing spondylitis causes high burden to patients and the healthcare system: results from a German claims database analysis. Rheumatol. Int. 38(11), 2121–2131 (2018).
- Rachid Rafia, Roberta Ara, Jon Packham et al. Healthcare costs and productivity losses directly attributable to ankylosing spondylitis. Clin. Exp. Rheumatol. 30(2), 246 (2012).
- Liudan Tu, Jayanti Chamling Rai, Shuangyan Cao et al. Costs and work limitation of patients with ankylosing spondylitis in China. Clin. Exp. Rheumatol. 32(5) (2014): 661–666.
- Blanch C. Economic burden of ankylosing spondylitis in Europe. A systematic review of the literature. Value. Health. 19(7), A541–A542 (2016).
- Malinowski Krzysztof Piotr, Paweł Kawalec. The indirect costs of ankylosing spondylitis: a systematic review and meta-analysis. Expert. Rev. Pharmacoecon. Outcomes. Res. 15(2), 285–300 (2015).
- Michael M Ward, Atul Deodhar, Lianne S Gensler et al. 2019 Update of the American College of Rheumatology/ Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis. Care. Res. (Hoboken). 71(10), 1285–1299 (2019).
- Désirée van der Heijde, Sofia Ramiro, Robert Landewé et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76(6), 978–991 (2017).
- James Cheng-Chung Wei, Chin-Hsiu Liu, Jui- Cheng Tseng et al. Taiwan Rheumatology Association consensus recommendations for the management of axial spondyloarthritis. Int. J. Rheum. Dis. 23(1), 7–23 (2020).
- Cheng-Yang Hsieh, Chien-Chou Su, Shih-Chieh Shao et al. Taiwan’s National Health Insurance Research Database: past and future. Clin. Epidemiol. 11, 349 (2019).
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45(6), 613–619 (1992).
- Shuo-Yan Gau, Yung-Heng Lee, Hsi-Kai Tsou et al. Patients with ankylosing spondylitis are associated with high risk of fibromyalgia: A nationwide population-based cohort study. Front. Med. 8, (2021).
- Tetsuya Tomita, Masayo Sato, Elizabeth Esterberg et al. Treatment Patterns and Health Care Resource Utilization Among Japanese Patients With Ankylosing Spondylitis: a Hospital Claims Database Analysis. Mod. Rheumatol. 1– 23 (2020).
- Tacjana Anna Barczyńska, Małgorzata Węgierska, Paweł Żuchowski et al. Coexistence of rheumatoid arthritis and ankylosing spondylitis. Reumatologia 53(5), 279 (2015).
- Ulf Lindström, Tor Olofsson, Sara Wedrén et al. Biological treatment of ankylosing spondylitis: a nationwide study of treatment trajectories on a patient level in clinical practice. Arthritis. Res. Ther. 21(1), 128 (2019).
- Palmer JB. Treatment patterns and costs for ant-TNF alpha therapy in patients with ankylosing spondylitis. Rheumatology. 2161–1149 (2015).
- Theresa Hunter, Krista Schroeder, David Sandoval et al. Persistence, discontinuation, and switching patterns of newly initiated TNF inhibitor therapy in ankylosing spondylitis patients in the United States. Rheumatol. Ther. 6(2), 207–215 (2019).
- Linda E Dean, Gareth T Jones, Alan G MacDonald et al. Global prevalence of ankylosing spondylitis. Rheumatology. 53(4), 650–657 (2014).
- El Maghraoui, Abdellah. Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur. J. Intern. Med. 22(6), 554– 560 (2011).
- Qiang Shi, Ko-Jen Li, Tamas Treuer et al. Estimating the response and economic burden of rheumatoid arthritis patients treated with biologic disease-modifying antirheumatic drugs in Taiwan using the National Health Insurance Research Database (NHIRD). PloS. One. 13(4) (2018).
- Josef S Smolen, Monika Schöls, Jürgen Braun et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann. Rheum. Dis. 77(1), 3–17 (2018).
- Jessica A Walsh, Xue Song, Gilwan Kim et al. Evaluation of the comorbidity burden in patients with ankylosing spondylitis treated with tumour necrosis factor inhibitors using a large administrative claims data set. J. Pharm. Health. Serv. Res. 9(2), 115–121 (2018c).