Research Article - Interventional Cardiology (2012) Volume 4, Issue 1
Timing of coronary collateral appearance during ST-elevation myocardial infarction
- Corresponding Author:
- Diederick E Grobbee
Julius Center for Health Sciences & Primary Care
University Medical Center Utrecht, The Netherlands
E-mail: d.e.grobbee@umcutrecht.nl
Abstract
Keywords
collateral circulation, myocardial infarction, preinfarction angina
Coronary collateral circulation offers a potentially important alternative source of blood supply during coronary occlusion. The angiographic appearance of collateral arteries during the early hours of acute myocardial infarction (MI), has been shown to reduce the size of infarction, promote microvascular perfusion and improve hemodynamic condition [1].
It has been shown in an animal experimental study that, during acute coronary occlusion, early reperfusion prevents or limits necrosis of jeopardized myocardium [2]. The earlier presence of collateral flow can result in smaller infarct size [3]. To our knowledge, the timing of the appearance of coronary collateral circulation during coronary occlusion in ST-elevation MI (STEMI), has not yet been studied. Results from a previous report have suggested that filling of collateral vessels increases acutely within 90 s during balloon occlusion and disappears soon after balloon deflation [4]. Another study showed that coronary collaterals might appear during the early hours of MI without, however, demonstrating the temporal progression of collateral appearance [5].
The timing of the angiographic appearance of coronary collaterals in patients with STEMI was studied. Clinical factors potentially associated with the early appearance of collaterals during acute coronary occlusion were also examined.
Patients and methods
Data was used from a cross-sectional study, in which each subject was seen at a particular point in time. Using subjects examined at different time intervals following MI, the data was used to investigate changes over time [6]. The angiographic appearance of coronary collaterals was assessed once for each patient, during coronary angiography soon after the MI. The angiographic assessment from the patients who have varying onset of MI, comprise the data for the evaluation of timing of the coronary collaterals appearance. This study design was chosen for ethical reasons.
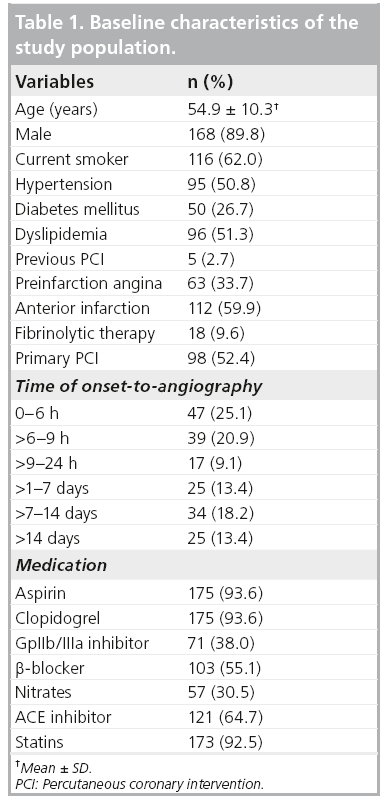
Using the October 2006 to October 2008 database of the National Cardiovascular Center (Harapan Kita Hospital, Jakarta, Indonesia), we randomly identified patients with STEMI who presented at the hospital within 14 days of onset of symptoms and underwent subsequent angiography. Patients were diagnosed with STEMI if they had chest pain of more than 30 min duration and electrocardiographic changes with ST-segment elevation >2 mm in at least two precordial leads or >1 mm in the limb-leads. Inclusion criteria were patients with a thrombolysis in MI flow grade of 0 or 1 in the culprit coronary artery. Primary percutaneous coronary interventions were performed in patients who presented within 12 h of the onset of symptoms. All patients received optimal drug treatment, including aspirin, clopidogrel, nitrates, ACE inhibitors, b-blockers and statins where appropriate. Exclusion criteria included a previous coronary bypass surgery, left main stenosis and a nonculprit lesion with a stenosis of more than 80% diameter.
Time-of-onset of MI was carefully determined by the occurrence of ischemic symptoms, defined as severe chest pain or chest discomfort lasting for more than 30 min. The discomfort was defined as sensations of constriction, crushing, oppression, compression or feelings of a heavy weight or squeezing in the chest. Patients whose onset of infarct-related pain could not be defined were excluded.
Preinfarction angina was defined as new-onset chest pain or chest pain with increasing intensity or frequency within 72 h before the onset of MI. The presence of diabetes mellitus (DM) was based on self-reported use of DM medication, or a previous record of a fasting plasma glucose concentration ≥126 mg/dl (7 mmol/l) or a nonfasting serum glucose >200 mg/dl (11 mmol/l). Hypertension was based on selfreported use of antihypertensive medication or a previous record of a systolic blood pressure >140 mmHg and/or a diastolic blood pressure >90 mmHg. Dyslipidemia was defined as a total cholesterol >240 mg/dl, or self-reported use of lipid-lowering medication before admission. The study protocol was reviewed and approved by the ethical committee of the hospital.
▪ Collaterals assessment
Angiograms were stored digitally in Digital Imaging and Communications in Medicine format (512 × 512 matrices). The criteria for the appropriateness of the angiograms to be reviewed were:
▪ Angiography was performed until contrast filling of the coronary veins;
▪ A minimum of two orthogonal views.
Patients whose angiograms could not be adequately evaluated were excluded.
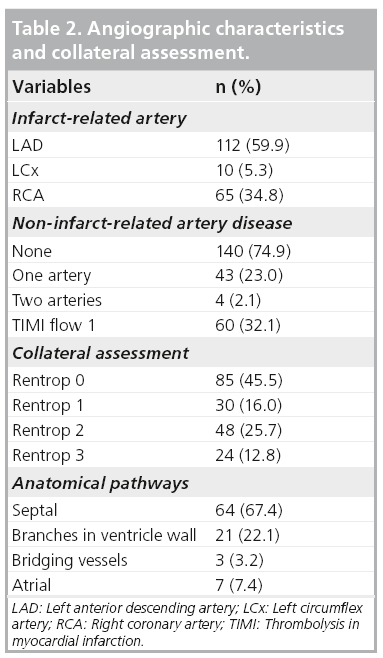
Coronary collaterals were graded by the degree of filling of the vessel beyond the stenosis according to the Rentrop classification:
▪ Grade 0: no visible filling;
▪ Grade 1: contrast medium passes through collateral channels but fails to opacify the epicardial vessel at any time;
▪ Grade 2: contrast material enters but fails to opacify the target epicardial vessel completely;
▪ Grade 3: contrast material enters and completely opacifies the target epicardial vessel [4].
Angiograms were assessed by a senior resident in training and an interventional cardiologist. In the event of a difference in interpretation, a third opinion was solicited. A random assessment of inter-observer variance showed high agreement between the researchers (k = 0.87; p < 0.001). Collateral grading was performed blind from the time of onset of MI.
▪ Statistical analysis
To determine the appearance of well-developed collaterals, we defined the presence of collaterals as those with a Rentrop flow grade 2 and 3. Differences between groups were assessed using chi-square for categorical variables; differences in continuous variables were evaluated using Student’s t-test. A two sided P value ≤0.05 was considered statistically significant. Statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
To analyze the temporal appearance of coronary collaterals, subjects were grouped according to time units. The time intervals were determined arbitrarily. To test the time relationship of collateral appearance, we used a Kendall’s t-b test.
To assess the clinical factors associated with the early appearance of collaterals, we selected a subgroup of patients who presented within 12 h of the onset of MI. Using logistic regression analyses, we examined preinfarction angina, hypertension, smoking, DM, dyslipidemia and age as potential determinants of collateral appearance. These clinical factors were selected on the recent insight into the potential determinant of coronary collateral presence [7–9]. Logistic regression analysis was used for statistical analysis in this subgroup.
Results
A total of 187 patients were included in this study. The baseline characteristics of the study population are shown in Table 1. It was found that 60% of subjects presented with anterior MI. Approximately 10% of subjects had received fibrinolytic therapy before the angiographic procedure. 60% of patients presented within 12 h. The duration from onset of MI to angiography ranged from 1.6 to 557 h (23 days), with a median of 9.9 h. The prevalence of preinfarction angina in the group of onset-to-angiography of 0–6 h, >6–9 h, >9–24 h, >1–7 days, >7–14 days, >14 days are 43, 44, 40, 56, 64 and 60%, respectively (p = 0.618).
Collateral connections were mostly found for the right coronary artery (RCA) with filling to the left anterior descending (LAD) artery (56%) and for LAD filling to the RCA (23%; Table 2). Most anastomoses were found via the septal connection. The frequency of collateral presence for the LAD, left circumflex and RCA was 37, 50 and 40%, respectively (p = 0.67).
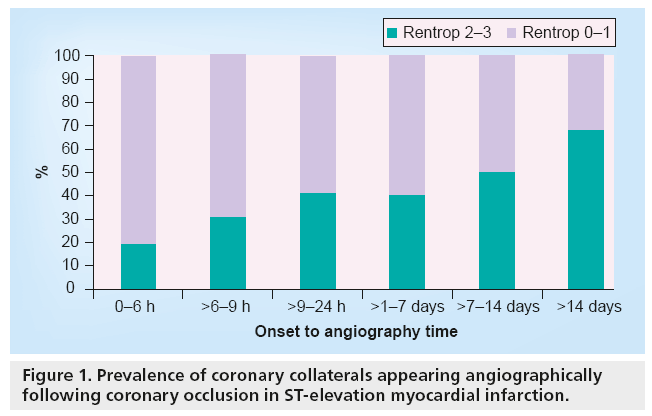
Figure 1 reveals that the presence of collaterals increased significantly with increasing time (p for trend < 0.001). In subjects presenting within 6 h of onset, the presence of collaterals was 19%. This increased to 31, 41, 40, 50 and 68% for assessments at 6–9 h, 9–24 h, 1–7 days, 7–14 days and >14 days, respectively.
▪ Determinants of collateral presence
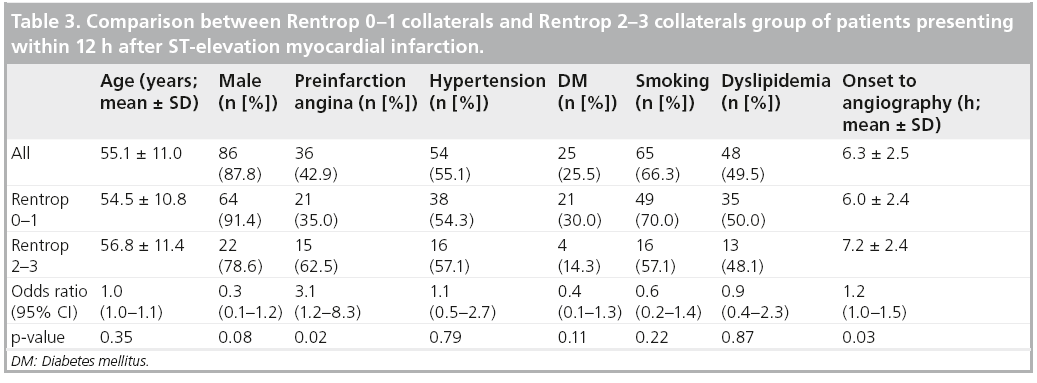
Determinants of collateral presence were examined in 98 subjects who underwent angiography within 12 h of onset of MI (mean duration from onset-to-angiography 6.3 ± 2.5 h). Table 3 compares subjects with and without collateral presence. Subjects with preinfarction angina more often had collaterals. In addition, preinfarction angina was shown to be an independent determinant of collateral presence, adjusted for time between onset of symptoms and angiography (odds ratio 3.1; 95% CI: 1.2–8.6).
The relationship between the presence of preinfarction angina and the presence of collaterals was then examined according to two different categories of the time between onset of symptoms and angiography: 0–6 h (n = 46) and 6–12 h (n = 52). The likelihood of the presence of collaterals following preinfarction angina was higher in the first 6 h after onset of symptoms than in the later 6-h period (odds ratio 7.7; 95% CI: 1.4–43.9 vs 1.8; 95% CI: 0.5–6.3).
Discussion
The results of this study in 187 patients, with detailed information on time between onset of symptoms and angiographic assessment of coronary collaterals, show that the presence of collaterals increases significantly during the first 24 h, with 40% of patients showing collaterals at 24 h versus 19% of patients after 6 h. Preinfarction angina was the independent determinant of the presence of collaterals during acute MI independent of time-of-onset, particularly in the early hours of infarction.
The presence of early angiographically visible collaterals (Rentrop 2 or 3) in this study was higher than in a previously reported study by Elsman et al. [1]. They examined a total of 1059 patients who underwent primary percutaneous coronary intervention within 6 h after onset of STEMI and reported a prevalence of collaterals of 10%.
The presence of early angiographically visible collaterals (Rentrop 2 or 3) in this study was higher than in a previously reported study by Elsman et al. [1]. They examined a total of 1059 patients who underwent primary percutaneous coronary intervention within 6 h after onset of STEMI and reported a prevalence of collaterals of 10%.
The nature of the occurrence of coronary collaterals within a short time after acute coronary occlusion may be questioned, since it is likely that the recruitment of new collaterals needs sufficient time to become functional. It is generally accepted that it takes a period of 3–5 days for a progressive occlusion of a coronary artery to result in the appearance of collaterals. In an animal study, Khouri et al. found that progressive occlusion of a coronary artery over 3–5 days was associated with more than a fourfold increase in retrograde flow through collaterals, compared with retrograde f low collected immediately after sudden coronary occlusion [10].
Collaterals do not form de novo, but result from luminal enlargement of pre-existing small arterioles that have an internal diameter of 30–50 μm [11]. In animal models of femoral artery occlusion, it has been shown that collateral enlargement occurs through an active process of positive remodeling [12]. The process begins minutes after the femoral occlusion, with the activation of the endothelial cells by shear stress. It is followed hours later by the induction and/or upregulation of adhesion molecules and the subsequent adhesion of blood monocytes. Remodeling further continues as the smooth muscle cells are transformed from contractile into proliferative and synthetic phenotypes. Mitosis of endothelial cells and smooth muscle cells starts approximately 24 h after occlusion. Maximal growth of collaterals has been observed during the first 3 weeks and is almost complete at 21 days after occlusion. To be visible angiographically in humans, collaterals need to reach a diameter of at least 0.5 mm [13].
The most likely explanation for the early appearance of collaterals in this study, is that the collaterals are already being recruited before the onset of symptomatic MI. It has been shown that plaque rupture and/or thrombus formation may start at least 3 days before an occlusive thrombosis occurs and results in symptoms of infarction [14,15]. Interestingly, the preceding diameter of stenoses of the culprit lesions was >50% and generally 60–70% [16]. A considerably higher degree of stenosis may repetitively occur by the waxing and waning of a thrombus in the ruptured plaque. This degree of stenosis creates a sufficient pressure gradient across the arteriolar connection and, subsequently, may trigger the process of arteriogenesis. Collaterals become easily recruitable with the presence of a stenosis of 80% or more [17,18]. Brief, repetitive, 2-min occlusions of a coronary artery sufficiently increase collateral flow and recruitability [19].
In addition, our findings suggest that collateral growth is accelerated following acute MI. Inflammation during infarction promotes arteriogenesis and the abundance of monocytes accelerates the growth of collaterals [20]. MCP 1, the most important chemokine in the regulation of migration and infiltration of monocytes, markedly increases 3 h after the onset of chest pain during acute MI [21]. The presence of MCP-1 in a collaterals network stimulates arteriogenesis. Ito et al. showed that infusion of MCP-1 into the proximal stump of an occluded femoral artery, greatly accelerates the speed of collateral development in an animal model [22]. It has also been shown that a higher level of MCP-1 is related to the appearance of visible collaterals in patients with acute MI [23].
Finally, luminal filling of the growing collaterals may be increased by NO-mediated vasodilatation. Minutes after onset of ischemia, endothelial NO synthase activity increases [24] and subsequently the NO level during early ischemia is increased. NO plays an important role in the initial phases of arteriogenesis after exposure to shear stress, mainly by vasodilatation of the collateral vessels [25].
In this study, the only clinical determinant associated with the appearance of collaterals during acute coronary occlusion was preinfarction angina. DM, hypertension, smoking and dyslipidemia were not associated with early collateral appearance. These findings confirm the previous reports on acute MI [26,27]. This association was observed most prominently in the patient group of early infarction. The strong association between preinfarction angina and early collateral appearance, supports the view that pressure gradients occurring before the onset of symptomatic infarction act as triggers for collateral recruitment. Some patients may not have developed collaterals, despite the presence of triggers, because they lack the intrinsic capacity to have preformed collaterals or to develop mature collaterals [28].
Other factors have shown no, or only inconsistent, relationships to collateral presence and occurrence in acute MI. Older age may result in reduced arteriogenesis. Previous studies have found that the prevalence of coronary collaterals is lower in older patients, particularly in those aged over 65 or 70 years [27,29]. This finding was not revealed in our study. This discrepant result might be due to the inclusion of fewer old patients in our study population (mean age 55 ± 11 years). According to Abaci et al., patients with DM develop fewer visible collaterals, despite the existence of more severe coronary artery disease [30]. However, Zbinden et al. reported the opposite in a study using quantitative collateral measurement [31].
Koerselman et al. found that a high systolic blood pressure is inversely associated with the presence of collaterals in a cross-sectional study among patients admitted for elective coronary angioplasty [9]. However, Kyriakides et al. found the opposite result in a study on coronary collaterals in relation to systemic hypertension and left ventricle hypertrophy [32]. Fujita et al. reported no association between hypertension and collaterals in acute MI [27].
The extent to which smoking affects the presence of collaterals was investigated by Koerselman et al. [8]. They found a higher prevalence of coronary collaterals in current smokers. An experimental study supports the view that nicotine can promote arteriogenesis [33]. Nevertheless, the association in patients with acute MI should be treated with caution, if only because smoking is also a strong determinant of severe coronary stenosis, which consequently may promote collateral formation.
To appreciate these results, some limitations of this study should be noted. First, the temporal appearance of collaterals in a cross-sectional study was evaluated, rather than by serial angiography in the same subjects. Consequently, the inherent inter-individual variability in developing collateral circulation may have affected the estimates of collateral presence over time. Second, the angiographic method we used to evaluate the presence of collaterals cannot identify vessels less than 100 μm in diameter. Third, since the study’s nature is observational and retrospective, it would be difficult to adjust all possible determinants of coronary collateral development. Furthermore, data variability on angiographic collateral grading due to nonpredefined rate and volume of contrast injection, durations of video recording and occurrence of inhibitively narrow windows for observation of the collaterals may present. To reduce the technical limitations, criteria for the appropriateness of the angiograms to be reviewed was applied.
Conclusion
The results of this study show that during STEMI, the appearance of angiographic visible collaterals is associated with the duration of occlusion. The presence of collaterals doubles between 6 and 24 h after onset of symptoms. The recruitment of collaterals is probably more rapid than previously thought. Preinfarction angina is the important clinical fact ors that associated with the early appearance of coronary collaterals.
Future perspective
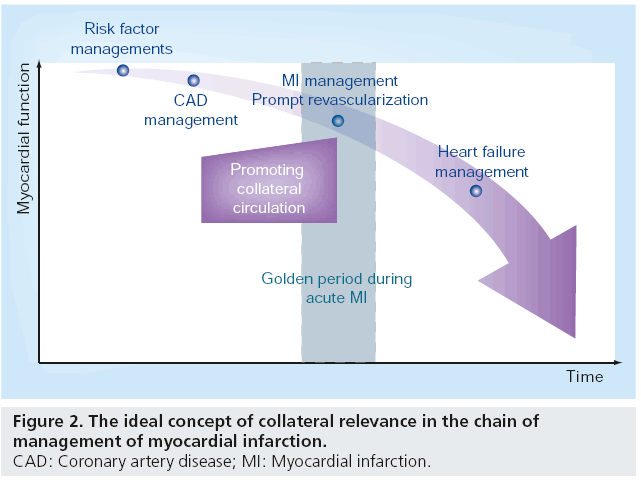
When an acute coronary occlusion occurs, the wavefront of MI commences and the infarct size increases as ischemia persists. The ideal scenario is that as soon as an acute coronary occlusion occurs, an immediate alternative pathway is available, thereby avoiding fatal destruction of the myocardium (Figure 2). The area at risk will be reduced, infarct size will be lower and subsequent fatal mechanical, hemodynamic and arrhythmic complications of STEMI will be reduced. In addition, successful acute revascularization – in terms of salvaged myocardium – can still be achieved after unavoidable logistical delay if more salvageable area is maintained through the presence of collateral flow.
Collaterals should ideally be present or readily recruitable if required, whenever coronary occlusion occurs. This means that efforts at manipulation, either to promote collateral presence or their recruitability, should be made before the severe ischemia or infarction occurs (Figure 2). In line with this point of view, collateral promotion should take place at any point before MI occurs in individuals with coronary artery disease. Numerous studies have been undertaken to explore the possibility of augmenting collateral function, mostly within the setting of peripheral artery disease. With the current knowledge of arteriogenesis, it is seems feasible to manipulate the growth of collaterals by means of either biochemical or biophysical intervention.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪ The earlier presence of collateral flow can result in smaller infarct size in acute coronary occlusion.
▪ The timing of the appearance of coronary collaterals during acute myocardial infarction (MI) has not yet been studied.
Methods
▪ A cross-sectional study included a total of 187 patients who suffered ST-elevation MI (STEMI) with varying time onset of symptoms.
Results
▪ Coronary collaterals were present in 19% of patients presenting within 6 h of onset.
▪ Presence of coronary collaterals increases significantly during the first 24 h after STEMI.
▪ Preinfarction angina was a significant determinant of the presence of collaterals during STEMI, independent of the time of onset of MI.
Conclusion
▪ The appearance of angiographic-visible collaterals is associated with the duration of occlusion during STEMI.
▪ Preinfarction angina is the most important clinical factor associated with the early appearance of coronary collaterals.
Future perspective
▪ The early presence of functional collaterals as alternative pathways during acute coronary occlusion will be the ideal situation in lifesaving and/or increasing the chance for better myocardial salvation. Based on the current knowledge regarding arteriogenesis, it is seems feasible to manipulate the growth of collaterals by means of either biochemical or biophysical intervention.
References
- Elsman P, van ‘t Hof AWJ, de Boer MJ et al. Role of collateral circulation in the acute phase of ST-segment elevation myocardial infarction treated with primary coronary intervention. Eur. Heart J. 25, 854–858 (2004).
- Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 56, 786–794 (1977).
- Nienaber C, Gottwik M, Winkler B, Schaper W. The relationship between the perfusion deficit, infarct size and time after experimental coronary artery occlusion. Basic Res. Cardiol. 78, 210–226 (1983).
- Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J. Am. Coll. Cardiol. 5, 587–592 (1985).
- Nitzberg WD, Nath HP, Rogers WJ et al. Collateral flow in patients with acute myocardial infarction. Am. J. Cardiol. 56, 729–736 (1985).
- Altman D. Practical Statistics for Medical Research. Chapman & Hall, London, UK (1991).
- Fujita M, Tambara K. Recent insights into human coronary collateral development. Heart 90, 246–250 (2004).
- Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, van der Graaf Y. Coronary collateral circulation: the effects of smoking and alcohol. Atherosclerosis 191, 191–198 (2007).
- Koerselman J, de Jaegere PP, Verhaar MC, van der Graaf Y, Grobbee DE. High blood pressure is inversely related with the presence and extent of coronary collaterals. J. Hum. Hypertens. 19, 809–817 (2005).
- Khouri EM, Gregg DE, McGranahan GM Jr. Regression and reappearance of coronary collaterals. Am. J. Physiol. 220, 655–661 (1971).
- Scholz D, Cai WJ, Schaper W. Arteriogenesis: a new concept of vascular adaptation in occlusive disease. Angiogenesis 4, 247–257 (2001).
- Scholz D, Ito W, Fleming I et al. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch. 436, 257–270 (2000).
- Rockstroh J, Brown BG. Coronary collateral size, flow capacity, and growth: estimates from the angiogram in patients with obstructive coronary disease. Circulation 105, 168–173 (2002).
- Rittersma SZ, van der Wal AC, Koch KT et al. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation 111, 1160–1165 (2005).
- Ojio S, Takatsu H, Tanaka T et al. Considerable time from the onset of plaque rupture and/or thrombi until the onset of acute myocardial infarction in humans: coronary angiographic findings within 1 week before the onset of infarction. Circulation 102, 2063–2069 (2000).
- Manoharan G, Ntalianis A, Muller O et al. Severity of coronary arterial stenoses responsible for acute coronary syndromes. Am. J. Cardiol. 103, 1183–1188 (2009).
- van Liebergen RA, Piek JJ, Koch KT, de Winter RJ, Schotborgh CE, Lie KI. Quantification of collateral flow in humans: a comparison of angiographic, electrocardiographic and hemodynamic variables. J. Am. Coll. Cardiol. 33, 670–677 (1999).
- Meier B, Luethy P, Finci L, Steffenino GD, Rutishauser W. Coronary wedge pressure in relation to spontaneously visible and recruitable collaterals. Circulation 75, 906–913 (1987).
- Billinger M, Fleisch M, Eberli FR, Garachemani A, Meier B, Seiler C. Is the development of myocardial tolerance to repeated ischemia in humans due to preconditioning or to collateral recruitment? J. Am. Coll. Cardiol. 33, 1027–1035 (1999).
- Heil M, Ziegelhoeffer T, Pipp F et al. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am. J. Physiol. Heart Circ. Physiol. 283, 2411–2419 (2002).
- Matsumori A, Furukawa Y, Hashimoto T et al. Plasma levels of the monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 are elevated in patients with acute myocardial infarction. J. Mol. Cell. Cardiol. 29, 419–423 (1997).
- Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ. Res. 80, 829–837 (1997).
- Park HJ, Chang K, Park CS et al. Coronary collaterals: the role of MCP-1 during the early phase of acute myocardial infarction. Int. J. Cardiol. 130, 409–413 (2008).
- Depre C, Fierain L, Hue L. Activation of nitric oxide synthase by ischemia in the perfused heart. Cardiovasc. Res. 33, 82–87 (1997).
- Frank MW, Harris KR, Ahlin KA, Klocke FJ. Endothelium-derived relaxing factor (nitric oxide) has a tonic vasodilating action on coronary collateral vessels. J. Am. Coll. Cardiol. 27, 658–663 (1996).
- Kurotobi T, Sato H, Kinjo K et al. Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J. Am. Coll. Cardiol. 44, 28–34 (2004).
- Fujita M, Nakae I, Kihara Y et al. Determinants of collateral development in patients with acute myocardial infarction. Clin. Cardiol. 22, 595–599 (1999).
- Regieli JJ, Nathoe HM, Koerselman J, van der Graaf Y, Grobbee DE, Doevendans PA. Coronary collaterals: insights in molecular determinants and prognostic relevance. Int. J. Cardiol. 116, 139–143 (2007).
- Nakae I, Fujita M, Miwa K et al. Agedependent impairment of coronary collateral development in humans. Heart Vessels 15, 176–180 (2000).
- Abaci A, Oguzhan A, Kahraman S et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 99, 2239–2242 (1999).
- Zbinden R, Zbinden S, Billinger M, Windecker S, Meier B, Seiler C. Influence of diabetes mellitus on coronary collateral flow: an answer to an old controversy. Heart 91, 1289–1293 (2005).
- Kyriakides ZS, Kremastinos DT, Michelakakis NA, Matsakas EP, Demovelis T, Toutouzas PK. Coronary collateral circulation in coronary artery disease and systemic hypertension. Am. J. Cardiol. 67, 687–690 (1991).
- Heeschen C, Weis M, Cooke JP. Nicotine promotes arteriogenesis. J. Am. Coll. Cardiol. 41, 489–496 (2003).