Clinical Trial Teport - Interventional Cardiology (2011) Volume 3, Issue 4
The Rapid Evaluation of Vessel Healing after Angioplasty (REVEAL) trial
- Corresponding Author:
- Davide Capodanno
Cardiology Department, Ferrarotto Hospital, University of Catania, Catania, Italy
Tel: +39 957 436 202
Fax: +39 953 624 29
E-mail: dcapodanno@gmail.com
Abstract
Keywords
CATANIA™ stent,early strut coverage,optical coherence tomography,polyzene-F,vessel healing
After promising results from pivotal clinical trials [1,2], drug-eluting stent (DES) implantation has rapidly extended to a ‘real-world’ population with more complicated clinical and lesion subsets, thus raising important questions about the mid- and long-term safety of these devices [3]. In particular, DES have been associated with a higher risk of late stent thrombosis (ST) compared with bare-metal stents (BMS) [4], a phenomenon not recognized in various pivotal trials due to the carefully selected population and limited follow-up duration. Delayed endothelialization associated with DES implantation may increase the risk of ST [5]. Although the use of dual antiplatelet therapy (DAPT) combining acetylsalicylic acid and a thienopyridine has greatly decreased the incidence of ST [6], this complication is still observed acutely and late after percutaneous coronary intervention (PCI) procedures [7,8]. Although premature discontinuation of thienopyridines is associated with a marked increase in ST, the role of extended use of DAPT beyond 12 months after DES implantation remains uncertain [9].
Background & rationale
The availability of first-generation DES (sirolimus- eluting stents [SES], Cypher®, Cordis, Johnson & Johnson, NJ, USA; and paclitaxeleluting stents [PES], Taxus®, Boston Scientific, MA, USA) has significantly improved the treatment options for coronary artery disease through decreasing rates of restenosis and target lesion revascularization (TLR) following PCI [1,2,10,11]. However, early enthusiasm was tempered by reports of higher rates of late ST [3,12], a complex and multifactorial phenomenon that seems to be correlated (adding to patient, lesion and technical factors) with the delayed, poor and nonuniform arterial healing observed after DES implantation [13–23]. One morphometric parameter that correlates with re-endothelization is the ratio of uncovered to total stent struts; specifically, a ratio of uncovered to total struts per section (RUTSS) >30% has been associated with a ninefold increase in probability of late ST [5]. Optical coherence tomography (OCT), with its high-resolution capacity (10 μm) and, when compared with intravascular ultrasound, superior visualization and differentiation of the vessel lumen and arterial wall interface [20,21], is able to detect the early kinetics of struts coverage [16,17,21,24,25].
Unlike BMS that develop circumferential coverage with an average thickness of 500 μm or more (1-mm late loss), which is generally well visualized with intravascular ultrasound and angiography, DES delay and prevent the hyperplastic response so that the average late lumen loss (LLL) for SES or PES can be lower than 100 μm [17]. Therefore, the amount of neointimal thickening could not be detectable with intravascular ultrasound because of its limited axial resolution and the presence of artifacts around struts [17].
In recent years, the interest of the interventional cardiology community has been focused on late ST because it is often a lifethreatening event, associated with a relevant incidence of death (20–40%), myocardial infarction (MI; 50–70%) and TLR [13]. Although several recent studies have reported lower late ST rates than originally expected with DES use compared to BMS use [1,4,10,26,27], the need for novel stents presenting a favorable safety profile while maintaining the low restenosis rates of DES remains unmet. Recently, a nanotechnology approach to reduce ST after stent implantation was to develop biocompatible polymers that promote the physiologic healing process after stent implantation [28].
The CATANIA™ (CAT) stent (CeloNova Biosciences, GA, USA) presents a chromium– cobalt alloy (Cr–Cb L605) balloon-expandable scaffold, with a modified open-cell design and a strut thickness of 65 or 74 μm (stent size: 2.00–2.75 or 3.00–4.00 μm, respectively), which is covered by a nanolayer (40 nm) of poly[bis (trif luoroethoxy)phosphazene] (Polyzene-F™, CeloNova Biosciences), a soft rubber-like inorganic polymer, highly biocompatible, with anti-inflammatory, antithrombotic and bacteria-resistant proprieties. This novel technical solution promises to decrease instent restenosis without increasing the risk of ST [29–31].
The Assessment of the Latest Non- Thrombogenic Angioplasty Stent (ATLANTA) first-in-man trial showed an excellent early and mid-term safety profile and high-level efficacy of the stent in the treatment of de novo coronary lesions, reducing late LLL, restenosis and TLR without the need for prolonged DAPT. The study, conducted in 55 patients, reported a 6-month LLL of 0.6 mm, whereas at 12-month follow-up no deaths or MIs were reported, and a clinically driven TLR rate of 3.6% was observed. No cases of ST were observed [29]. Of note, OCT analysis was performed in 15 patients, demonstrating that 99.5% of struts were fully covered at 6 months, thus documenting how this stent is close to mimicking a biologically inert structure between blood and vessel walls [30]. In another study, the 30-day and 6-month risk-adjusted outcomes for patients who received the CAT stent (n = 254) were compared with the outcomes of a historical cohort of patients who received BMS (n = 552). At 30 days, use of BMS versus the CAT stent resulted in borderline significant differences with respect to major adverse cardiac and cerebrovascular events (MACCE) and cardiac death or MI. At 6 months, BMS showed a statistically significant higher adjusted risk of MACCE (HR: 2.79; 95% CI: 1.20–6.48; p = 0.017) and no differences with respect to the subcomponent end points. The cumulative incidence of definite ST (Academic Research Consortium defined) at 6 months was 0.39% for the CAT stent and 2.35% for the BMS [31].
Study design
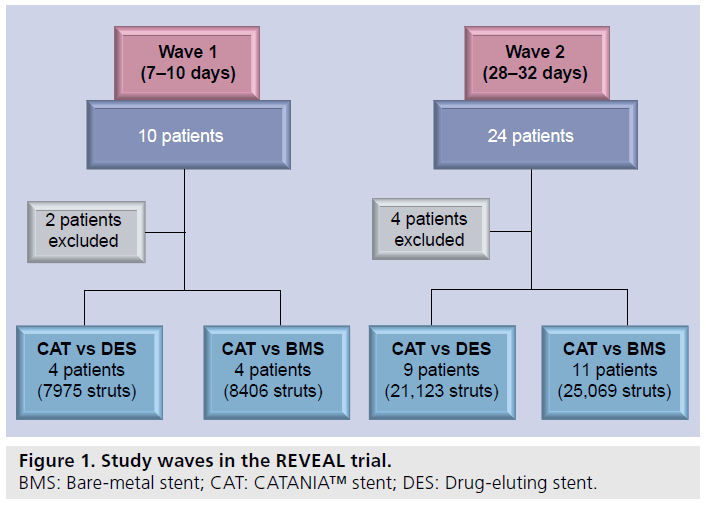
The Rapid Evaluation of Vessel Healing after Angioplasty (REVEAL) trial was a prospective head-to-head OCT study in which the temporal patterns and degrees of neointimal coverage after coronary implantation of a CAT stent, DES and cobalt–chromium BMS were analyzed [32,101]. A total of 34 patients were enrolled with at least two short (<20 mm) angiographically signif icant de novo American College of Cardiology/American Heart Association type A or type B lesions, located in remote vessels or in the same vessel but in two different coronary segment. Patients underwent PCI at the Ferrarotto Hospital (Catania, Italy) and randomly received a CAT stent in one lesion and a DES or BMS in the other lesion. The DES used were the SES 3160L stainless steel Cypher, with strut thickness of 140 μm and polymer (polyethelyne co-vinyl acetate and poly-n-butyl methacrylate) thickness of 12.6 μm, and the PES 316L stainless steel TAXUS Libertè®, with a strut thickness of 97 μm and polymer (polystyrene- b-isobutylene-b-styrene) thickness of 16.0 μm. The stent choice was randomly established with the use of sealed envelopes containing a computer-generated randomization sequence for the coronary vessel. Overlapping stent implantation was permitted only as a bailout for safety reasons. Enrolled patients (18–75 years of age; both male and female) had to have clinical symptoms and electrocardiographic signs compatible with stable coronary artery disease, such as angina pectoris and reversible electrocardiographic changes, or acute coronary syndrome (either unstable angina [UA]/non-ST elevation MI [NSTEMI] or ST elevation MI [STEMI]). Clinical and instrumental (electrocardiographic, echocardiographic, angiographic) data were collected and stored into a computerized database (Department of Cardiology, Ferrarotto Hospital) by specialized medical personnel.
The study was conducted according to the Declaration of Helsinki. The study protocol was approved by the local institutional ethics committee and all patients provided written informed consent before catheterization to take part in the study. Periprocedural antithrombotic therapy consisted of nonfractioned heparin at standard dosages, clopidogrel at a loading dose of 600 mg and acetylsalicylic acid. Platelet glycoprotein IIb/IIIa inhibitors were administered only in acute coronary syndrome. After PCI, DAPT (100 mg acetylsalicylic acid and 75 mg clopidogrel daily) was administered for 1 month in case of CAT/BMS implantation and for 12 months in case of CAT/DES implantation. Patients were divided into two cohorts of ten (wave 1) and 24 patients (wave 2) that were followed-up with an OCT evaluation at 7–10 days and 28–32 days, respectively (Figure 1). OCTs were obtained using the Lightlab M2 system (M2 Cardiology Interface System, LightLab Imaging Inc., MA, USA), that uses a 1310-nm broadband light source and the principles of interferometry to produce images with an axial resolution of 15 μm and a lateral resolution of 25 μm, through a nonocclusive technique recently developed by Rome Heart Research (Rome, Italy) [33]. This technique provides the manual injection of a iso-osmolar contrast media (iodixanol, 320 mg/ml of iodine; Visipaque™, GE Healthcare, Ireland) through the guiding catheter at an infusion rate between 2 and 4 ml/s, based on the run-off of the artery and the online assessment of OCT image quality. This contrast media is recommended both for its low arrhythmogenic potential and for its high viscosity that help to prolong the imaging time. Large series have shown that the blood can be completely displaced from the artery during the entire acquisition period, allowing a better quality OCT to be obtained [33]. This imaging protocol has shown ease and safety with a maximal volume per pullback of 35 ml. Criteria for stopping the infusion included the presence of marked ST-segment elevation, QRS widening and a decrease >30% of baseline heart rate [33]. Previous nonfractioned heparin administration (50 UI/kg), coronary catheterization (7 F guiding catheter) and crossing of the stenosis with conventional coronary guidewire for safety reasons, a wire-type imaging catheter (ImageWire, LightLab Imaging Inc.) was positioned inside the stented area. The ImageWire is like a coronary wire with a 0.006 inch (0.15 mm) fiberoptic core that rotates inside a sheath with a diameter of 0.016 inches (0.41 mm). The image was pulled back at 2-mm/s speed and OCT frames were acquired at a frame rate of 15.6/s, digitally stored and subsequently analyzed independently by a trained examiner in a validated core laboratory (Rome Heart Research). Frames with a suboptimal visualization of stent struts, with artifacts determining the inability to analyze almost 90% of stent struts in a crosssection, were discarded. After analysis of all the acquired OCTs, two patients of wave 1 and four patients of wave 2 were excluded for poor OCT image quality by the aforementioned independent core laboratory. Of the remaining eight patients of wave 1, four underwent implantation of CAT/BMS and four underwent implantation of CAT/DES (Figure 2). Of the remaining 20 patients of wave 2, 11 underwent implantation of CAT/BMS and 9 underwent implantation of CAT/DES (Figure 2). Both stent area (SA) and lumen area (LA) inside all stent struts were measured by manual trace at 0.125-mm intervals (all frames), resulting in the analysis of thousands of struts and strongly powered comparisons. Through the difference between SA and LA (SA minus LA) the neointimal hyperplasia (NIH) area was calculated. Percentage NIH (% NIH) was calculated as (SA – LA)/SA × 100. Stent coverage was defined as the measured NIH thickness >0 μm, which is uniformly apposed rim of new tissue on stent struts and absence of any detectable overwhelming thrombus, identified by a linear shape above the stent struts. Consequently, a NIH thickness equal to 0 μm was defined as stent strut exposure. According to a recent review, strut and polymer thicknesses were considered in addition to OCT resolution when assessing malapposition of a stent [34,35]. Since Polyzene-F is applied to the CAT stent as a 40-nm surface modification, metal struts (65–74 μm, depending on stent size) accounted almost entirely for overall thickness. In addition, to achieve a more accurate assessment of malapposition, OCT resolution (15 μm) was considered. Consequently, malapposed CAT struts were defined as those with a maximum distance >90 μm between the inner strut surface and the adjacent vessel surface.
The primary end point of the study was the percentage of covered stent struts with regard to the total amount of stent struts analyzed. Secondary end points included:
▪▪ The rates of covered struts on the total amount of analyzed struts for the comparisons between CAT and BMS at 7–10 and 28–32 days;
▪▪ The rates of cross sections with RUTSS >30%, struts covered with thrombus, struts malapposed and struts uncovered/malapposed for the comparisons between CAT versus DES and CAT versus BMS at 7–10 and 28–32 days;
▪▪ The mean and maximum % NIH comparing CAT versus DES and CAT versus BMS at 28–32 days.
Data analysis
A total of 566 struts, 283 per stent type, had to be analyzed with a = 0.01 (type I error) to obtain a 99% chance of detecting an absolute 20% different percentage in the rates of stent strut coverage at 7–10 days between CAT and DES, with an estimated stent strut coverage in 30% of DES struts. Assuming a dropout rate due to patients lost to follow-up or stent exclusion from the analysis due to suboptimal stent strut visualization, and in order to overcome possible unexplained individual factors that could be responsible for higher or lower neointimal growth, a goal of five patients/ten lesions was set for the comparison between CAT and DES and five patients/ten lesions for the comparison between CAT and BMS in wave 1. Second, it was also calculated that 494 struts, 247 per stent type, had to be analyzed with a = 0.01 to obtain a 99% chance of detecting an absolute 20% different rate in stent strut coverage at 28–32 days between CAT and DES, with estimated stent strut coverage in 60% of DES struts. For the same reasons as before, a goal of 12 patients/24 lesions was set for the comparison between CAT and DES and 12 patients/24 lesions for the comparison between CAT and BMS in wave 2 [32]. Continuous variables were presented as mean ± standard deviation and compared using Student’s unpaired t-test. Categorical variables were presented as counts and percentages and were compared with the χ2 test where appropriate (expected frequency >5) or, otherwise, with the Fisher’s exact test.
All probability values reported are two-sided, and a p-value <0.05 was considered significant. All data were processed using the Statistical Package for Social Sciences, version 15 (SPSS, IL, USA).
Results
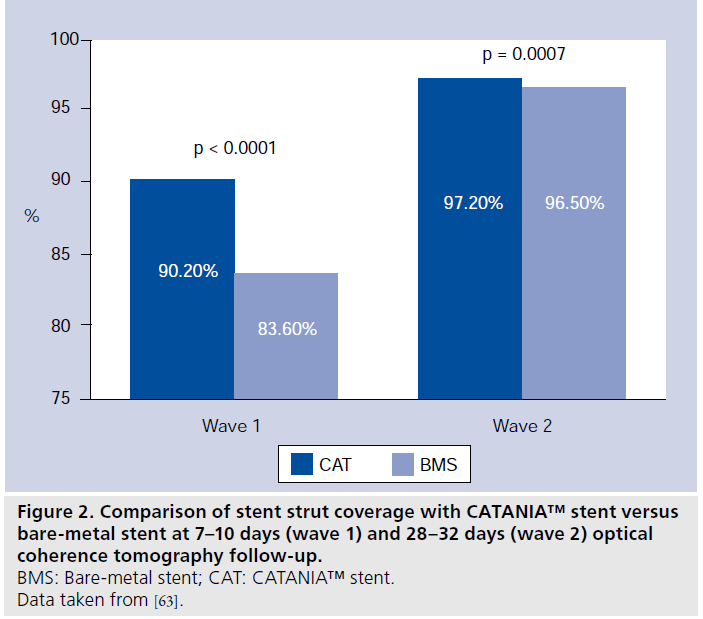
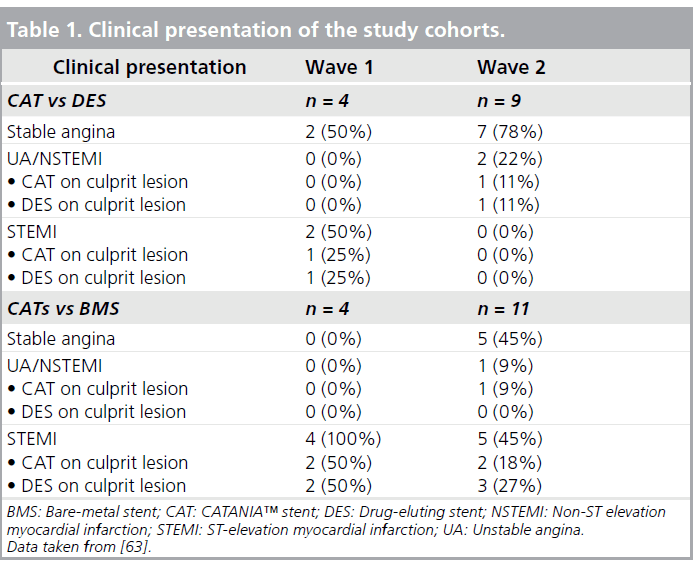
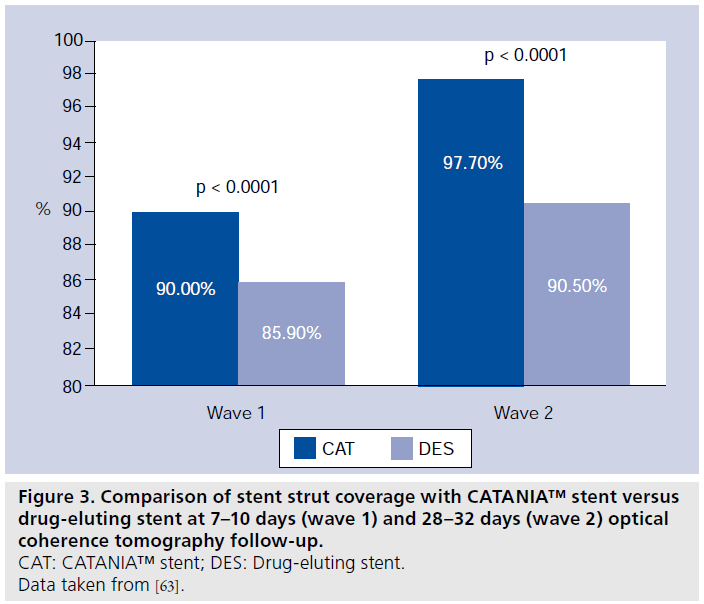
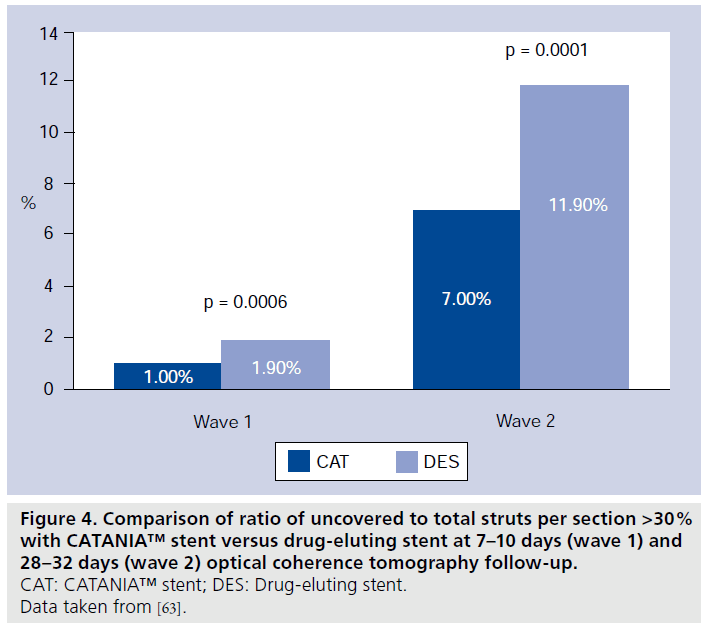
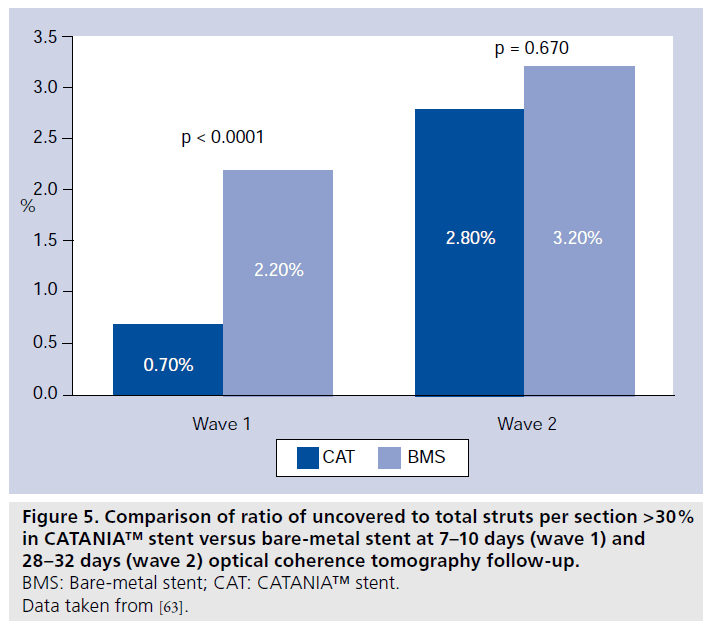
Patients of wave 1 presented a mean age of 61.8 ± 9.4 years and were predominantly male (88%). Among them, six (75%) had STEMI and two (25%) stable angina (Table 1). In patients presenting with MI, the randomization procedure ensured that the same number of culprit lesions were treated with CAT or the control. Quantitative coronary angiography analysis did not display significant differences among groups with regards to pre- and post-procedural vessel and lesion measurements. At 7–10 days, CAT demonstrated the best strut coverage, compared with both DES (90.0 vs 85.9%; p < 0.0001; 7975 struts analyzed; four patients) and BMS (90.2 vs 83.6%; p < 0.0001; 8406 struts analyzed; four patients) (Figures 2 & 3) [32]. At this OCT follow-up time, a significantly lower percentage of RUTSS >30% (1.0 vs 1.9%; p < 0.0006) (Figure 4), malapposed struts (0.5 vs 1.9%; p < 0.0001), and uncovered and malapposed struts was observed with CAT compared with DES (0.1 vs 0.5%; p < 0.0005), whereas no difference between CAT and DES was noted in terms of struts with thrombotic adhesions (0.5 vs 0.8%; p = 0.887) [32]. At the same OCT follow-up time, in patients treated with CAT/ BMS, CAT demonstrated a lower percentage of RUTSS >30% (0.7 vs 2.2%; p < 0.0001) (Figure 5), malapposed struts (0.4 vs 7.4%; p < 0.0001), uncovered and malapposed struts (0.1 vs 4.1%; p < 0.0001) and struts covered with thrombus (0.5 vs 1.1%; p = 0.0018) than BMS [32].
Patients of wave 2 presented with a mean age of 63.4 ± 10.4 years and were predominantly male (75%). Among them, five (25%) had STEMI, three (15%) UA/NSTEMI and 12 (60%) stable angina (Table 1). Again, in patients presenting with MI, the randomization procedure ensured that the same number of culprit lesions were treated with CAT or the control. At quantitative coronary angiography there were no significant differences among groups with regards to preprocedural and postprocedural vessel and lesion measurements. At 28–32 days, the higher percentage of coverage of CAT compared with DES (97.7 vs 90.5%; p < 0.0001; 21,123 struts analyzed; nine patients) and BMS (97.2 vs 96.5%;p < 0.0001; 25,069 struts analyzed; 11 patients) was confirmed (Figures 2 & 3) [32]. At this OCT follow- up time, a significantly lower percentage of RUTSS >30% (7 vs 11.9%; p < 0.0001) (Figure 4), malapposed struts (5.2 vs 9.5%; p < 0.0001), and uncovered and malapposed struts (2.9 vs 5.3%; p < 0.0001) was observed in CAT compared with DES, whereas no differences were noted in terms of strut covered with thrombus (0.3 vs 0.2%; p = 0.098) [32]. A significantly lower rate of malapposed struts (4.4 vs 1.2%; p < 0.0001) and uncovered and malapposed struts (1.6 vs 0.5%; p < 0.0001) was found in CAT compared with BMS; no differences were observed in terms of RUTSS >30% (2.8 vs 3.2%; p = 0.670) and strut covered with thrombus (0.5 vs 0.5%; p = 0.330) (Figure 5) [32]. Furthermore, at 28–32 days OCT follow-up, mean and maximum % NIH were similar between CAT and DES (mean% NIH: 17.0 ± 9.1 vs 10.4 ± 4.7 μm; p = 0.080; maximum% NIH: 31.6 ± 13.4 vs 25.2 ± 7.3 μm; p = 0.258) and between CAT and BMS (mean% NIH: 17.0 ± 12.6 vs 16.0 ± 9.2 μm; p = 0.720; maximum% NIH: 30.8 ± 14.6 vs 26.2 ± 10.9 μm; p = 0.368) [32].
Conclusion
The REVEAL study aimed to investigate the early stages of the re-endothelization process. At 1 week after stent implantation, regardless of the type of stent used (CAT, BMS or DES), high endothelization rates were found with more than 80% of coverage in each stent. At 1 month follow-up, CAT and BMS, although the latter at a lower speed, showed continuous vessel healing 1 week after PCI, with almost complete stent strut coverage (97.2 and 96.5%, respectively). At the same follow-up time, DES showed a degree of coverage close to that present at the earlier follow-up time-point, indicating the presence of a plateau in the speed of neointimal proliferation after DES implantation. This rate of coverage, adding to previously published OCT data regarding DES coverage at 3 and 6 months [14,17,36,37], remains almost unchanged for several months, thus justifying the current recommendation of prescribing DAPT for at least 12 months after DES implantation.
Future perspective
Although ST is clearly a multifactorial phenomenon [13,38,39], attention has been recently focused on the speed and degree of stent surface endothelial coverage after implantation. The efforts of coronary bioengineers are aimed at researching new technical solutions to reduce the magnitude of ST, affecting not only early- and mid-term safety, but also, ST being detectable at a constant rate of 0.6% per year up to 3 years after implantation, long-term safety [7]. Second-generation DES have more biocompatible polymers and have demonstrated impressive safety results at mid- and long-term follow-up [40–42]. However, second-generation stents have not completely addressed the issues related to DES use [43]. New therapeutic strategies are currently are under investigation in preclinical and clinical trials, building on the knowledge and experiences gained from the first- and second-generation DES: durable polymers DES [44], biodegradable polymers DES [45–48], polymer-free DES [49], stents with novel coatings [50–53] and completely biodegradable stents [54,55].
Several different biotechnology solutions are currently under examination but it is clear that only time will tell which is the most appropriate and that no single stent design and polymer type will be suitable for all patients and lesion types. Based on this background, CAT could serve as an effective new-generation therapeutic option, and this is currently under examination in the ATLANTA II trial, a large real world population with encouraging preliminary results and long follow-up time (2 years) [56]. Moreover, when considering the choice of stent, patient characteristics and, in particular, the individual ability to be prescribed with long-term DAPT, should be taken into account. Although the optimal duration of DAPT has not been well established, DAPT beyond 1 year has become the usual preventive treatment indicated after DES implantation [6]. It is well known that premature DAPT discontinuation is associated with a higher rate of ST [3,9,57,58] and that prolonged DAPT is associated with an increased risk of bleeding [59,60] and some limitations, such as the inability to perform early noncardiac surgery [61,62]. The short period of DAPT required after CAT implantation (only 1 month) makes post-PCI patient management more manageable and free from DAPT-related adverse events. This short duration of DAPT is justified by the REVEAL OCT analysis (both at 7–10 days and at 28–32 days) with demonstration of a greater velocity and homogeneity of strut reendothelization with the CAT stent, compared with both DES and BMS. This finding, while it could be predictable in the comparison between CAT and DES, is a surprising and encouraging result in the comparison between CAT and BMS. In addition, the REVEAL study suggests an innovative approach in coronary biotechnology research since it suggests how it is possible to study in vivo human coronary responses to stent implantation and how it is feasible to systematically obtain useful OCT chronologic and morphologic information even in the period which immediately follows PCI.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Stent properties
▪▪ Chromium–cobalt platform.
▪▪ Open-cell design.
▪▪ Strut thickness 65/74 μm (stent size 2.00–2.75/3.00–4.00)
▪▪ Polyzene-F™ ultra-thin coverage (40 nm):
– Low platelet apposition (low induction of glycoprotein IIb/IIIa [GP IIa/IIIb] receptors)
– Low affinity to fibrinogen, fibronectin and von Willebrand factor
– High albumin adsorption
– Low inflammatory response (no complement factor activation)
– Bacterial resistance
–Not immunoreactive.
Study design
▪▪ Prospective head-to-head optical coherence tomography (OCT) study.
▪▪ Comparison between CATANIA™ (CAT) stent and bare-metal stent (BMS) or CAT and drug-eluting stent (DES).
▪▪ Two waves with different OCT follow-up time:
– OCT follow-up at 7–10 days
– OCT follow-up at 28–32 days
Inclusion criteria
▪▪ 18–75 years of age.
▪▪ Multifocal/multivessel coronary artery disease.
▪▪ Lesion length <20 mm.
▪▪ Lesion Type A or Type B (American College of Cardiology/American Heart Association lesion angiographic classification).
▪▪ Lesion treatable with either CAT or BMS/DES.
▪▪ Single stent implantation/bail-out overlapping stent implantation.
Exclusion criteria
▪▪ Chronic kidney disease (serum creatinine >2.0 mg/dl).
▪▪ Left ventricular ejection fraction <30%.
▪▪ Previous percutaneous coronary intervention (PCI) <6 months.
▪▪ Cardiogenic shock.
▪▪ Suspected or documented systemic and/or infectious disease.
▪▪ Cardiac and extracardiac disease requiring surgical repair.
▪▪ Hypersensivity to contrast media or any PCI-related drugs/products.
▪▪ Extreme coronary tortuosity.
▪▪ Diffuse and severe coronary calcifications.
▪▪ Debulking device.
Results
▪▪ 7–10 days OCT follow-up (wave 1):
– CAT versus DES: better stent strut coverage with CAT (90.0 vs 85.9%; p < 0.0001; 7975 struts analyzed; four patients) and significantly lower ratio of uncovered to total struts per section (RUTSS) >30% (1.0 vs 1.9%; p < 0.0006);
– CAT vs BMS: better stent strut coverage with CAT (90.2 vs 83.6%; p < 0.0001; 8406 struts analyzed; four patients) and significantly lower percentage of RUTSS >30% (0.7 vs 2.2%; p < 0.0001).
▪▪ 28–32 days OCT follow-up (wave 2):
– CAT vs DES: better stent strut coverage with CAT (97.7 vs 90.5%; p < 0.0001; 21,123 struts analyzed; nine patients) and significantly lower percentage of RUTSS >30% (7 vs 11.9%; p < 0.0001);
– CAT vs BMS: better stent strut coverage with CAT (97.2 vs 96.5%; p < 0.0001; 25,069 struts analyzed; 11 patients) and no differences in terms of RUTSS >30% (2.8 vs 3.2%; p = 0.670).
Future perspective
▪▪ CAT could serve as an effective new-generation therapeutic option, particularly in some subsets.
▪▪ The Rapid Evaluation of Vessel Healing after Angioplasty (REVEAL) study suggests an innovative approach in stent biotechnology research that may allow the study of new stents in the early phases after implantation in order to gain important information relevant to trial design and clinical development.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Moses JW, Leon MB, Popma JJ et al.; for the SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Ellis SG, Cox DA et al.; for the TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126–2130 (2005).
- Holmes DR Jr, Kereiakes DJ, Laskey WK et al. Thrombosis and drug-eluting stents. J. Am. Coll. Cardiol. 50(2), 109–118 (2007).
- Finn AV, Joner M, Nakazawa G et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115(18), 2435–2441 (2007).
- Grines CL, Bonow RO, Casey DE Jr et al. A science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. J. Am. Coll. Cardiol. 49(6), 734–739 (2007).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Roukoz H, Bavry AA, Sarkees ML et al. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am. J. Med. 122(6), 581, e1–e10 (2009).
- Pfisterer M, Brunner-LaRocca HP, Buser PT et al.; for the BASKET-LATE Investigators.Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents. J. Am. Coll. Cardiol. 48(12), 2584–2591 (2006).
- Caixeta A, Leon MB, Lansky AJ et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J. Am. Coll. Cardiol.54(10), 894–902 (2009).
- Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N. Engl. J. Med. 354(5), 483–495 (2006).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Holmes DR Jr, Kereiakes DJ, Garg S et al.Stent thrombosis. J. Am. Coll. Cardiol.56(17), 1357–1365 (2010).
- Takano M, Inami S, Jang IK et al. Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am. J. Cardiol. 99(8), 1033–1038 (2007).
- Ozaki Y, Okumura M, Ismail TF et al. The fate of incomplete stent apposition with drug-eluting stents: an optical coherence tomography-based natural history study. Eur. Heart J. 31(12), 1470–1476 (2010).
- Suzuki Y, Ikeno F, Koizumi T et al. In vivo comparison between optical coherence tomography and intravascular ultrasound for detecting small degrees of in-stent neointima after stent implantation. J. Am. Coll. Cardiol. Interv. 1(2), 168–173 (2008).
- Matsumoto D, Shite J, Shinke T et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur. Heart J. 28(8), 961–967(2007).
- Chen BX, Ma FY, Luo W et al. Neointimal coverage of bare-metal and sirolimus-eluting stents evaluated with optical coherence tomography. Heart 94(5), 566–570 (2008).
- Oyabu J, Ueda Y, Ogasawara N, Okada K, Hirayama A, Kodama K. Angioscopic evaluation of neointima coverage: sirolimus drug-eluting stent versus bare metal stent. Am. Heart. J. 152(6), 1168–1174 (2006).
- Awata M, Kotani JI, Uematsu M et al. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation comparison with bare-metal stents. Circulation 116(8), 910–916 (2007).
- Kotani JI, Awata M, Nanto S et al. Incomplete neointimal coverage of sirolimus-eluting stents. angioscopic findings. J. Am. Coll. Cardiol. 47(10), 2108–2111 (2006).
- Kume T, Akasaka T, Kawamoto T et al. Assessment of coronary arterial plaque by optical coherence tomography. Am. J. Cardiol. 97(8), 1172–1175 (2006).
- Jang IK, Bouma BE, Kang DH et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 39(4), 604–609 (2002).
- Prati F, Regar E, Mintz GS et al.; PW for the Expert’s OCT Review Document. Expert review document on methodology, terminology, and clinical application of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary artery and atherosclerosis. Eur. Heart J. 31(4), 401–415 (2010).
- Capodanno D, Prati F, Pawlowsky T et al. Comparison of optical coherence tomography and intravascular ultrasound for the assessment of in-stent tissue coverage after stent implantation. EuroIntervention 5(5), 538–543 (2009).
- Kirtane AJ, Gupta A, Iyengar S et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119(25), 3198–3206 (2009).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591), 937–948 (2007).
- Garg S, Serruys PW. Coronary stents. looking forward. J. Am. Coll. Cardiol. 56(10), S43–S78 (2010).
- La Manna A, Capodanno D, Cera M et al. Optical coherence tomographic results at six-month follow-up evaluation of the CATANIA coronary stent system with nanothin Polyzene-F surface modification (from the Assessment of The Latest Non-Thrombogenic Angioplasty Stent [ATLANTA] trial). Am. J. Cardiol. 103(11), 1551–1555 (2009).
- Tamburino C, La Manna A, Di Salvo ME et al. First-in-man 1-year clinical outcomes ofthe CATANIA coronary stent system with nanothin Polyzene-F in de novo native coronary artery lesions: the ATLANTA (Assessment of The Latest Non-Thrombogenic Angioplasty stent) trial. J. Am. Coll. Cardiol. Interv. 2(3), 197–204 (2009).
- Capodanno D, La Manna A, Di Salvo ME, Sanfilippo A, Corcos T, Tamburino C. Early and mid-term clinical outcomes with the CATANIA coronary stent system vs bare metal stents in patients with coronary artery disease. Cardiovasc. Revasc. Med. 10(4), 216–220 (2009).
- Tamburino C, Capodanno D, La Manna A, Di Salvo M, Sanfilippo A, Prati F. Rapid Evaluation of Vessel hEaling After angiopLasty (REVEAL) trial: rationale, objectives and design. J. Cardiovasc. Med. (Hagerstown) 11(1), 53–58 (2010).
- Prati F, Cera M, Ramazzotti V, Imola F, Giudice R, Albertucci M. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention 3(3), 365–370 (2007).
- Tanigawa J, Barlis P, Di Mario C. Intravascular optical coherence tomography: optimisation of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention 3(1), 128–136 (2007).
- Nakazawa G, Finn AV, Joner M et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 118(11), 1138–1145 (2008).
- Xie Y, Takano M, Murakami D et al. Comparison of neointimal coverage by optical coherence tomography of a sirolimus-eluting stent versus a bare-metal stent three months after implantation. Am. J. Cardiol. 102(1), 27–31 (2008).
- Kim JS, Jang IK, Fan C et al. Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by optical coherence tomography. The ENDEAVOR OCT trial.J. Am. Coll. Cardiol. Interv. 12(2),1240–1247 (2009).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Lüscher TF, Steffel J, Eberli FR et al. Drug-eluting stent and coronary thrombosis. biological mechanisms and clinical implications. Circulation 115(8), 1051–1058 (2007).
- Garg S, Serruys PW, Onuma Y et al. Three year clinical follow up of the XIENCE V Everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions. The SPIRIT II trial. Am. Coll. Cardiol. Interv. 2(12), 1190–1198(2009).
- Stone GW, Midei M, Newman W et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: 2-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation 119(5), 680–686 (2009).
- Pendyala LK, Yin X, Li J, Chen JP, Chronos N, Hou D. The first-generation drug-eluting stents and coronary endothelial dysfunction. Am. Coll. Cardiol. Interv. 2(12), 1169–1177(2009).
- Joner M, Nakazawa G, Finn AV et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol. 52(5), 333–342 (2008).
- Serruys PW, Silber S, Garg S et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N. Engl. J. Med. 363(2), 136–146 (2010).
- Garg S, Sarno G, Serruys PW et al. The twelve-month outcomes of a biolimus eluting stent with a biodegradable polymer compared with a sirolimus eluting stent with a durable polymer. EuroIntervention 6(2), 233–239 (2010).
- Chevalier B, Silber S, Park SJ et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial – Phase 2. Circ. Cardiovasc. Interv. 2(3), 188–195 (2009).
- Grube E, Schofer J, Hauptmann KE et al. A novel paclitaxel-eluting stent with an ultrathin abluminal biodegradable polymer 9-month outcomes with the JACTAX HD stent. J. Am. Coll. Cardiol. Interv. 3(4), 431–438 (2010).
- Ormiston JA, Abizaid A, Spertus J et al., NEVO ResElution-I Investigators. Six-month results of the NEVO Res-Elution I (NEVO RES-I) trial: a randomized, multicenter comparison of the NEVO sirolimus-eluting coronary stent with the TAXUS Liberté paclitaxel-eluting stent in de novo native coronary artery lesions. Circ. Cardiovasc. Interv. 3(6), 556–564 (2010).
- Tada N, Virmani R, Grant G et al. Polymer-free biolimus A9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting Cypher stent in a porcine model. Circ. Cardiovasc. Interv. 3(2), 174–183 (2010).
- Windecker S, Simon R, Lins M et al. Randomized comparison of a titanium-nitride-oxide-coated stent with a stainless steel stent for coronary revascularization: the TiNOX trial. Circulation 111(20), 2617–2622 (2005).
- Moschovitis A, Simon R, Seidenstucker A et al. Randomised comparison of titanium-nitride-oxide coated stents with bare metal stents: five year follow-up of the TiNOX trial. EuroIntervention 6(1), 63–68 (2010).
- Aoki J, Serruys PW, van Beusekom H et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the HEALING-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) Registry. J. Am. Coll. Cardiol. 45(10), 1574–1579 (2005).
- Duckers HJ, Silber S, de Winter R et al. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the HEALING-II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention 3(1), 67–75 (2007).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373(9667), 897–910 (2009).
- Okamura T, Garg S, Gutierrez-Chico JL et al. In-vivo evaluation of stent strut distributionpatterns in the bioabsorbable everolimus-eluting device: an OCT ad hoc analysis of the Revision 1.0 and Revision 1.1 stent design in the ABSORB clinical trial. EuroIntervention 5(8), 932–938 (2010).
- La Manna A, Sanfilippo A, Di Salvo ME et al. One-year outcomes of CATANIA coronary stent system with nanothin polyzene-F in a real-world unselected population: assessment of the latest non-thrombogenic angioplasty stent 2 (Atlanta-2) study. Presented at: EuroPCR. Paris, France, 25–28 May 2010.
- Kimura T, Morimoto T, Nakagawa Y et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation 119(7), 987–995 (2009).
- Airoldi F, Colombo A, Morici N et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 116(7), 745–754 (2007).
- Berger PB, Bhatt DL, Fuster V et al.; CHARISMA Investigators. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 121(23), 2575–2583 (2010).
- Ben-Dor I, Torguson R, Scheinowitz M et al. Incidence, correlates, and clinical impact of nuisance bleeding after antiplatelet therapy for patients with drug-eluting stents. Am. Heart J. 159(5), 871–875 (2010).
- van Kuijk JP, Flu WJ, Schouten O et al. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. Am. J. Cardiol. 104(9), 1229–1234 (2009).
- Berger PB, Kleiman NS, Pencina MJ et al.; for the EVENT investigators. frequency of major noncardiac surgery and subsequent adverse events in the year after drug-eluting stent placement. results from the EVENT (Evaluation of Drug-Eluting Stents and Ischemic Events) Registry. J. Am. Coll. Cardiol. Interv. 3(9), 920–927 (2010).
- La Manna A, Prati F, Capodanno D et al. Head-to-head comparison of early vessel healing by optical coherence tomography after implantation of different stents in the same patient. J. Cardiovasc. Med. 12, 328–333 (2011).
- REVEAL Study: Vessel Healing After Angioplasty http://clinicaltrials.gov/ct2/show/ NCT00799344
▪ A study on the pathological predictors of late stent thrombosis.
▪▪ First-in-man trial of the CATANIA™ stent in human coronary arteries.
▪ Website