Research Article - Neuropsychiatry (2018) Volume 8, Issue 1
The Neural Basis of Self-Touch in a Pain-Free Situation
- Corresponding Author:
- Professor Yoshiaki Kikuchi, PhD & D Med Science
Department of Frontier Health, Science, Division of Human Health Sciences, Graduate School of Tokyo Metropolitan University, 7-2-10, HigashiOgu, Arakawa-Ku, Tokyo, 116-0012, Japan
Tel & Fax: +81-3-3819-7270
Abstract
Abstract
Objective: Self-touch is thought to be an act of coping with harmful or stressful situations, based on the mechanism which suppresses somatosensory perception as well as somatosensory cortex activity, and sympathetic activity. In addition, this suppression can be observed in even nonpainful and non-stressful situations. However, its detailed neural mechanism remains unknown. Several studied have shown, not only that the descending pain modulatory system (DPMS) plays critical roles in painful situations, but also that there is intrinsic functional connectivity in the DPMS in even non-painful situations. Based on these findings, we hypothesized that the neural system consisting of the anterior cingulate cortex (ACC), amygdala and rostral ventromedial medulla (RVM) would play a basic role in self-touch, and we here investigated interactive effects of these regions in a pain-free self-touch situation.
Methods: We used functional magnetic resonance imaging (fMRI) to investigate brain activity induced by mere self-touch (rubbing the left hand with the right) in a pain-free and stress-free situation, and carried out the Physio-Physiological Interaction (PPI) analysis to investigate the modulatory effects of brain activity.
Keywords
Self-touch, Self-soothing, Descending pain modulatory system (DPMS), Bodily self, Anterior cingulate cortex (ACC), Amygdala, Rostral ventromedial medulla (RVM), Secondary somatosensory cortex (SII)
Abbreviations
T: Tesla; MRI: Magnetic Resonance Imaging; BOLD: Blood Oxygenation Level Dependent; TR: Repetition Time; TE: Echo Time; FOV: Field of View; FDR: False Discovery Rate; FWE: Family Wise Error; SVC: Small Volume Correction
Introduction
In monkeys, infants deprived of their mothers exhibit a striking decrease in social interactions with others and an increase in self-comforting behaviors [1-3]. In adults, nonverbal “selfadaptors” are unintentional movements that involve self-touching behaviors such as stroking or rubbing one’s own hand, and are associated with a lack of conscious awareness, occurring in response to situational anxiety and stress [4-8]. In another example, we often grasp a painful hand with the other hand to reduce the pain. This selftouch is also unintentionally and automatically induced by the painful stimulation. Accordingly, self-touch is considered to be an act of coping with harmful or stressful situations, and is therefore important to the ‘survival of the organism’ [9].
Experimental studies have shown that self-touch suppresses painful sensations [10,11]. Kammers, et al. [10] investigated the influence of touch on paradoxical pain using the thermal grill illusion (TGI), showing that self-touch suppresses thermal pain. They induced the TGI by placing the participant’s index and ring fingers in hot water, and the middle finger in cold water. This results in a paradoxical feeling of painful heat in the middle finger. It was shown that the illusioninduced pain is reduced when the fingers of the other hand touched the fingers used to induce the illusion. Even in pain-free tactile perception, many experimental studies provide considerable evidence for sensory attenuation via self-touch in healthy individuals [12,13]. These findings support the view that self-touch suppresses not only feelings of pain but also somatosensory perception more generally, with some cooccurring self-soothing (i.e., anti-sympathetic) effects being demonstrated. In addition, this view suggests that inhibitory effects on somatosensory afferents and sympathetic activity commonly underlie these various kinds of selftouch phenomena.
Neuroimaging data show that self-generated tactile stimuli attenuate neuronal responses in regions of the somatosensory cortex (including SI and SII regions) [11-14] associated with sensory attenuation. In addition to deactivation of SI and SII, the anterior cingulate cortex (ACC) is also deactivated. However, this phenomenon has only been discussed in terms of its role in emotional awareness, without a discussion of its regulatory role in sensory and cortical suppression, for example that self-generated stimuli are less tickly or painful than are externally produced stimuli [11-13]. Predictive forward models are generally assumed to underlie such attenuation, with the model involving a prediction about the sensory consequences of an action [12,13].
In contrast, studies of pain control have shown that the ACC plays a key role in cortical control of the brainstem opioid system, which comprises the periaqueductal grey (PAG) and the rostral ventromedial medulla (RVM) [14-16], during both opioid and placebo analgesia [17,18]. These neural structures constitute the descending pain modulatory system (DPMS) [19]. In particular, both the ACC and amygdala are opioid-rich. They contribute to and influence the expression of behavioral opioid analgesia via connections with the RVM, which plays a critical role in pain inhibition through interactions with the spinal cord. The RVM shapes spinal processing in a top-down fashion through direct presynaptic actions on the dorsal horn terminals of primary afferent fibers. Based on these, it is suggested that mere self-touch automatically induces the ACC-amygdala-RVM (AAR) system activity not only in painful situations but also in pain-free situations.
Furthermore, stress induces analgesia through descending inhibitory pathways from the amygdala and RVM to the spinal cord [20]. This suggests that stress-induced analgesia is modulated by the endogenous opioid system, and this system may play an important role in soothing effects. Indeed, the RVM is thought to be involved in cardiovascular and respiratory regulation [21,22]. In animal studies, contexts that induce stress have been described as important for activation of the endogenous opioid system [23]. There are indications that the self-soothing effects of certain animal behaviors might result from the release of opioids [24]. Moreover, involvement of endogenous opioids and anti-sympathetic effects has also been implicated in human self-soothing behaviors [24,25]. These findings suggest that the role of the AAR system is not just to regulate somatosensory perception including pain perception, but also to modulate the general state of the organism. In fact, intrinsic functional connectivity among regions of the DPMS including ACC and RVM has also been demonstrated in even non-painful situations [26].
Based on these considerations, it is suggested that there is a common neural basis underlying various kinds of self-touch in painful, painfree, stressful or stress-free situations, and the AAR system plays a critical role in this process. In addition, it has been suggested that SII is involved in the effects of self-touch [10,27], given that SII plays a critical role in multisensory/motor integration across both hands. In fact, human neuroimaging studies have shown modulation of blood oxygenation level-dependent (BOLD) responses in SII during bimanual self-touch [12]. Therefore, synchronous activity of the bilateral SII is also suggested to play an important role in the neural mechanisms underlying self-touch.
Here, we investigated the involvement of the AAR system and synchronous activity of the bilateral SII in simple self-touch (rubbing the back of the left hand with the palm of the right) that is usually observed in everyday situations rather than painful or stressful situations. We investigated two ways of rubbing the hand, which involved either circular (C) or backand- forth (BF) rubbing, and investigated the averaged contrast of (C + BF), focusing on neural activity independent of hand motion as far as possible. Subsequently, we investigated the modulatory effects of the amygdala (source of modulation) on the connection from the rostral ACC (rACC, source of connectivity) to the RVM, and the modulatory effect of coherent bilateral SII activity to the RVM. These effects were investigated with the physio-physiological interaction (PPI) method [28].
Methods and Materials
▪ Participants
Twelve healthy female participants (31.5 ± 3.7 years) took part in the experiment. All participants were right-handed according to the Chapman test (13.3 ± 0.6) and had no history of neurological or psychiatric disorders. Participants provided informed consent to participate in the present study. The Research Ethics Committee of Tokyo Metropolitan University approved this study and all methods were carried out in accordance with the approved guidelines.
▪ Stimuli, trial protocol, and procedure
We used two ways of rubbing the hand, which involved either circular (C) or back-and-forth (BF) rubbing of the back of the left hand with the palm of the right. The participant was instructed to relax and close her eyes without thinking about anything specific. Three seconds before condition onset, the researcher let the participant know whether the next condition to be performed was C or BF by placing the participant’s right palm softly on the back of her left hand and moving her right hand in the appropriate manner (C or BF) for 2 sec. A session consisted of 8 trials (2 conditions × 4 times), with the trials counterbalanced across participants. A block-design paradigm was applied, with each trial lasting 32 sec and resting for 8 sec.
▪ fMRI data analysis
Scanning was conducted using a 3.0T MRI system (Achieva Quasar Dual, Philips). BOLD T2*-weighted MR signals were measured with a gradient echo-planar imaging (EPI) sequence (TR = 4,000 msec, TE = 35 msec, flip angle = 90°, FOV = 23 cm2, scan matrix = 128 × 128, total scan time = 324 sec, slice thickness = 5 mm, 25 slices per volume). Image processing was conducted with Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm8). EPI images were realigned and normalized to MNI (Montreal Neurological Institute) stereotactic space. Normalized images were smoothed using an 8 mm full-width half-maximum Gaussian kernel. The data were temporally convolved with the hemodynamic response function (HRF) and high-pass filtered with a cut-off period of 128 sec.
Each C and BF condition was modeled with a separate regressor. We tested significance in activation/deactivation for the contrast of (C + BF) vs. baseline with a height threshold of p<0.0005 and an extent threshold of p<0.05 (FDR, corrected), to focus on neural activity independent of hand motion as far as possible. Subsequently, among the averaged brain regions showing significant deactivation and activation, we selected regions specifically involved in motor (e.g., left primary motor cortex (MI) and right CB), somatosensory (e.g., right SI and left CB), and emotion (e.g., insula) processing as regions of interest (ROIs, 5 mm radius sphere centered at peak coordinates) for PPI analysis. Moreover, we searched the local maximum points within the nearest 8 mm from the coordinates of the rACC (-10 34 16) [29], right amygdala (16 0 -16) [30,31], and RVM (-6 -35 -39) [31], in the averaged activation/deactivation map. We then selected the spherical regions within a 5 mm radius centered at these points and investigated significant activation/deactivation at p<0.05 (FWE, SVC) as ROIs for the PPI analysis.
We also set additional ROIs that showed significant positive and negative connectivity to the RVM ROI seed for (C + BF) vs. baseline with CONN software (version 13, http://www.web.mit.edu/swg/software.htm). Functional imaging data were first band-pass filtered with a range of 0.01–0.10 Hz. Voxel-by-voxel functional time-series data for the whole brain were regressed onto time-series data for the RVM seed region with general linear model (GLM) analysis, removing confounding effects related to white matter and cerebrospinal fluid BOLD signals, head motion (realignment parameters), and the main condition effects (hemodynamic model convolved with the HRF). For each participant, we calculated connectivity between the RVM seed and voxels across the whole brain for the (C + BF) condition. In the second-level between-subjects analysis, we tested whether functional connectivity with the RVM ROI was significant (for each region of positive and negative connectivity, with a height threshold of p<0.0005 and an extent threshold of p<0.05, FDR, corrected). Finally, among the brain regions showing significant connections, we set additional ROIs that are also related to somatosensory processing (e.g., SII and left CB).
We applied a PPI analysis [28] to (C + BF) in SPM8. In this GLM analysis assessing AAR system involvement, the modulatory effects of the amygdala (source of modulation) on the connection from the rACC (source of connectivity) to target regions were calculated across the whole brain for each participant. These effects were modeled as follows. The signal time series for any given voxel (Xi) was regressed on XrACC, denoting the mean-corrected vector containing the activation time series obtained as the first eigenvariate in the rACC ROI, as well as the interaction term XrACC × Xamy, wherein Xamy contains the activation time series obtained as the first eigenvariate in the ROI for the amygdala. The interaction term equals the element-byelement product of the mean-corrected vectors. To calculate this term, the rACC and amygdala signal time series were deconvolved [32] to compute the underlying neural signal, and then the interaction term was calculated by convolving the product of the neural signals with the HRF. The parameter estimate (β1) for XrACC × Xamy is thought to reflect the amygdala’s modulatory effect on connectivity from the rACC. The t-map based on the null hypothesis “β1 = 0” was created using the following statistical model:
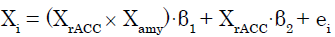 (1)
(1)
Where in β1 and β2 are parameter estimates and ei is an error term. Connectivity analysis results from individual participants were subjected to a group analysis with a random effects model. For each of the ROIs except the rACC and right amygdala, we then tested whether the eigenvariate value was significant (p<0.05, FWE, SVC).
To investigate the modulatory effect of coherent bilateral SII activity, we specifically set ROIs (left SII and right SII) and their interaction in the regression model. The subsequent regression model was expressed as follows [28,33]:
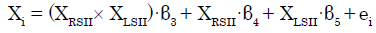 (2)
(2)
where XRSII and XLSII denote the time series of the two ROIs. The parameter estimate (β3) for XRSII × XLSII is the effect of the synchronous activity (interaction) of both SIIs on connectivity with Xi. The t-map based on the null hypothesis was set as “H0: β3 = 0” For each of the ROIs except for the left and right SII, we then tested whether the eigenvariate value was significant (p<0.05, FWE, SVC).
Subsequently, to assess the relationship between activation/deactivation of significantly modulated ROIs and strength of the modulatory effect (β1 or β3), we conducted a simple linear regression analysis with the eigenvariate value for the ROI as the dependent variable and the modulatory effect as the independent variable (p<0.05). Moreover, we checked the residuals by performing the Shapiro-Wilk (S-W) test of normality (p<0.05), and calculated the Durbin- Watson (D-W) statistic for the null hypothesis of no autocorrelation (using SPSS ver. 20, IBM).
Finally, we investigated activation/deactivation relationships between the right SI and the left CB for (C + BF). We conducted a simple linear regression analysis with the eigenvariate value for the right SI ROI as the dependent variable and that of the left CB ROI as the independent variable (p<0.05). Moreover, we checked the residuals by performing the S-W test of normality (p<0.05), and calculated the D-W statistic for the null hypothesis of no autocorrelation.
Results
▪ Activation and deactivation during selfrubbing
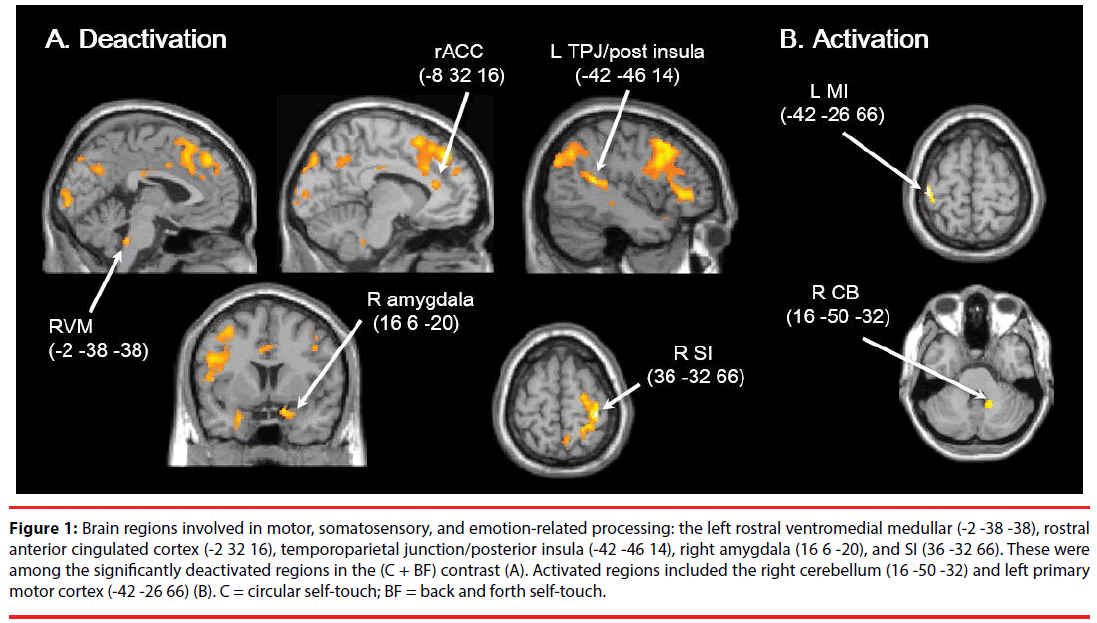
Whole brain analysis showed that significant deactivation was observed in the left ventrolateral prefrontal cortex (VLPFC), orbitofrontal cortex (OFC), temporal pole, dorsomedial prefrontal cortex (DMPFC), posterior insula/temporoparietal junction (TPJ) (-42 -46 14), inferior parietal lobe (IPL)/TPJ, temporal region/ superior temporal sulcus (STS), right SI (36 -32 66), and lateral occipital cortex (LOC), as well as the dorsolateral prefrontal cortex (DLPFC) bilaterally (Table 1, Figure 1A). In contrast, significant activation was observed in the left MI (-42 -26 66) and right CB (16 -50 -32) (Table 1, Figure1B). Moreover, the ROI analyses showed significant deactivation in the left rACC (-8 32 16) (p = 0.002, t = 5.38, SVC, Euclidean distance (Ed) from rACC (-10 34 16) = 2.83 mm), left RVM (-2 -38 -38) (p = 0.001, t = 5.21, SVC, Ed from RVM (-6 -35 -39) = 5.10 mm), and the right amygdala (16 6 -20) (p = 0.002, t = 5.55, SVC, Ed from amygdala (16 0 -16) = 7.21 mm). Among these regions, those involved in motor, somatosensory, and emotion processing included the right SI (36 -32 66), amygdala (16 6 -20), CB (16 -50 -32), left MI (-42 -26 66), RVM (-2 -38 -38), posterior insula/TPJ (-42 -46 14), and rACC (-8 32 16).
Figure 1: Brain regions involved in motor, somatosensory, and emotion-related processing: the left rostral ventromedial medullar (-2 -38 -38), rostral anterior cingulated cortex (-2 32 16), temporoparietal junction/posterior insula (-42 -46 14), right amygdala (16 6 -20), and SI (36 -32 66). These were among the significantly deactivated regions in the (C + BF) contrast (A). Activated regions included the right cerebellum (16 -50 -32) and left primary motor cortex (-42 -26 66) (B). C = circular self-touch; BF = back and forth self-touch.
| Brain area | L/R | x | y | z | Voxels |
|---|---|---|---|---|---|
| Deactivation | |||||
| Dorsolateral prefrontal cortex | R | 34 | 36 | 42 | 183 |
| 24 | 26 | 56 | |||
| 24 | 38 | 46 | |||
| L | -32 | 34 | 42 | 2095 | |
| Ventrolateral prefrontal cortex | L | -50 | 14 | 32 | |
| Orbitofrontal cortex | L | -34 | 16 | -20 | 161 |
| Temporo-parietal cortex | L | -26 | 10 | -26 | |
| -30 | 12 | -36 | |||
| Dorsomedial prefrontal cortex | L | -10 | 42 | 42 | 844 |
| 0 | 42 | 42 | |||
| Posterior insula/Temporo-parietal junction |
L | -42 | -46 | 14 | 1098 |
| IPL/TPJ | L | -52 | -40 | 24 | |
| -34 | -76 | 38 | |||
| Temporal/Superior temporal sulcus | L | -68 | -30 | -2 | 270 |
| -62 | -14 | -6 | |||
| -58 | -36 | -6 | |||
| Primary somatosensory cortex, SI | R | 36 | -32 | 66 | 839 |
| Lateral occipital complex | R | 20 | -92 | 22 | 717 |
| 14 | -82 | 48 | |||
| 10 | -90 | 8 | |||
| Activation | |||||
| Primary motor cortex, MI | L | -42 | -26 | 66 | 156 |
| Cerebellum | R | 16 | -50 | -32 | 142 |
Table 1: Deactivation and activation of brain areas associated with self-rubbing.
▪ Negative and positive functional connectivity with RVM
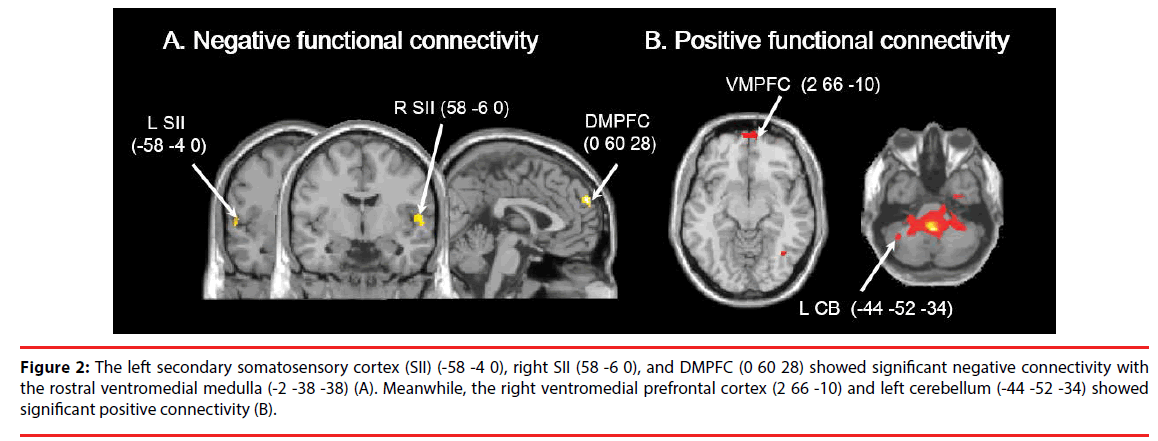
There was significant negative connectivity of the RVM with the DMPFC, left SII (-58 -4 0), and right SII (58 -6 0; Table 2, Figure 2A), and positive connectivity with the left CB (-44 -52 -34) and right VMPFC (2 66 -10; Table 2, Figure 2B).
Figure 2: The left secondary somatosensory cortex (SII) (-58 -4 0), right SII (58 -6 0), and DMPFC (0 60 28) showed significant negative connectivity with the rostral ventromedial medulla (-2 -38 -38) (A). Meanwhile, the right ventromedial prefrontal cortex (2 66 -10) and left cerebellum (-44 -52 -34) showed significant positive connectivity (B).
| Brain area | L/R | x | y | z | Voxels |
|---|---|---|---|---|---|
| Negative connectivity | |||||
| Dorsomedial prefrontal cortex | - | 0 | 60 | 28 | 82 |
| Secondary somatosensory cortex, SII | R | 58 | -6 | 0 | 57 |
| L | -58 | -4 | 0 | 55 | |
| Positive connectivity | |||||
| Dorsal pons/medulla | R | 2 | -42 | -36 | 1154 |
| 8 | -36 | -28 | |||
| 12 | -24 | -34 | |||
| Lateral occipital complex | R | 20 | -92 | 36 | 270 |
| 32 | -84 | 42 | |||
| 20 | -86 | 46 | |||
| L | -6 | -102 | 10 | 78 | |
| -12 | -102 | 18 | |||
| Ventral pons | R | 10 | -8 | -30 | 65 |
| Ventromedial prefrontal cortex | R | 2 | 66 | -10 | 66 |
| Cerebellum | L | -44 | -52 | -34 | 55 |
Table 2: Functional connectivity with the RVM.
▪ Relationship between the right SI and left CB
Activity in the right SI was positively correlated with that in the left CB (adjusted R2 = 0.620, t = 4.355, p = 0.001; S-W statistic = 0.978, p = 0.973; D-W statistic = 2.009).
▪ PPI analysis and regions showing activity correlated with the modulatory effect
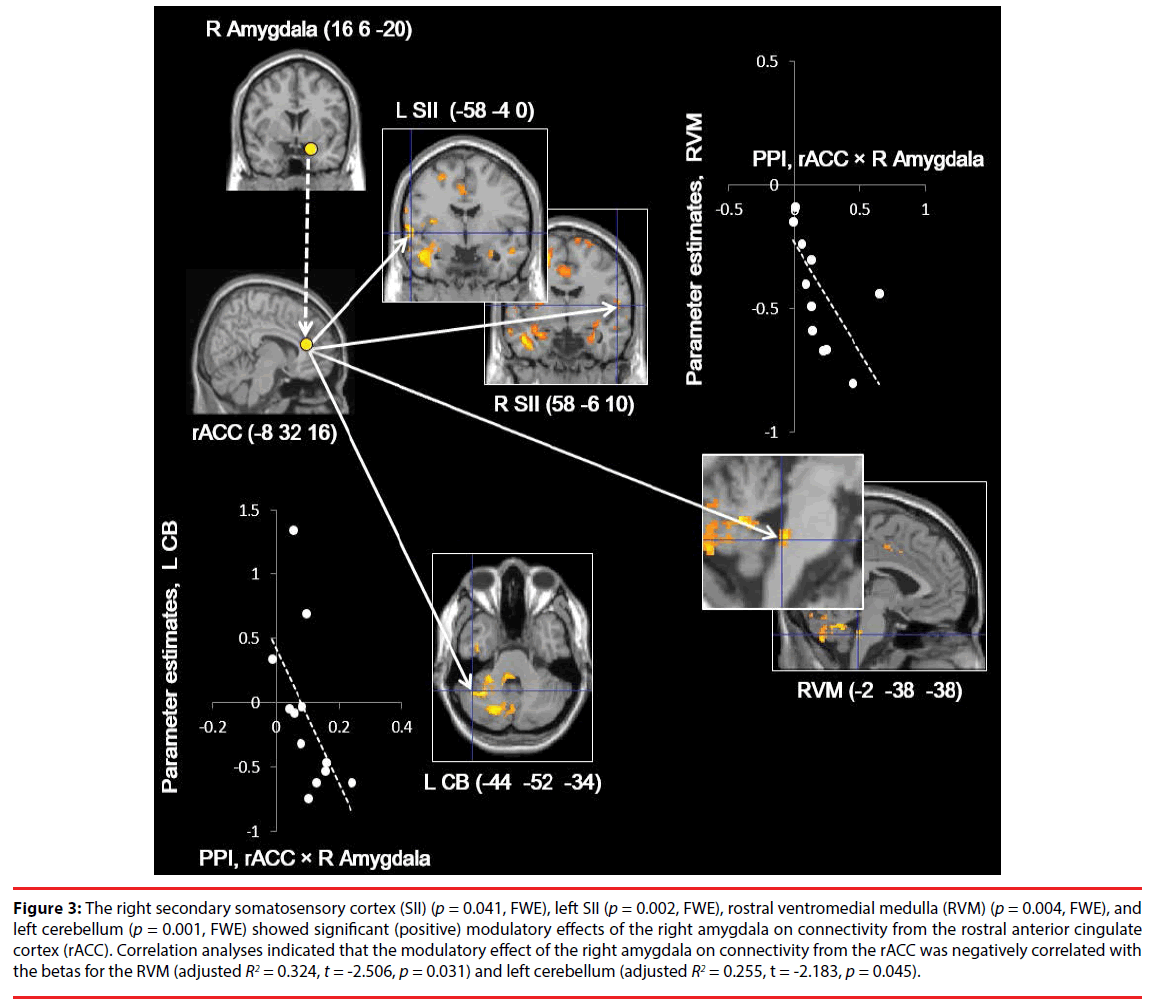
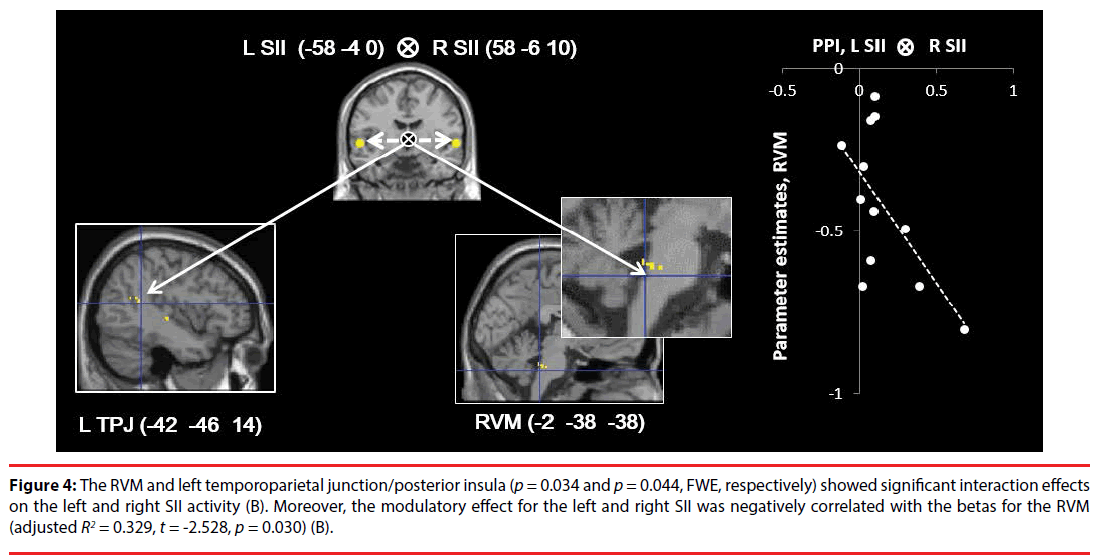
The right SII (p = 0.041, FWE), left SII (p = 0.002, FWE), RVM (p = 0.004, FWE), and CB (-44 -52 -34) (p = 0.001, FWE) (Figure 3A) showed significant (positive) modulatory effects of the right amygdala on connectivity from the rACC. There were no significant modulatory effects on the other ROIs (Figure 3). In addition, the RVM and left TPJ/posterior insula (p = 0.034 and p = 0.044, FWE, respectively) showed significant interaction effects on left and right SII activity (coherent SII activity) (Figure 4), while there were no significant modulatory effects on the other ROIs.
Figure 3: The right secondary somatosensory cortex (SII) (p = 0.041, FWE), left SII (p = 0.002, FWE), rostral ventromedial medulla (RVM) (p = 0.004, FWE), and left cerebellum (p = 0.001, FWE) showed significant (positive) modulatory effects of the right amygdala on connectivity from the rostral anterior cingulate cortex (rACC). Correlation analyses indicated that the modulatory effect of the right amygdala on connectivity from the rACC was negatively correlated with the betas for the RVM (adjusted R2 = 0.324, t = -2.506, p = 0.031) and left cerebellum (adjusted R2 = 0.255, t = -2.183, p = 0.045).
Figure 4: The RVM and left temporoparietal junction/posterior insula (p = 0.034 and p = 0.044, FWE, respectively) showed significant interaction effects on the left and right SII activity (B). Moreover, the modulatory effect for the left and right SII was negatively correlated with the betas for the RVM (adjusted R2 = 0.329, t = -2.528, p = 0.030) (B).
Correlation analyses indicated that the modulatory effect (β1) of the right amygdala on connectivity from the rACC was negatively correlated with the beta values for the RVM (adjusted R2 = 0.324, t = -2.506, p = 0.031, S-W statistic = 0.889, p = 0.114; D-W statistic = 1.188) and left CB (adjusted R2 = 0.255, t = -2.183, p = 0.045, S-W statistic = 0.870, p = 0.057; D-W statistic = 2.842) in whole brain deactivation (Figure 3), while there were no significant correlations for the other ROIs. Moreover, the modulatory effects of the left and right SII (β3) were negatively correlated with the beta values for the RVM (adjusted R2 = 0.329, t = -2.528, p = 0.030, S-W statistic = 0.957, p = 0.739; D-W statistic = 1.868) (Figure 4), while there was no significant correlation for the other ROI.
Discussion
We observed modulation of neural connectivity to the RVM from the rACC by the right amygdala, with RVM activity decreasing in proportion to the strength of the modulatory effect (β1). This suggests that the rACC suppresses RVM activity, which may successively suppress somatosensory afferent signals through interactions with the spinal cord via the right amygdala.
The rACC plays a key role in cortical control of the brainstem during both opioid and placebo analgesia [17,18]. The brainstem opioid system consists of a network of regions, including the PAG and RVM [15,16]. The ACC has one of the highest levels of opioid receptor binding in the cortex [34], and positron emission tomography (PET) studies indicate that the binding potential is specifically highest in the rACC [35,36]. A positive correlation has been shown between behavioral opioid analgesia and opioid-induced suppression of neuronal responses to noxious stimuli in the right amygdala and the RVM, which are the key DPMS structures [20]. The RVM plays a critical role in both inhibition and facilitation of pain through interactions with the spinal cord. Both off- and on-cells in the RVM project to the spinal dorsal horn, indicating that they exert modulatory influences on nociceptive/ non-nociceptive inputs [37]. RVM on-cells are directly inhibited by opioids, and it is suggested that these cells express mu-opioid receptors [38]. Moreover, increased RVM neuronal responses to noxious stimuli observed in human imaging studies indicate on-cell activity [39,40]. Therefore, we suggest that decreased responses in the RVM observed in the present study reflect inhibited on-cell activity through the AAR system activation. In fact, the AAR system can function even in the absence of painful/ stressful stimulation, because intrinsic functional connectivity among the DPMS regions including ACC and RVM has been demonstrated in such situations [26]. In addition, anatomical evidence that RVM neurons directly project to medullary and spinal cord cardiovascular sites suggests that RVM neurons elicit increases in arterial pressure by inhibiting parasympathetic control of the heart at the level of the medulla, and by activating direct descending pathways to spinal cord sympathetic preganglionic neurons [22,41,42]. Moreover, the RVM may also function in the control of respiration through direct connections to the phrenic nucleus [43,44]. Based on these facts, the present results suggest the possibility that self-touch regulates the autonomic nervous system, producing an antisympathetic effect in even a pain-free and stress-free situation. As with a possible input pathway to the AAR system in self-touch, sensory/motor-related information processed in the SII may be conveyed to the ACC and amygdala which are major regions in the AAR system, via the insula which directly connects to the SII.
In addition to the RVM, neural connectivity to the left CB from the rACC was also modulated by the right amygdala, and this activity decreased in proportion to the strength of the modulatory effect (β1), suggesting that the rACC suppresses left CB activity via the right amygdala. Moreover, right SI activity was positively correlated with left CB activity. Therefore, deactivation of both the RVM and left CB, interactively modulated by the rACC via the right amygdala, and may contribute to suppression of right SI activity. This suggests that the AAR system is involved in effective suppression of somatosensory cortex activity during self-touch. However, this suppression mechanism has only ever been discussed in terms of the internal forward model without discussion of its regulatory role in sensory and cortical suppression. In addition, neural connectivity to each of the left and right SII from the rACC was modulated by the right amygdala, suggesting that SII activity is synchronized with interactive activity between the rACC and right amygdala. This suggests that sensory/motor interactive processing in both SII regions is specifically important for the AAR system activation. Moreover, the SII region has significantly higher BP opiate receptor activity than SI/MI, a level comparable to the ACC, suggesting that the cortical anti-nociceptive effects of opiates are mediated not only by the rACC, right amygdala, and RVM, but also by SII, if it can be assumed that opioid binding mediates anti-nociception in this structure [44].
The amygdala receives information about the external environment from the sensory thalamus and sensory cortices. The basolateral cortex of the amygdala is reciprocally connected with cortical regions, particularly the midline prefrontal cortices (including the rACC and VMPFC), as well as sensory areas such as SI and SII [45]. The amygdala plays a central role in encoding and maintaining sensory associations with potential threat [46]. Therefore, deactivation of the right amygdala may reflect reduced threat value, suggesting that even mere self-touch can create a certain safe internal state for the self.
It has been suggested that autonomic control and efference copy signals [47] originate in the rACC, which can be interpreted as the “visceromotor cortex” given its function in the autonomic modulation of bodily arousal to meet behavioral demands [48-51]. The neural basis of interoceptive prediction signals is thought to be an output from the VMPFC, which has robust connections with limbic regions, including the amygdala, hypothalamus, midbrain, brainstem (including the RVM), and spinal cord areas, which are involved in regulation of the internal bodily state (physiological state) [52,53]. This is complemented by a parallel and partly reciprocal system of anti-sympathetic efferent drive operating through the VMPFC [48,54,55]. Moreover, the VMPFC connects certain categories of events based on memory records to somatosensory units in the somatosensory cortex (SI, SII) and to interoceptive units in the insula. In addition, VMPFC processes may use the self as a point of reference. The internal body representation may ultimately provide the primary reference, a material self, for interaction with the environment. The significant functional connectivity between the VMPFC and the RVM suggests that self-touch regulates the internal state based on the self as a reference.
The SII sub-region , which we observed to be correlated with the RVM, is part of the parietal operculum (OP4), which is associated with sensory/motor integration, including incorporating sensory feedback into motor actions, unlike the other sub-regions [56,57]. In addition, early interhemispheric somatosensory integration primarily occurs in SII, and therefore has behavioral importance in terms of bimanual object manipulation and exploration. While SII neurons are predominantly contralateral dominant, they have moderate to well-defined bilateral receptive fields that are usually larger than the receptive fields for SI neurons [58]. Coherent SII activity is thought to be actuated as a corresponding bodily state induced and maintained by temporally synchronous sensory/ motor signals from both hands, which are spatially symmetrical with respect to the body axis. Furthermore, this coherence is important for the maintenance of the body schema [59]. Moreover, coherent SII activity significantly modulated the left TPJ, which is involved in body ownership [60]. This suggests that the bodily self is specifically important for the effects observed, together with VMPFC involvement. In addition, synchronous SII activity is shown to bias the routing of signals towards the ACC [61]. In our study, stronger bilateral SII activity coherence was associated with more RVM deactivation. Accordingly, coherent SII activity is thought to be an important factor for inducing the AAR system activity based on the bodily self. Our study suggested that this mechanism depends not only on the AAR system but also that bilateral coherence is associated with an enhanced sense of self-awareness.
The present study indicated the possibility that mere self-touch induces activation of the AAR system, which suppresses somatosensory afferents from the touched hand and regulates the sympathetic nervous system, in a pain-free and stress-free situation. It should be noted here that the neural mechanism observed in the present study, which exerts control over the RVM through modulatory effects of the rACC and amygdala, and bilateral SII activity, could be activated by mere self-touching behaviors, i.e., touching and rubbing the back of the left hand with the palm of the right in pain-free/ stress-free normal situations. Therefore, this mechanism is expected to be activated under the same self-touch situations, potentially explaining the effects of self-soothing behaviors, as well as attenuation of somatosensory perception and somatosensory cortex activity in pain-free/stressfree situations.
There are some limitations in the present study. First, simultaneous measurements of the autonomic responses were not performed in the present study, although we discussed the possibility that the AAR system activation is involved in antisympathetic effects associated with self-touch. To confirm it, simultaneous recording of the autonomic information such as heart rate and respiration should be performed. Second, it was not confirmed how opioid is actually involved in the effects associated with self-touching, in the present study. Some PET studies might be useful to confirm it.
Author contributions
Y.K. and M.N. analyzed the data, interpreted the results and wrote the paper. Y.K., M.S., and M.N. designed the experiment and collected the data. Y.K., M.S., A.M., and T.I. conducted the experiment.
Competing financial interests
The authors declare no competing financial interests.
References
- Cameron JL. Critical periods for social attachment: deprivation and neural systems in rhesus monkeys. Soc. Res. Child. Development 2-054 (2001).
- Sabatini MJ, Ebert P, Lewis DA, et al. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J. Neurosci 27(12), 3295-3304 (2007).
- Knudsen EI, Heckman JJ, Judy L, et al. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proc. Natl. Acad. Sci. USA 103(27), 10155-10162 (2016).
- Ekman P, Friesen WV. Nonverbal leakage and clues to deception. Psychiatry 32(1), 88-102 (1969).
- Ekman P, Friesen WV. Nonverbal behavior and psychopathology. In: Friedman RJ, Kats MM, editors. The psychology of depression: contemporary theory and research. Washington DC, Winston & Sons, USA (1974).
- Waxer PH. Nonverbal cues for anxiety: an examination of emotional leakage. J. Abnorm. Psych 86(3), 306-314 (1977).
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Person. Individ. Diff 6(1), 21-29 (1985).
- Knapp ML, Hall JA. Nonverbal communication in human interaction. 5th ed. Wadsworth, Thomas Learning (2007).
- Harrigan JA. Self-touching as an indicator of underlying affect and language processes. Soc. Sci. Med 20(11), 1161-1168 (1985).
- Kammers MP, de Vignemont F, Haggard P. Cooling the thermal grill illusion through self-touch. Curr. Biol 20(20), 1819-1822 (2010).
- Wang Y, Wang JY, Luo F. Why self-Induced pain feels less painful than externally generated pain: distinct brain activation patterns in self- and externally generated pain. PLoS. ONE 6(8), e23536 (2011).
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat. Neurosci 1(7), 635-640 (1998).
- Blakemore SJ, Wolpert DM, Frith CD. The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. Neuroimage 10(4), 448-459 (1999).
- Chapman CE. Active versus passive touch: factors influencing the transmission of somatosensory signals to primary somatosensory cortex. Can. J. Physiol. Pharmacol 72(5), 558-570 (1994).
- Fields H. State-dependent opioid control of pain. Nat. Rev. Neurosci 5(7), 565-575 (2004).
- Petrovic P. Opioid and placebo analgesia share the same network. Semin. Pain. Med 3(1), 31-36 (2005).
- Zubieta JK, Ketter TA, Bueller JA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch. Gen. Psychiatry 60(11), 1145-1153 (2003).
- Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat. Rev. Neurosci 16(7), 403-418 (2015).
- Denk F, McMahon B, Tracy I. Pain vulnerability: a neurobiological perspective. Nat. Neurosci 17(2), 192-200 (2014).
- Hopkins E, Spinella M, Pavlovic ZW, et al. Alterations in swim stress-induced analgesia and hypothermia following serotonergic or NMDA antagonists in the rostral ventromedial medulla of rats. Physiol. Behav 64(3), 219-225 (1998).
- Hadziefendic S, Haxhiu MA. CNS innervation of vagal preganglionic neurons controlling peripheral airways: a transneuronal labeling study using pseudorabies virus. J. Autonom. Nerv. Syst 76(2-3), 135-145 (1999).
- Gowen MF, Ogburn SW, Suzuki T, et al. Collateralization of projections from the rostral ventrolateral medulla to the rostral and caudal thoracic spinal cord in felines. Exp. Brain. Res 220(2), 121-123 (2012).
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev 1(4), 429-438 (1994).
- Mason W. A. Stereotypies: a critical review. Anim. Behav 41(1), 1015-1031 (1991).
- Gračanin A, Bylsma LM, Vingerhoets AJ. Is crying a self-soothing behavior? Front. Psychol 5(1), 502 (2014).
- Kong J, Tu PC, Zyloney C, et al. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav. Brain. Res 211(2), 215-219 (2010).
- Hogendoorn H, Kammers M, Haggard P, et al. Self‑touch modulates the somatosensory evoked P100. Exp. Brain. Res 233(10), 2845-2858 (2015).
- Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6(3), 218-229 (1997).
- Atlas LY, Wager TD. A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions. Handb. Exp. Pharmacol 225, 37-69 (2014).
- Etkin A, Egner T, Peraza DM, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51(6), 871-882 (2006).
- Eippert F, Bingel U, Schoell ED, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63(4), 533-543 (2009).
- Gitleman DR, Penny WD, Ashburner J, et al. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage 19(1), 200-207 (2003).
- Di X, Biswal BB. Modulatory interactions of resting-state brain functional connectivity. PLoS. ONE 8(8), e71163 (2013).
- Vogt BA, Wiley RG, Jensen EL. Localization of Mu and delta opioid receptors to anterior cingulate afferents and projection neurons and input/output model of Mu regulation. Exp. Neurol 135(2), 83-92 (1995).
- Jones AK, Qi LY, Fujirawa T, et al. In vivo distribution of opioid receptors in man in relation to the cortical projections of the medial and lateral pain systems measured with positron emission tomography. Neurosci. Lett 126(1), 25-28 (1991).
- Willoch F, Schindler F, Wester HJ, et al. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain 108(3), 213-220 (2004).
- Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J. Neurophysiol 74(4), 1742-1759 (1995).
- Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience 48(3), 533-543 (1992).
- Dunckley P, Wise RG, Fairhurst M, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J. Neurosci 25(32), 7333-7341 (2005).
- Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis. Arthritis. Rheum 61(9), 1226-1234 (2009).
- Cox BF, Brody MJ. Subregions of rostral ventral medulla control arterial pressure and regional hemodynamics. Am. J. Physiol 257(3 Pt 2), R635-640 (1989).
- Babic T, Ciriello J. Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventromedial medulla. J. Comp. Neurol 469, 391-412 (2004).
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motorneurons in rats. J. Comp. Neurol 347(1), 64-86 (1994).
- Baumgärtner U, Buchholz HG, Bellosevich A, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage 30(3), 692-699 (2006).
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol55(3), 257-332 (1998).
- Costafreda SG, Brammer MJ, David AS, et al. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain. Res. Rev 58(1), 57-70 (2008).
- Harrison NA, Gray MA, Gianaros PJ, et al. The embodiment of emotional feelings in the brain. J. Neurosci 30(38), 12878-12884 (2010).
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int. J. Psychophysiol 73(2), 88-94 (2009).
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29(2), 537-545 (2001).
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain. Struct. Funct 214(5-6), 535-549 (2010).
- Anil KS, Suzuki K, Critchley H. An interoceptive predictive coding model of conscious presence. Front. Psychol 2(1), 395 (2011).
- Barbas H, Saha S, Rempel-Clower N, et al. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC. Neurosci 4(1), 25 (2003).
- Barrett LF, Bar M. See it with feeling: affective predictions during object perception. Philos. Trans. R. Soc. Lond. B. Biol. Sci 364(1521), 1325-1334 (2009).
- Nagai Y, Critchley HD, Featherstone E, et al. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage 22(1), 243-251 (2004).
- Wager TD, Waugh CE, Lindquist M, et al. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 47(3), 821-835 (2009).
- Rizzolatti G, Wolpert DM. Motor systems. Curr. Opin. Neurobiol 15(6), 623-625 (2005).
- Eickhoff SB, Jbabdi S, Caspers S, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J. Neurosci 30(18), 6409-6421 (2010).
- Iwamura Y. Bilateral receptive field neurons and callosal connections in the somatosensory cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci 355(1394), 267-273 (2000).
- van Stralen HE, van Zandvoort MJE, Dijkerman HC. The role of self-touch in somatosensory and body representation disorders after stroke. Philos. Trans. R. Soc. Lond. B. Biol. Sci 366(1581), 3142–3152 (2011).
- Heydrich L, Blanke O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain 136(Pt 3), 790-803 (2013).
- Hauck M, Lorenz J, Engel AK. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J. Neurosci 27(35), 9270-9277 (2007).