Case Report - International Journal of Clinical Rheumatology (2023) Volume 18, Issue 9
The many faces of IG4 related disease: two unusual case presentation
Nehal–El Ghobashy*1, Esraa seif2, Rana Hussein3, Geilan Mahmoud4*
Rheumatology department, Faculty of medicine, Cairo University
Rheumatology department, Faculty of medicine, Cairo University
E-mail: nehalhamdy7@gmail.com
Received: 27-Aug-2023, Manuscript No. fmijcr-23-114125; Editor assigned: 29- Aug-2023, Pre-QC No. fmijcr-23-114125 (PQ); Reviewed: 12-Sep-2023, QC No. fmijcr-23-114125; Revised: 14-Sep-2023, Manuscript No. fmijcr-23-114125 (R); Published: 21-Sep-2023, DOI: 10.37532/1758- 4272.2023.18(9).245-252
Abstract
First case: Systemic Lupus Erythematosus (SLE) overlap with IgG4 related orbital disease (IgG4 ROD): A 25 –year-old female known case of SLE for the last 8 years diagnosed according 2012 SLICC classification criteria. She presented to us with recurrent left eye protrusion associated with pain and no any other local or systemic manifestations and showed incomplete response to corticosteroids. A computed tomographic scan of the orbit showed left dacryoadenitis. Lacrimal gland biopsy showed mixed acute and chronic inflammatory process with marked collagenization and sclerosis. Immunohistochemistry showed finding compatible with IgG4 related disease and serum level of IgG4 subclass was 156 mg/dl. Pulse intravenous methylprednisolone was administrated followed by rituximab with a dose of 2 g with 2 weeks apart with dramatic response and no recurrence throughout 8 months of follow up. Second case: IG4- related sclerosing cholangitis: A 65-year –old male patient presented with abdominal pain, picture of obstructive jaundice and marked weight loss. CT abdomen showed intrahepatic biliary dilation, hepatomegaly. ERCP showed stenosis in biliary tract after which stenting was done. 1-month later recurrence of symptoms. MRCP showed: Malfunctioning CBD stent with moderate intrahepatic biliary dilation with cholangitis .abrupt termination of the dilated biliary tree noted near the portahepatis region attributed to chronic inflammation and fibrosis. Picture of chronic pancreatitis. Liver biopsy showed: picture of cholestatic liver disease with portal tract fibrosis and relative increased IG4 plasma cells. Pulse intravenous methylprednisolone was administrated followed by mycophenolate mofetyl of a dose 2ag per day with marked improvement and no recurrence throughout 3-month follow up.
Introduction
Case presentation (1)
A 25-year-old female, with SLE fulfilling the 2012 classification criteria for SLE [1] presented to the rheumatology outpatient clinic of Kasr Alainy Hospital of Cairo University with recent gradual onset of left eye protrusion associated with eye pain with no visual symptoms or other systemic complaints.
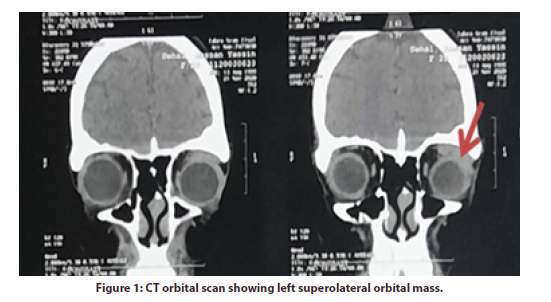
She was diagnosed at the rheumatology outpatient clinic as SLE in 2012 with polyarthritis, maculopapular skin rash, proteinuria with 24-hour urinary proteins 5g/24 hours, low complement 3 (C3), positive speckled antinuclear antibody (ANA) and positive anti-double-stranded DNA (antids DNA); fulfilling the 2012 classification criteria for SLE [1]. In June 2018, the patient came complaining of left eye protrusion associated with eye pain. She denied any recent history of trauma, fever, weight loss or other constitutional manifestations. The ophthalmological examination of the left eye showed proptosis, mild increase of the intraocular pressure (IOP 21 mmHg), relative afferent pupillary defect (RAPD) grade 1, with cup to disc ratio (CDR) 0.4 with evident temporal pallor; findings that signify optic nerve dysfunction secondary to stretch of the optic nerve caused by proptosis. There was left mild eye dryness evident by presence of punctate keratopathy (minute epithelial corneal erosions) because of proptosis that lead to lagophthalmos (incomplete eyelid closure) while Schirmer test type 1 was 13mm (normal > 10mm) denoting good tear production. There was no conjunctival injection or chemosis as signs for infection and the overlying skin was normal. Thyroid function tests were within normal limits (thyroid stimulating hormone (TSH) was 2.5mIU/L, free T3 80 ng/dL and free T4 was 0.9ng/ dL). Hepatitis B surface antigen (HBsAg), hepatitis C virus antibodies (HCV-Ab) and tuberculin skin test were negative.Serological tests revealed a positive ANA, positive anti-dsDNA (136 IU/ml; normal <25 IU/mL) and positive anti-smith antibodies. Anti-Ro, Anti-La, cytoplasmic antineutrophil cytoplasmic antibodies (C-ANCA) and perinuclear antineutrophil cytoplasmic antibodies (P-ANCA) were negative (CT) scan of the orbits showed a well-defined soft tissue lesion at the superolateral aspect of the left orbit measuring 3 x 3 x 1.3 cm with homogeneous contrast enhancement and tiny calcific focus displacing the superior and lateral recti muscles as well as the optic nerve medially with consequent protrusion of the left eye globe with intact bony orbital boundaries, the right eye showed no abnormalities; giving a conclusion of left dacryoadenitis (Figure 1).
An inflammatory non-infectious cause was suggested based on the presence of an autoimmune disease in the absence of history of trauma, signs or laboratory markers of infection. Pulse intravenous methylprednisolone (1gm) was given daily for three consecutive days, followed by oral prednisone at 0.5 mg/kg/day for one month followed by gradual tapering, with continuation on HCQ and MMF with the same doses. However, the patient showed partial clinical improvement, with recurrence of proptosis after 3 months.
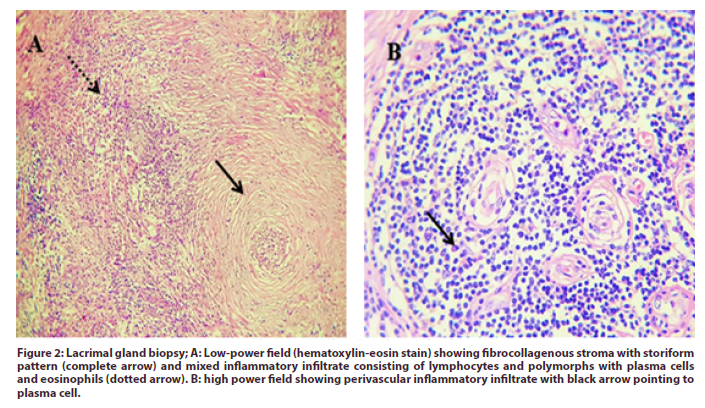
Due to the partial response to steroid therapy, biopsy was recommended. Lacrimal gland biopsy showed the picture of mixed acute and chronic inflammatory process with marked collagenization and sclerosis. The fibrocollagenous tissue showed a nodular infiltrate of mixed inflammatory cells, mostly perivascular, formed of lymphocytes and plasma cells with polymorphs (Figure 2) and immune-phenotyping was advised. The specimen was treated with; CD20, a marker for B cells, CD138 as a marker for plasma cells, IgG4 and CD3 as a marker for T lymphocytes.
Figure 2: Lacrimal gland biopsy; A: Low-power field (hematoxylin-eosin stain) showing fibrocollagenous stroma with storiform pattern (complete arrow) and mixed inflammatory infiltrate consisting of lymphocytes and polymorphs with plasma cells and eosinophils (dotted arrow). B: high power field showing perivascular inflammatory infiltrate with black arrow pointing to plasma cell.
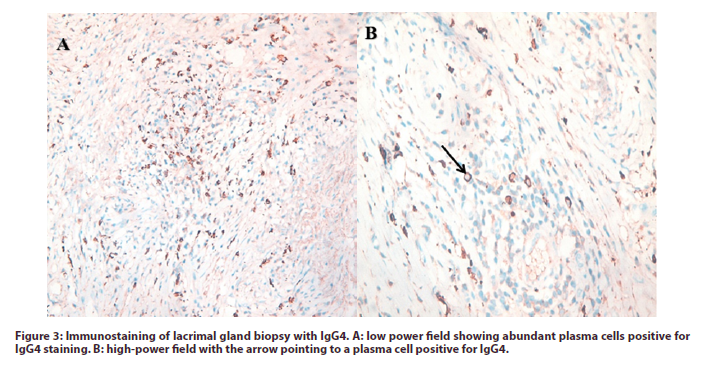
The results were positive for plasma cells with IgG4 subtypes more than 40% of plasma cells with more than 10 cells in the high power field and positive dense lymphocytes with scattered lymphoid follicles; compatible with IgG4-RD (Figure 3). Serum IgG4 level was assessed and the result was 156mg/dl (normal range: 8-135 mg/dl).
According to comprehensive diagnostic criteria of IgG4-R [2]; our patient was diagnosed as a case of IgG4- RD fulfilling the clinical presentation, radiological findings, histopathological features and immunostaining tests. Also, the case met the all newly established European League Against Rheumatism/American College of Rheumatology 2019 (ACR/EULAR) criteria of IgG4-RD with a total score of 21 [3].
A diagnosis of IgG4-related orbital disease (IgG4- ROD) associated with SLE was made. Pulse intravenous methylprednisolone (1gm) was given daily for three consecutive days, followed by oral prednisone 60 mg/d (1 mg/kg/d) for one month that was gradually tapered. Two doses of rituximab (1000 mg) were administrated intravenously two weeks apart. The patient showed dramatic response with no flare of her lupus or recurrence of proptosis throughout a follow up period of 10 months. Her routine labs including complete blood picture, ALT, AST, serum creatinine, and urine analysis; all were within normal ranges with ESR 11mm/h, Serum C3 was 94 mg/dL and 24 hour urinary proteins was 0.2 g/24h. Last follow up was in August 2021; the patient was regular on oral prednisolone 5 mg/d, HCQ 300mg/d, MMF 2g/d.
Case (2)
65- year old male patient presented with upper abdominal pain, jaundice, weight loss and generalized lymphadenopathy. Initial laboratory results showed total bilirubin: 2.04mg/dl (0.2-1.2), direct bilirubin: 1.24mg/dl (0-0.3), indirect bilirubin: 0.8mg/dl (0- 0.9), ALT: 18U/L (10-49), AST: 79(0-34) U/L, Gamma GT: 705 U/L (0-73). Serum albumin: 2.6g/ dl (3.2-4.8), serum creatinine 0.78. His CBC was within normal, elevated ESR: 80mm/hr. CT abdomen showed: hepatosplenomegaly, intra hepatic biliary duct dilatation. ERCP and stenting was done with improvement of his symptoms. One-month later recurrence of symptoms with elevation of liver enzymes and direct bilirubin. Laboratory investigations showed negative viral hepatitis. Negative immune profile including antinuclear antibodies (ANA), anti DNA anti bodies (ADNA Ab), anti-smooth antibodies, Liver kidney anti bodies (LKAB), normal c3 & c4 and negative anti phospholipid antibodies.
MRCP showed malfunctioning CBD stent associated with moderate intra hepatic biliary duct dilatation with cholangitis. Abrupt termination of the dilated biliary tree noted near the portahepatis region possibly attributed to chronic inflammation and fibrosis. Hepatomegaly with undue enlargement of the caudate lobe. Picture of chronic pancreatitis and Splenomegaly.
PET scan showed: abdomen and pelvis: intrahepatic biliary duct dilatation with metabolic FDG uptake along the biliary tree achieving up to 6.2 SUV max. Small active right anterior cardio-phrenic lymph node measuring 14 mm. metabolically active upper abdominal lymph nodes that are seen at (gastro-hepatic, porta hepatis and porto caval regions ) measuring up to 24 mm and achieving 13.09 SUV max. A biliary stent is noted with its terminal end at the 2nd part of duodenum. Active left external iliac lymph node measuring 20 mm and achieving 3.8 SUV max mostly reactive. Chest and musculoskeletal: free.
Ultrasound guided core biopsy form left external iliac lymph node showed: Reactive follicular and sinus hyperplasia, chronic lymphadenitis rich in plasma cells. Immunophenotyping showed: IGG4 is positive in 50 % of plasma cells. CD20 showed positive follicles and CD3 showed positive inter follicular areas. Conclusion: picture compatible with IG4 related lymphadenopathy.
Liver biopsy: examination of the prepared slides revealed 4 portal areas that are expanded by fibrosis with area of fibrosis. There is mild bile ductular reaction. Portal areas show moderate inflammatory infiltrate with some plasma cells. There is moderate cellular and canalicular cholestasis. The fibrotic areas shows moderate inflammatory reaction with moderate plasma cells. No evidence of steatosis or hemosiderosis. No evidence of granulomas or viral inclusions. No evidence of dysplasia. Immunohistochemical staining for IgG and IgG 4 revealed positive IgG4 > 30 % of the IgG positive cells. Conclusion: picture of chronic cholestatic liver disease with portal tract fibrosis and relative increased IgG 4 plasma cells.
The patient received IV pulse methylprednisolone 1 gram for 3 days followed by 0.5 mg /kg of oral prednisolone. Mycophenolate Mofetyl was added as steroid sparing drug in a dose of 2 gram daily. During the follow up the patient was markedly improved with no recurrence. His laboratory investigation at last visit follow up: total bilirubin: 0.8 mg/dl (0-1.2), direct bilirubin: 0.3 mg/ dl (0- 0.3), indirect bilirubin: 0.5mg/dl (0-0.9), ALT: 40U/L (0-41), AST: 34 U/L (0-40) and normal serum albumin.
Discussion
Case (1)
SLE has a variety of ocular manifestations, with keratoconjunctivitis sicca being the most common manifestation. Orbital involvement in SLE is relatively rare. Causes of unilateral orbital involvement in SLE include trauma, vascular causes such as cavernous sinus thrombosis or retrobulbar hematoma, infections such as orbital cellulitis , tumors such as lymphoma, and autoimmune causes such as thyroid eye disease, Sjögren syndrome, ANCA associated vasculitis and IgG4-RD [4-6].
There are several reports of SLE cases presenting with unilateral proptosis due to orbital pseudotumors including those due to idiopathic orbital inflammation (IOI) [7-9], orbital myositis [10] and only one case due to dacryoadenitis [11].
IgG4-RD is a fibro-inflammatory disease characterized by tumefactive lesions affecting almost all organs. IgG4- related orbital disease (IgG4-ROD) is most frequently reported in the lacrimal gland, but also in the orbital soft tissue, extraocular muscles, eyelids, sclera, optical and trigeminal nerves and orbital bones with overall orbital involvement representing approximately 17 to 23%. Involvement of the lacrimal gland is commonly bilateral although simultaneous affection may not occur. Associated salivary gland involvement is common with extra-ophthalmic involvement in 70 to 80 % [12-15].
According to the diagnostic criteria, IgG4-ROD accounts for nearly half of the cases that were previously diagnosed as orbital pseudotumors or idiopathic orbital inflammation [15, 16].
The association between SLE and IgG4-RD has been rarely reported. It was first reported as autoimmune pancreatitis presenting with SLE [17], two other cases had IgG4- related kidney disease associated with SLE [18, 19] and one case report described IgG4- related lung disease and SLE [20]. To our knowledge there is no previously reported case of SLE overlapping with IgG4 -ROD.
IgG4-RD is characterized clinically by the presence of tumefactive lesions in a typical organ. Radiological findings such as a sausage-shaped pancreas and periaortitis affecting the infrarenal aorta are strongly suggestive of IgG4-RD; however, radiological findings in isolation are never sufficient for diagnosis. The core pathologic features include lymphoplasmacytic infiltrate enriched in IgG4-positive plasma cells, and a variable degree of fibrosis that has a characteristic "storiform" pattern and obliterative phlebitis. However, these pathologic features may not be found typically in the lacrimal glands [12, 14, 21-23].
IgG4-RD may be confused with malignancy, infection or other immune-mediated conditions, such as Sjögren’s syndrome or ANCA associated vasculitis. These mimics may also exhibit elevated serum level of the IgG4 subclass and/or nonspecific infiltration of IgG4-positive cells. Therefore, diagnosis of IgG4-RD should be done on the basis of clinical presentation, radiological findings, histopathological features and immunostaining tests. Recently, it was reported that the presence of an elevated serum IgG4 level is no longer considered essential for the diagnosis of IgG4-RD and that the histopathological features remain the gold standard for diagnosis [2, 3].
The exact etiopathogenesis of IgG4-RD is still unknown. It has been suggested that the fibrotic and inflammatory processes that drive IgG4-RD are attributed to a combination of Th2 cells and regulatory T cells (Treg cells) which is contrary to most autoimmune disorders where the inflammatory process is propagated by T helper 1 (Th1) and/or Th17 subsets [15].
There is an emerging consensus that the IgG4 antibodies in this disease are not pathogenic, but rather an epiphenomenon, possibly having an anti-inflammatory role. IgG4 has been termed as “odd” antibody due to the fact of its unique structures in the hinge region that are thought to be responsible for its different biological properties, binding characteristics, and reduced effector function, compared to other IgG subclasses [15].
Single amino acid changes in the constant region of IgG4 are responsible for structural differences and occurrence what is called Fab fragment (Fragment, antigen binding) exchange phenomenon. The resulting antibodies are bispecific and can cross-link two different antigens instead of two antigens of the same kind. Consequently, IgG4 competes for antigen binding with other antibody subclasses, therefore effectively inhibits antibody effector mechanisms and prevents the formation of antibody-antigen immune complexes. Furthermore, it prevents complement activation or immune cell mediated mechanisms such as antibody dependent cellular cytotoxicity (ADCC) [15, 24, 25].
As during SLE progression, the inflammatory response induced by each autoreactive IgG subtype depends on its ability to activate effector function, IgG4 autoantibodies may bind autoantigens completely with other subclasses of autoantibodies. Thus, compared with other subclasses of autoantibodies, IgG4 autoantibodies may attenuate disease progression in SLE by suppressing complement consumption and inflammatory cytokine production. Therefore, SLE and IgG4- RD may overlap even in the absence of systemic activity or changes in biomarkers of acute inflammation and disease activity [26].
According to several studies ESR and CRP were elevated in the majority of IgG4-RD patients, and ESR exerted an excellent performance in predicting disease relapse. However, other studies showed that serum ESR and CRP levels are not necessarily elevated in patients with IgG4-RD with multiple organ involvement [27-30].
Hypocomplementemia in our patient could be either due to IgG4-RD or SLE. Although the emergence of immune complexes in the circulation with complement consumption is a typical feature of SLE, it has also been reported in IgG4-RD [15, 30-32].
In addition, Pan et al., [33] reported that serum levels of antinuclear IgG4 were higher in SLE patients with positive correlation with serum levels of total IgG4, albumin, and C3 and negative correlation with 24-hour urinary protein and concluded that IgG4 autoantibody may dampen the inflammatory response in SLE.
Moreover, the B-cell-activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL) BAFF/APRIL system that is known to be a key factor in the development of SLE, may be involved in the pathogenesis of IgG4-RD as the serum levels of these cytokines were elevated in patients with IgG4-RD. These findings indicate the presence of possible autoimmune mechanisms in IgG4-RD and may suggest a possible mechanism of the overlap with SLE [15, 34, 35].
Management of IgG4- ROD follows different strategies. A systematic review including 35 articles suggested that glucocorticoids (GC) are the preferred first-line systemic treatment with an initial dose of 0.5–1 mg/kg/day [36].
Rituximab is the most effective biologic disease modifying anti-rheumatic drug (DMARD) in IgG4- ROD especially in severe refractory or vision-threatening disease. For induction therapy two doses of 1000 mg each are given two weeks apart combined with GC. It may be used as maintenance therapy every 6 months for duration of two years [36, 37]. There is a case report of a SLE patient presenting with orbital pseudotumor that was refractory to cyclophosphamide and showed great response to rituximab [9]. This case could have been IgG4-ROD but no biopsy was obtained in this case.
In less severe cases of IgG4-ROD, MMF or infliximab can be considered as maintenance or steroid-sparing therapy while the efficacy of conventional DMARDs such as methotrexate and azathioprine is limited, so early initiation of rituximab is recommended in case of refractory and organ- or life-threatening disease [36]. Radiotherapy or surgery should be considered in localized refractory disease in patients with compressive optic neuropathy or proptosis [36, 38].
As B cell seems to have a role in the pathophysiology of SLE and IgG4- RD, B cell targeted therapies have emerged for management of both diseases [39, 40]. Recently, a case of SLE nephritis overlapping with IgG4- related kidney disease was successfully treated with belimumab [41].
The association between IgG4-RD and malignant lymphoma has been reported either concurrent or asynchronous, including; diffuse large B-cell, follicular, lymphoplasmacytic lymphoma and mucosal-associated lymphoid tissue (MALT) lymphoma especially in IgG4- ROD. Recently, a case of MALT lymphoma was reported that occurred in the right lacrimal gland 11 years after the diagnosis of IgG4-related dacryoadenitis in the left lacrimal gland [42]. High index of suspicion should be considered when rapidly enlarging or clinically refractory masses or lymphadenopathy are encountered in patients with IgG4-RD [28, 43].
Conclusion
We present an unusual case of SLE overlapping with IgG4 –ROD manifesting by unilateral dacryoadenitis that showed partial response to glucocorticoids and great response to rituximab.
Case (2)
IgG4-related sclerosing cholangitis (IgG4-SC) is the biliary manifestation of IgG4-related disease (IgG4- RD), a systemic fibro inflammatory condition that is characterized by mass lesions and/or strictures with classical histopathological findings in involved organs [44].
IgG4-SC has a male preponderance, usually presenting in the fifth and sixth decades of life. It is the most common extra pancreatic manifestation in patients with autoimmune pancreatitis (AIP type 1). Isolated IgG4-SC accounts for only 8% of cases in Western cohorts [45].
Clinical presentation depends on disease activity and organ involvement. Patients usually present with obstructive jaundice (70% to 80%), weight loss, and abdominal pain. No specific symptoms allow reliable differentiation from other causes of biliary obstruction. Those with concomitant AIP can have symptomatic pancreatic exocrine and endocrine insufficiency [46].
IgG4-SC can involve any part of the biliary tree, and it is best characterized by cholangiography
[47]. Particular features include long (>1/3 length) and multifocal strictures, mild upstream dilatation, and proximal biliary disease with diffuse pancreatic swelling and a thin, narrowed pancreatic duct. However, endoscopic retrograde cholangiopancreatography (ERCP) alone is a poor discriminator (88% specificity, 45% sensitivity) to differentiate IgG4-SC from PSC and CCA [48].
Serum IgG4 concentrations are raised (>1.4 g/L) in 65% to 80% at diagnosis.However, elevated IgG4 is seen in 5% to 25% of inflammatory, autoimmune, and malignant conditions and 5% of healthy individuals [49]. Serum IgG4 levels greater than 5.6 g/L increase the specificity and positive predictive value for differentiating IgG4-SC from PSC and CCA to 100%.Patients with a normal serum IgG4 have a distinct clinical phenotype, with a reduced risk for relapse and fewer organs involved [50].
Similar histopathological findings (lymphoplasmacytic infiltrate, obliterative phlebitis, and storiform fibrosis) are evident in all organs affected by IgG4-RD.11 In IgG4-SC, more than 10 IgG41 cells/HPF in a biopsy or more than 50 IgG41 cells/HPF in a resection specimen, and an IgG4/ IgG ratio greater than 40%, in the context of two of the earlier features, are considered diagnostic [51]. Limitations to interpretation include patchy distribution of disease, insufficient tissue sampling, and fibrotic phenotype. An abundance of IgG41 cells alone is not sufficient for diagnosis and can also be seen in PSC and CCA. [52].
Urgent treatment is appropriate in IgG4-SC, even when asymptomatic, to prevent infectious cholangitis and permanent fibrosis. The mainstay of treatment is systemic corticosteroids, despite an absence of randomized placebo controlled trials. Steroid use has been shown to induce remission quicker, more consistently, and with a lower relapse rate than a conservative approach [53]. International consensus suggests initiation with prednisolone 30 to 40 mg daily for 4 weeks, reducing by 5 mg every 2 weeks, depending on response [54].
Biliary stenting is useful to decompress the biliary tree in those with symptomatic obstructed biliary strictures.2 Stents can be removed and/or fall out spontaneously once steroid therapy is effective. Immunosuppressive drugs are used such as azathioprine & mycophenolate mofetyl are used to prevent relapse [55].
References
- Petri M, Orbai AM, Alarcón GS et al. Derivation and Validation of Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 64, 2677-2686 (2012).
- Umehara H, Okazaki K, Masaki Y et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 22, 21-30 (2012).
- Wallace ZS, Naden RP, Chari S et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis. 79, 77-87 (2020).
- Palejwala NV, Walia HS, Yeh S. Ocular Manifestations of Systemic Lupus Erythematosus: A Review of the Literature. Autoimmune Dis. 2012, 290898 (2012).
- Silpa-archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 100, 135-141 (2016).
- Dammacco R. Systemic lupus erythematosus and ocular involvement: an overview. Clin Exp Med. 18, 135-149 (2018).
- Gray RE, Jenkins EA, Hall MA et al. Recurrent acute proptosis in atypical systemic lupus erythematosus. Clin Rheumatol. 8, 528-532 (1989).
- Siebert S, Srinivasan U. Proptosis can be the presenting feature of systemic lupus erythematosus. Ann Rheum Dis. 63, 908-909 (2004).
- Escudero González CM, Rodríguez Montero S, Martínez Pérez R et al. [Resistant orbital pseudotumor treated with rituximab in a patient with systemic lupus erythematosus. A case presentation]. Reumatol Clin. 6, 214-216 (2010).
- Chan AJ, Rai AS, Lake S. Orbital myositis in systemic lupus erythematosus: A case report and literature review. Eur J Rheumatol. 7, 135–137 (2020).
- Brenner EH, Shock JP. Proptosis secondary to systemic lupus erythematosus. Arch Ophthalmol Chic Ill. 91, 81–82 (1974).
- Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum. 43, 806-817 (2014).
- Wu Albert, Andrew NH, McNab AA et al. IgG4-Related Ophthalmic Disease: Pooling of Published Cases and Literature Review. Curr Allergy Asthma Rep. 15, 27 (2015).
- Ebbo M, Patient M, Grados A et al. Ophthalmic manifestations in IgG4-related disease: Clinical presentation and response to treatment in a French case-series. Medicine (Baltimore). 96, e6205 (2017).
- Koneczny I. Update on IgG4-mediated autoimmune diseases: New insights and new family members. Autoimmun Rev. 19, 102646 (2020).
- Andrew NH, Sladden N, Kearney DJ et al. An analysis of IgG4-related disease (IgG4-RD) among idiopathic orbital inflammations and benign lymphoid hyperplasias using two consensus-based diagnostic criteria for IgG4-RD. Br J Ophthalmol. 99, 376-381 (2015).
- Kobayashi S, Yoshida M, Kitahara T et al. Autoimmune pancreatitis as the initial presentation of systemic lupus erythematosus. Lupus. 16, 133-136 (2007).
- Zaarour M, Weerasinghe C, Eter A et al. An Overlapping Case of Lupus Nephritis and IgG4-Related Kidney Disease. J Clin Med Res. 7, 575-581 (2015).
- Wada Y, Matsuo K, Ito Y et al. A Case of Concurrent IgG4-Related Kidney Disease and Lupus Nephritis, in: IgG4-Related Kidney Disease. 303-311 (2016).
- Chang LC, Wu CH, Hsu CL et al. IgG4-Related Lung Disease in a Patient with Systemic Lupus Erythematosus: A Case Report. Vol. 33, 6 (2008).
- Deshpande V, Zen Y, Chan JK et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol Off JUS Can Acad Pathol Inc. 25, 1181-1192 (2012).
- Khosroshahi A, Wallace ZS, Crowe JL et al. Second International Symposium on IgG4-Related Disease, International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol Hoboken NJ. 67, 1688-1699 (2015).
- Lin W, Lu S, Chen H et al. Clinical characteristics of immunoglobulin G4–related disease: a prospective study of 118 Chinese patients. Rheumatology. 54, 1982-1990 (2015).
- Vidarsson G, Dekkers G, Rispens T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front Immunol. 5, 520 (2014).
- Chiu ML, Goulet DR, Teplyakov A et al. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 8, 55 (2019).
- Pan Q, Xiao H, Shi L et al. IgG4 Autoantibodies Attenuate Systemic Lupus Erythematosus Progression by Suppressing Complement Consumption and Inflammatory Cytokine Production. Fron Immunol. 11, 1047 (2020).
- Pieringer H, Parzer I, Wöhrer A et al. IgG4- related disease: an orphan disease with many faces. Orphanet J Rare Dis. 9, 110 (2014).
- Wallace ZS, Deshpande V, Mattoo H et al. IgG4-Related Disease: Baseline clinical and laboratory features in 125 patients with biopsy-proven disease. Arthritis Rheumatol Hoboken NJ. 67, 2466-2475 (2015).
- Kasashima S, Kawashima A, Kasashima F et al. Inflammatory features, including symptoms, increased serum interleukin-6, and C-reactive protein, in IgG4-related vascular diseases. Heart Vessels. 33, 1471-1481 (2018).
- Tang J, Cai S, Ye C et al. Biomarkers in IgG4-related disease: A systematic review. Semin Arthritis Rheum. 50, 354-359 (2020).
- Saeki T, Kawano M, Mizushima I et al. The clinical course of patients with IgG4-related kidney disease. Kidney Int. 84, 826-833 (2013).
- Peng L, Lu H, Zhou J et al. Clinical characteristics and outcome of IgG4-related disease with hypocomplementemia: a prospective cohort study. Arthritis Res Ther. 23, 102 (2021).
- Pan Q, Guo L, Wu J et al. Association between IgG4 Autoantibody and Complement Abnormalities in Systemic Lupus Erythematosus. Mediators Inflamm. 2016, e2196986 (2016).
- Kiyama K, Kawabata D, Hosono Y et al. Serum BAFF and APRIL levels in patients with IgG4-related disease and their clinical significance. Arthritis Res Ther. 14, R86 (2012).
- Vincent FB, Morand EF, Schneider P et al. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 10, 365-373 (2014).
- Detiger SE, Karim AF, Verdijk RM et al. The treatment outcomes in IgG4-related orbital disease: a systematic review of the literature. Acta Ophthalmol (Copenh). 97, 451-459 (2019).
- Wu A, Andrew NH, Tsirbas A et al. Rituximab for the treatment of IgG4-related orbital disease: experience from five cases. Eye. 29, 122-128 (2015).
- Glass LRD, Freitag SK. Management of orbital IgG4-related disease. Curr Opin Ophthalmol. 26, 491-497 (2015).
- Bag-Ozbek A, Hui-Yuen JS. Emerging B-Cell Therapies in Systemic Lupus Erythematosus. Ther Clin Risk Manag. 17, 39-54 (2021).
- Yamamoto M. B cell targeted therapy for immunoglobulin G4-related disease. Immunol Med. 1–7 (2021).
- Yamamoto M, Aochi S, Suzuki C et al. A case with good response to belimumab for lupus nephritis complicated by IgG4-related disease. Lupus. 28, 786-789 (2019).
- Bledsoe JR, Wallace ZS, Stone JH et al. Lymphomas in IgG4-related disease: clinicopathologic features in a Western population. Virchows Arch. 472, 839-852 (2018).
- Liu Y, Fu J, Ning X et al. Malignancy Risk of Immunoglobin G4-Related Disease: Evidence from a Large Cohort Multicenter Retrospective Study. Rheumatol Ther. 8, 1207-1221 [2021].
- Culver EL, Chapman RW. IgG4-related hepatobiliary disease: an overview. Nat Rev Gastroenterol Hepatol. 13, 601-612 (2016).
- Ghazale A, Chari ST, Zhang L et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 134, 706-715 (2008).
- Huggett MT, Culver EL, Kumar M et al. Type 1 autoimmune pancreatitis and IgG4- related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 109,1675-1683 (2014).
- Nakazawa T, Ohara H, Sano H et al. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 32, 229 (2006).
- Kalaitzakis E, Levy M, Kamisawa T et al. Endoscopic retrograde cholangiography does not reliably distinguish IgG4-associated cholangitis from primary sclerosing cholangitis or cholangiocarcinoma. Clin Gastroenterol Hepatol. 9, 800-803.e2 (2011).
- Culver EL, Sadler R, Simpson D et al. Elevated serum IgG4 levels in diagnosis, treatment response, organ involvement, and relapse in a prospective IgG4-related disease UK cohort. Am J Gastroenterol. 111, 733-743 (2016).
- Boonstra K, Culver EL, de Buy Wenniger LM et al. Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology. 59, 1954-1963 (2014).
- Deshpande V, Zen Y, Chan JK et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 25, 1181-1192 (2012).
- Bateman AC, Culver EL. IgG4-related disease-experience of 100 consecutive cases from a specialist centre. Histopathology. 70, 798-813 (2017).
- Hart PA, Kamisawa T, Brugge WR et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 62, 1771-1776 (2014).
- Khosroshahi A, Wallace ZS, Crowe JL et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 67, 1688-1699 (2015).
- Hart PA, Topazian MD, Witzig TE et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 62, 1607-1615 (2013).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref