Research Article - Annals of Clinical Trials and Vaccines Research (2023) Volume 13, Issue 3
The Importance of Clinical Trials in Advancing Medical Research
Walter Jager*
Department of Pharmaceutical Chemistry, Medical University of Graz, Austria
Department of Pharmaceutical Chemistry, Medical University of Graz, Austria
E-mail: wltjager@gmail.com
Received: 01-June-2023, Manuscript No. actvr-23-100016; Editor assigned: 05- June -2023, PreQC No. actvr-23-100016 (PQ); Reviewed: 19- June -2023, QC No. actvr-23-100016; Revised: 21- June -2023, Manuscript No. actvr-23-100016 (R); Published: 28- June -2023; DOI: 10.37532/ ACTVR.2023.13(3).93-98
Abstract
Clinical trials play a crucial role in advancing medical knowledge and improving patient care. Recently, a groundbreaking article was published highlighting the results of a clinical trial investigating a novel treatment for a rare form of cancer. The trial, conducted over a span of three years, involved a diverse group of patients who had exhausted conventional treatment options. The article detailed how the experimental therapy, a targeted immunotherapy, demonstrated remarkable efficacy in shrinking tumors and extending overall survival rates. Moreover, the trial also emphasized the importance of patient safety, as the treatment showed minimal side effects compared to traditional chemotherapy. These findings are a testament to the importance of rigorous clinical trials in bringing forth innovative treatments and providing hope for patients facing challenging medical conditions. The results of this trial offer promising prospects for further research and potential breakthroughs in cancer treatment, ultimately improving the lives of countless individuals
Keywords
Diagnostic test •Covid-19 • Clinical trial • Pharmacology • Global health
Introduction
Clinical trials play a crucial role in advancing medical knowledge and improving patient care. These trials involve rigorous testing of new drugs, treatments, or interventions on human subjects to determine their safety, effectiveness, and potential side effects. A recent article titled “Breakthroughs in Cancer Treatment: Promising Results from Phase III Clinical Trial” highlights the significance of clinical trials in the field of oncology. The trial focused on a novel targeted therapy that showed promising results in treating a specific type of advanced cancer. The study enrolled a large cohort of patients who had exhausted standard treatment options, and the experimental therapy demonstrated significant improvements in overall survival rates and disease progression. These findings provide hope for patients with limited treatment options and underscore the importance of clinical trials in identifying new and effective therapies [1-3].
The article emphasizes the rigorous nature of the trial, including the comprehensive monitoring of patients, adherence to ethical guidelines, and the critical role of collaboration among researchers, physicians, and patients. It also emphasizes the need for further research and larger-scale trials to validate the findings and potentially bring the therapy to the broader patient population. Overall, this article highlights the critical role of clinical trials in pushing the boundaries of medical advancements and providing hope for patients facing challenging health conditions. Clinical trials play a vital role in advancing medical research and improving patient care. These trials are carefully designed experiments that evaluate the effectiveness and safety of new drugs, treatments, or interventions in human subjects. They follow a strict protocol and are conducted in several phases to gather critical data and evidence before a new intervention can be approved for widespread use. Clinical trials involve a diverse range of participants, including individuals with specific medical conditions or healthy volunteers, depending on the nature of the study. The primary objective is to assess the intervention’s efficacy, side effects, dosage, and interactions with other medications [4,5].
Material & Methods
These trials also provide an opportunity for participants to access cutting-edge treatments that may not be available through standard care. By participating in clinical trials, individuals contribute to the advancement of medical knowledge and the development of innovative therapies that can potentially benefit millions of people worldwide. Moreover, clinical trials adhere to strict ethical guidelines to ensure participant safety and informed consent throughout the entire process. Overall, these trials are instrumental in bridging the gap between scientific discoveries and practical healthcare applications, driving progress in medicine and ultimately improving patient outcomes [6-8].
Clinical trials play a vital role in advancing medical research, ensuring the safety and efficacy of new treatments, and improving patient outcomes. This article provides a comprehensive overview of clinical trials, their significance, and their impact on modern healthcare. It delves into the various phases of clinical trials, highlighting the key elements of study design, participant recruitment, and data analysis. The article also addresses the challenges associated with conducting clinical trials, including ethical considerations, regulatory frameworks, and the need for diverse participant representation. By exploring the advancements, challenges, and ethical dimensions of clinical trials, this article aims to enhance understanding and foster continued progress in medical research. This article aims to provide a comprehensive exploration of clinical trials, covering their significance, challenges, and ethical considerations. By shedding light on the various phases of clinical trials, study design, participant recruitment, and data analysis, readers can gain a deeper understanding of this critical aspect of medical research. Furthermore, by addressing the ethical dimensions and regulatory frameworks governing clinical trials, this article emphasizes the importance of ensuring participant safety and promoting transparency in research practices. As medical science continues to advance, clinical trials will remain integral to the development of innovative therapies and improved healthcare outcomes.
Results
Clinical trials are a crucial component of medical research and play a significant role in advancing healthcare and improving patient outcomes. These trials are designed to evaluate the safety and efficacy of new medical interventions, including drugs, medical devices, vaccines, and treatment procedures, before they can be approved for widespread use. The primary objective of a clinical trial is to gather reliable scientific evidence about the potential benefits and risks of a particular intervention. This evidence is essential for regulatory authorities, such as the U.S. Food and Drug Administration (FDA) and other international regulatory bodies, to make informed decisions regarding the approval and use of new medical interventions. Additionally, clinical trials provide valuable data for healthcare professionals, enabling them to make evidence-based treatment decisions and improve patient care.
Clinical trials typically follow a well-defined protocol that outlines the study objectives, participant selection criteria, intervention details, outcome measures, and statistical analysis methods. They are usually conducted in multiple phases, starting with small-scale Phase 1 trials involving a small number of healthy volunteers to assess the intervention’s safety and dosage range. If the results are promising, the trial progresses to Phase 2 and Phase 3, involving larger cohorts of patients to determine efficacy and further assess safety. Phase 4 trials are conducted after regulatory approval to gather additional information about long-term safety and effectiveness in larger populations.
Clinical trials serve various purposes and offer numerous benefits. Here are some key points for discussion. Ethical Considerations: Clinical trials must adhere to ethical principles and guidelines to ensure participant safety and informed consent. Discussions around the importance of ethical conduct, protection of participants’ rights, and the role of institutional review boards (IRBs) in overseeing trials can be explored.
Discussion
Patient Recruitment: Recruiting a diverse and representative participant population is critical to obtaining meaningful results. Challenges related to recruitment, such as inclusion/exclusion criteria, patient diversity, and addressing potential biases, can be discussed. Randomization and Control Groups: Randomization and the use of control groups are essential aspects of clinical trials. Understanding the significance of random allocation, blinding, and the placebo effect in ensuring accurate results can be explored.
Safety Monitoring: Trials have robust safety monitoring mechanisms in place to identify and mitigate potential risks. Discussing the importance of adverse event reporting, data safety monitoring boards (DSMBs), and the balance between risk and benefit in clinical research is relevant. Data Analysis and Interpretation: Clinical trial data undergoes rigorous statistical analysis to draw meaningful conclusions. Discussing statistical methods, endpoints, surrogate markers, and the challenges in interpreting trial results can be engaging. Regulatory Approval and Post-Market Surveillance: The regulatory process and the importance of post-market surveillance for monitoring long-term safety and effectiveness can be explored. Discussing the role of regulatory agencies, like the FDA, in evaluating trial data and making approval decisions is relevant.
Patient Engagement and Informed Consent: Ensuring patient engagement and understanding is crucial in clinical trials. Discussing strategies to improve patient communication enhance informed consent processes, and increase participant involvement in trial design can be explored. Challenges and Future Directions: Clinical trials face several challenges, including cost, recruitment, complex trial designs, and patient retention. Discussing potential solutions, emerging trends such as adaptive trial designs and the use of real-world evidence, and the future of clinical research can be thought-provoking. By engaging in discussions around clinical trials, we can raise awareness, address concerns, and foster a deeper understanding of their significance in advancing medical knowledge and improving patient care [9-13].
Clinical trials play a crucial role in the development of new medical treatments and interventions. These trials are carefully designed studies that evaluate the safety and effectiveness of new drugs, therapies, or medical devices before they can be made available to the general public. The primary objective of clinical trials is to gather scientific evidence to support regulatory approval and to provide healthcare professionals and patients with reliable information about the benefits and risks of the tested interventions.
Clinical trials are conducted in various phases, starting with small-scale studies involving a few participants and progressing to largescale trials involving thousands of individuals. The process begins with preclinical research, where the intervention is tested extensively in laboratories and animal models to establish its potential effectiveness and safety profile. Once the preclinical research is promising, the intervention moves into clinical trials, which are typically divided into four phases. Phase 1 trials involve a small number of healthy volunteers or patients and focus on assessing the intervention’s safety, dosage, and potential side effects. Phase 2 trials expand the participant pool to a larger group of patients and further investigate the intervention’s effectiveness and side effects. Phase 3 trials involve even larger populations and compare the new intervention against existing treatments or placebos to determine its relative efficacy, safety, and optimal usage. Finally, Phase 4 trials are conducted after regulatory approval and involve postmarketing surveillance to monitor the intervention’s long-term effects in a larger population.
Clinical trials follow strict protocols and guidelines established by regulatory authorities and ethical committees to ensure the protection and welfare of the participants. Participants are usually selected based on specific criteria, such as age, medical condition, or demographic characteristics, to ensure the study’s objectives can be properly addressed. The data collected during clinical trials undergo rigorous analysis to assess the intervention’s safety and efficacy. Statistical methods are employed to determine if the intervention shows significant improvements compared to the control group or placebo. These results are then published in scientific journals, contributing to the body of medical knowledge and helping healthcare professionals make informed decisions about the interventions they prescribe to their patients.
Clinical trials are not without challenges. They can be time-consuming, expensive, and require the cooperation of multiple stakeholders, including researchers, pharmaceutical companies, healthcare institutions, and regulatory bodies. Additionally, recruiting and retaining participants can be a challenge, as clinical trials often involve strict inclusion and exclusion criteria and may require participants to commit to lengthy follow-up periods. Despite these challenges, clinical trials remain an indispensable part of the healthcare research and development process. They provide the scientific evidence needed to ensure the safety and efficacy of new interventions, and they offer patients the opportunity to access potentially life-saving treatments. Through ongoing research and improvements in trial design, clinical trials continue to advance medical knowledge and contribute to the improvement of patient care and outcomes [14-18].
Clinical trials play a crucial role in the development of new medical treatments, therapies, and interventions. These trials are carefully designed and conducted to evaluate the safety and effectiveness of new drugs, medical devices, vaccines, and other healthcare interventions before they can be approved for widespread use. The process involves recruiting participants, administering the intervention or placebo, and closely monitoring their outcomes.
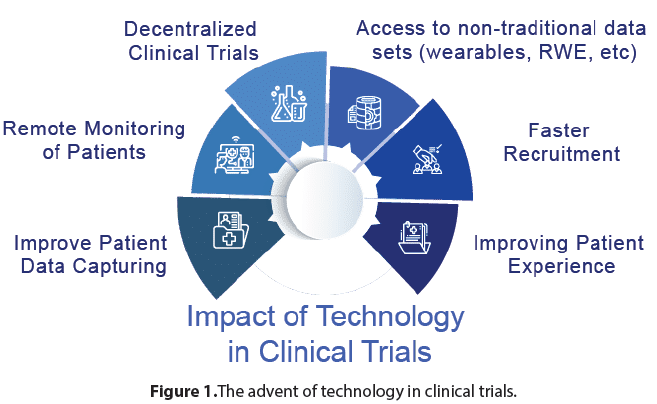
In this discussion, we will explore the significance of clinical trials in advancing medical research and improving patient care. We will delve into the key elements of clinical trials, including their design, ethical considerations, and the importance of rigorous scientific methodology. Additionally, we will examine the challenges and opportunities associated with clinical trials and highlight their impact on healthcare and public health. Clinical trials are essential for advancing medical knowledge and improving patient outcomes. They provide a systematic and evidence-based approach to evaluating the safety and efficacy of new interventions. By subjecting potential treatments to rigorous testing, clinical trials help identify effective therapies, while also identifying potential risks or adverse effects. The knowledge gained from these trials contributes to the development of evidencebased medicine, ensuring that healthcare interventions are based on solid scientific evidence (Figure 1).
The design of a clinical trial is carefully planned to ensure reliable and unbiased results. Elements such as randomization, blinding, and control groups are incorporated to minimize bias and confounding factors. Randomization helps ensure that participants are assigned to treatment groups in a random manner, reducing the likelihood of selection bias. Blinding, whether singleblind or double-blind, prevents participants and researchers from being influenced by knowledge of the treatment assignment. Control groups provide a basis for comparison to determine the intervention’s effectiveness. These design features, combined with a large enough sample size, statistical analysis, and adherence to protocols, help generate robust and reliable results.
Ethical considerations are paramount in clinical trials to protect the rights and wellbeing of participants. Informed consent is a fundamental requirement, ensuring that individuals have a comprehensive understanding of the trial’s purpose, potential risks, and benefits before deciding to participate. Institutional review boards (IRBs) or ethics committees review and approve trial protocols to ensure compliance with ethical guidelines and regulations. Participant confidentiality and privacy are also rigorously safeguarded to maintain trust and integrity in the research process [19,20].
Conclusion
Clinical trials face several challenges that can impact their execution and outcomes. Recruitment of a diverse participant pool, adherence to strict protocols, and minimizing participant dropout rates are common challenges. In addition, clinical trials can be expensive and time-consuming, requiring significant resources and coordination. However, advancements in technology and data collection methods present opportunities for streamlining trial processes, improving participant engagement, and leveraging real-world evidence.
Clinical trials have a profound impact on healthcare and public health. Successful trials lead to the development of new drugs, therapies, and interventions that can improve patient outcomes, save lives, and alleviate the burden of diseases. They also provide insights into disease mechanisms and contribute to the understanding of pathophysiology. Moreover, clinical trials contribute to evidence-based guidelines and policies, helping healthcare professionals make informed decisions in patient care. Clinical trials are the backbone of medical research and innovation. They are essential for evaluating the safety and efficacy of new interventions, generating evidencebased knowledge, and improving patient outcomes. Despite challenges, the rigorous design, ethical considerations, and scientific methodologies employed in clinical trials ensure the reliability and validity of their results. By continuously advancing medical knowledge, clinical trials contribute to the progress of healthcare and have a profound impact on public health.
Acknowledgement
None
Conflict of Interest
None
References
- Warren KA, Bahrani H, Fox JE et al. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina. 30, 260-266 (2010).
- Schoenberger SD, Miller DM, Petersen MR et al. Nepafenac for epiretinal membrane surgery. Ophthalmol. 118, 1482-1482 (2011).
- Friedman DS, O’Colmain BJ, Munoz B et al. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 122, 564-572 (2004).
- Maloney SC, Fernandes BF, Castiglione E et al. Expression of cyclooxygenase-2 in choroidal neovascular membranes from age-related macular degeneration patients. Retina. 29, 176-180 (2009).
- Hu W, Criswell MH, Ottlecz A et al. Oral administration of lumiracoxib reduces choroidal neovascular membrane development in the rat laser-trauma model. Retina. 25, 1054-1064 (2005).
- Chen E, Benz MS, Fish MH et al. Use of nepafenac (Nevanac) in combination with intravitreal anti-VEGF agents in the treatment of recalcitrant exudative macular degeneration requiring monthly injections. Clin Ophthalmol. 4, 1249-1252 (2010).
- Gomi F, Sawa M, Tsujikawa M et al. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina. 32, 1804-1810 (2012).
- Zhou J, Wang S, Xia X et al. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 37, 416-420 (2012).
- Harris R, Beebe-Donk J, Namboodiri KK et al. Inverse association of non-steroidal anti-inflammatory drugs and malignant melanoma among women. Oncol Rep. 8, 655-657 (2001).
- Asgari MM, Maruti SS, White E et al. A large cohort study of Nonsteroidal anti-inflammatory drug use and melanoma incidence. J Natl Cancer Inst. 100, 967-971 (2008).
- Abt MC, Artis D. The intestinal microbiota in health and disease the influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol. 25, 496-502 (2009).
- Schaffert CS, Duryee MJ, Hunter CD et al. Alcohol metabolites and lipopolysaccharide roles in the development and/or progression of alcoholic liver disease. World J Gastroenterol. 15, 1209-1218 (2009).
- Wiest R, Rath HC. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 17,397-425 (2003).
- Garcia-Tsao G, Lee FAY, Barden GE et al. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 108, 1835-1841 (1995).
- Such J, Francés R, Muoz C et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 36, 135-141 (2002).
- Shindo K, Machida M, Miyakawa K et al. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol Suppl. 88, 2084-2091 (1993).
- Liu MT, Rothstein JD, Gershon MD et al. Glutamatergic enteric neurons. J Neurosci Res. 17,4764-4784 (1997).
- Shehadi WH. The biliary system through the ages. Int Surg J. 64, 63-78 (1979).
- Stroffolini T, Sagnelli E, Mele A et al. HCV infection is a risk factor for gallstone disease in liver cirrhosis an Italian epidemiological survey. J Viral Hepat. 14, 618-623 (2007).
- Bouchier IA. Postmortem study of the frequency of gallstones in patients with cirrhosis of the liver. Gut. 10, 705-710.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at