Review Article - Interventional Cardiology (2012) Volume 4, Issue 1
Selecting the optimal closure device in patients with atrial septal defects and patent foramen ovale
- Corresponding Author:
- David Roy
St George’s Hospital, Blackshaw Road, London, UK
E-mail:david.roy@stgeorges.nhs.uk
Abstract
Keywords
atrial septal defect closure, patent foramen ovale closure, stroke, transcatheter device closure
Patent foramen ovale (PFO) is a normal fetal communication between the right and left atria that persists postpartum. It is common, occuring in 20–40% of the population. Occasionally, PFOs can lead to paradoxical embolism that can manifest as a stroke or systemic arterial embolism [1–4]. Atrial septal defects (ASD) are far less prevalent than PFO; however, it remains one of the most common congenital cardiac defects and the majority of these are ostium secundum defects. These occur either from excessive resorption of the septum primum or from deficient growth of the septum secundum. Patients with ASDs often present with exercise intolerance or palpitations, but occasionally present with overt right ventricular failure, especially in older patients and paradoxical embolism [5–7]. Percutaneous closure of both PFOs and secundum ASDs is increasingly becoming the treatment of choice where closure is indicated, and while there is good evidence for ASD closure, the closure of symptomatic and asymptomatic PFOs remains highly controversial. A multitude of devices is available for percutaneous closure of both defects, but there is limited data on the comparative efficacy of these devices. This article reviews the indications and percutaneous device options for PFO and ASD closure.
PFO
▪ Indications for PFO closure
Cryptogenic stroke
A cryptogenic stroke is defined as a stroke of unknown cause, despite extensive investigations to exclude other causes (for example atrial fibrillation [AF], carotid and aortic atheroma, carotid dissection, space occupying lesions and intracerebral hemorrhage) [8–10]. The relationship between PFO and cryptogenic stroke is, however, well established [11]. The largest body of evidence comes from a meta-analysis that compared cryptogenic stroke with stroke from known causes, and in 22 studies the odds ratio for PFO was 3.16. Recurrent neurological events in patients with PFO and previous cryptogenic stroke have also been reported in 6–8% of patients and can be as high as 15% in the presence of atrial septal aneurysm (ASA) [10–12]. Windecker et al. reported a trend towards risk reduction in the combined end point of death, transient ischemic attack (TIA) and stroke in an uncontrolled cohort series that compared medical therapy with PFO closure following cryptogenic stroke (24.3 vs 8.5%, respectively) [13].
The only prospective randomized controlled trial (RCT) – the keenly anticipated CLOSURE 1 study by Furlan et al., did not demonstrate superiority for PFO closure in patients <60 years old with the STARFlex versus medical therapy alone for cryptogenic stroke [14]. The stroke rates at 2 years were not significantly different: 3.1% in the device group versus 3.4% in those treated with medical therapy. Notably, the device arm also showed significantly higher rates of AF (5.7 vs 0.7%). A major criticism of this trial has been that in 80% of the patients with multiple stroke or TIA, there were explanations other than paradoxical embolism for neurological events. The inclusion of TIAs and strokes, rather than true strokes only, has also been questioned, as has the fact that the increased rate of AF in the device group may have increased the rate of stroke [14,15]. There are still a number of further RCTs of PFO closure for stroke prevention in progress, in particular the RESPECT (Amplatzer® device, USA) the PC-Trial (Amplatzer device, outside the USA) and the REDUCE trial (HELEX® device). These trials, with more stringent inclusion criteria and extended follow-up periods, may shed more light on this treatment strategy for so called cryptogenic stroke in the presence of a PFO.
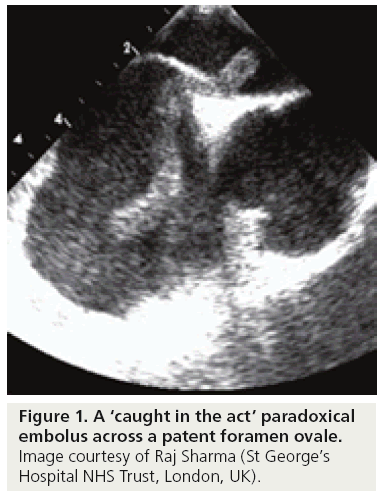
A study by Rigatelli et al. proposed the alternative theory that PFO in combination with ASA may lead to thrombus formation due to ‘slow flow’ rather than paradoxical embolism [16]. While this may be true of large ASAs, images similar to Figure 1 of a large thrombus across a PFO, or a ‘caught in the act’ paradoxical embolus, are compelling images that are frequently observed in case reports and present a strong case for PFO closure, especially in young patients where no other likely explanation for stroke can be found [17–20].
Systemic arterial embolism
Systemic arterial emboli, other than cryptogenic strokes, have been reported in patients with PFOs and include emboli in the lower limbs, visceral organs and coronary arteries. While there is no evidence for PFO closure in these settings, in young patients with symptoms that suggest concomitant venous thrombosis, it is reasonable to conclude that the PFO is the source of paradoxical embolism. Gersony et al. and Meier et al. have described PFO and ASD closure in the acute setting of acute myocardial infarction in young patients with normal coronary arteries in whom no other cause of arterial thrombosis could be identified [21–24].
Migraine with aura
Migraine with aura is associated with causes of left-to-right shunting, including PFO. It is postulated that in some people who experience migraine with aura, a trigger substance passes across a shunt but, if this is so, the trigger has not yet been identified. If this mechanism is responsible for some cases, it cannot account for all, because not all migraine sufferers have a shunt and many people with a shunt do not suffer from migraines. There are a number of nonrandomized, observational series that have reported that in patients with migraine who have had PFO closure for the indication of paradoxical embolism, symptoms of migraine have improved dramatically. Although the placebo effect could not be excluded, some of the the earlier studies reported this effect of device closure prior to any known association of PFO and migraine [25–32].
The publication of the first randomized study of PFO and migraine in 2008, did not make this association any clearer. The MIST trial was a prospective, multicenter, randomized, doubleblind controlled trial that compared the effect of closure of moderate or large PFOs using the STARFlex device with sham intervention in patients with severe and frequent migraine. The MIST trial differed from earlier observational studies because patients with a history of stroke were excluded. In the MIST trial, follow-up continued for 6 months, with patients receiving aspirin and clopidogrel for the first 3 months and the headache analysis phase carried out during the second 3-month period [33].
The preliminary findings of the study seemed encouraging, with 60.2% of patients found to have a right-to-left shunt (RLS), the majority of which were classified as a moderate or large PFO. In total, 147 patients were randomized to either implantation of a STARFlex device or sham intervention. The results comparing the implant and sham groups showed no effect on the primary end point of cessation of migraine or secondary end points of improvement in migraine in either the intention-to-treat analysis or per-protocol population. The implant arm also had a high rate of procedural complications [33].
Since the findings of MIST were published, a number of further observational studies have reported striking improvements in migraine post-PFO closure. Trabattoni et al. presented data from a single-center series, in which a total of 305 consecutive patients underwent PFO closure following an ischemic event confirmed by brain CT or MRI, followed by confirmation of a PFO. Of this cohort, 25% also reported having moderate to severe migraines prior to PFO closure. Trabattoni reported a significant mid- and long-term reduction of migraine intensity and frequency in over 80% of patients [34]. Similar findings have been shown by Rigatelli et al. and Vigna et al., and it seems that the patients more likely to have reduction in migraines are those with clinically proven ischemic events [35–37]. The role and potential benefits of PFO closure among patients with severe migraine in the absence of previous ischemic events, remain questionable and warrant future investigation with appropriately powered, well-designed and executed randomized trials.
Platypnea orthodeoxia syndrome
Platypnea orthodeoxia syndrome is a rare condition that occurs when the patient suffers from dyspnea and hypoxia after assuming a position of upright posture. It is thought to occur due to abnormal right-to-left shunting across a septal defect. The anatomical defect can be a PFO, ASD or a fenestrated ASA. The combination of this defect with a functional component (often lung or abdominal pathology) that causes a deformity of the atrial septum with upright posture, allows abnormal shunting of blood from the right to left atrium and hence the development of symptoms. There are many case reports supporting device closure in this unusual syndrome [38,39].
Decompression illness
Decompression illness (DCI) is when changes from high to low ambient pressure cause the nucleation of nitrogen bubbles into the arterial system, causing embolization. This is most commonly exhibited in scuba divers but can occur in extreme high-altitude flying [11,40,41]. Wilmshurst et al. first noticed an association of PFO with DCI in scuba divers [40]. This finding has since been further examined and several studies have found a correlation between the prevalence of PFO and the occurrence of cerebral DCI. Torti et al. found the risk of DCI to be fivetimes higher in those with a PFO diagnosed by transesophageal echocardiography (TEE) in 230 scuba divers [41]. To date, there are no prospective RCTs examining the benefit of closing PFOs in scuba divers, although device closure for those with PFO has been considered [42]. There is no consensus of opinion on how to manage scuba divers who have been diagnosed with a PFO; however, modification of the dive profile, device closure or stopping diving are all reasonable management strategies.
▪ Diagnosis & imaging for PFO
Echocardiography
If the indication for PFO closure is cryptogenic stroke, appropriate neurological-led investigations to exclude other mechanisms of potential stroke should be carried out. These should include brain and carotid imaging, as well as a thrombophilia screen. Transthoracic echocardiography (TTE) often demonstrates a potential substrate for stroke, such as dilated cardiomyopathy, old myocardial infarction, mitral stenosis and a dilated left atrium. However, a direct source of embolus is rarely found. With color flow mapping, TTE has limited accuracy for the diagnosis of a PFO. A bubble contrast echo (TTE or TEE) is the gold standard and requires an experienced operator to ensure adequate vagal maneuvers have been performed to detect whether bubbles cross the inter-atrial septum when the right atrial pressure is transiently elevated. A cardiac shunt is diagnosed by the presence of bubbles in the left atrium within the first three cardiac cycles. Pulmonary level shunts produce bubbles after three cycles and do not require provocation. Typically, the procedure should be performed two- or three-times to provide a confident diagnosis [43–45].
TEE
TEE allows detailed anatomical assessment of the left atrium and interatrial septum. It is the gold standard for the diagnosis of left atrial appendage thrombus and for visualizing other atrial masses that may have caused stroke. TEE also allows detailed evaluation of aortic arch atheroma and thrombus. It is therefore considered by some to be mandatory test for patients with cryptogenic stroke. The technique is also crucial for assessing the anatomy of a PFO, which is highly variable and has important implications when considering an appropriate closure device. Real-time 3D echocardiography is especially promising in this respect. Some PFOs consist of a long tunnel, with the septa tightly opposed, whilst others open widely. In some PFO tunnels, the septum primum is held away from the septum secundum by a fold of tissue on the left atrial side, a so-called ‘PFO with fixed opening’, or ‘held open PFO.’ TEE allows identification of an atrial septal aneurysm (ASA). The mobile portion of the ASA lies within the septum primum and may cause this area to retract, undermining the stability of device closure. Some operators therefore intentionally oversize the device in this situation. TEE is also useful for identifying other structures that may cause technical difficulties during device closure, such as a large eustacian valve or chiari network [46–49]. Many operators are happy to perform PFO closure under fluoroscopy only, without intraprocedural echo guidance or with intracardiac echo, as long as a TEE has been performed preprocedurally [47,48].
Intracardiac echo
Intracardiac echocardiography (ICE) has become the intraprocedural imaging modality of choice for many operators for PFO and ASD closure. It is performed using an 8 or 9 Fr catheter, which is introduced via a second femoral venous sheath. There are many advantages of ICE compared with TEE imaging. The image quality is often superior to TEE because the transducer is within the right atrium enabling high resolution and accurate assessment of the interatrial septum. The use of ICE also obviates the need for an echocardiographer and often a general anesthetic, making the procedure faster and potentially cheaper. The obvious disadvantages of ICE include the need for insertion of a second venous sheath and the expense of singleuse ultrasound catheters. Another disadvantage is that during cases with complex anatomy, it may be challenging to manipulate both the ICE catheter and the closure device at the same time [50].
Transcranial doppler
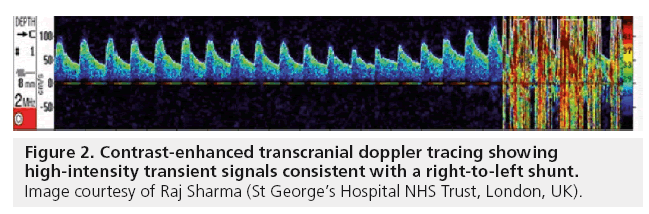
More recently, contrast enhanced transcranial Doppler ultrasound (c-TCD) has become a technique for PFO screening in some centers. It involves using agitated saline or galactose based contrast agents to look for microbubbles or high intensity transient signals (Figure 2) in the middle cerebral artery, vertebrobasilar system and, more recently, described the carotid artery in order to confirm the presence of a RLS. It has become particularly popular with neurologists due to the clear advantages of patient comfort in this noninvasive technique. The method is occasionally limited by poor temporal windows of the middle cerebral artery, which is reported to occur in approximately 10% of patients, but may depend on the expertise of the operator [51–53].
Recent literature reports the diagnostic accuracy of c-TCD to be excellent when compared with TEE, with experienced centers reporting sensitivities of 70–100% [53]. Best ‘timing’ from contrast injection until identification of the first high intensity transient signals in the main coronary artery has also been offered to separate RLS from intracardiac and extracardiac causes but this topic remains controversial and there is no consensus regarding this criterion in the literature. There is also no universally accepted standard as to how many microbubbles constitute a clinically relevant RLS. Regardless of these issues, c-TCD has emerged as a very effective screening tool for detecting RLS, while TEE should be used for further characterization of the anatomy. TEE and c-TCD are complementary methods and should be applied jointly, especially if they are to be used in the percutaneous treatment of PFO [53].
Van et al. have suggested a method for standardizing what constitutes an appropriate Valsalva maneuver during c-TCD by blowing into a manometer and achieving a pressure of 40 mmHg [54]. This was performed in a study comparing c-TCD to ICE intraprocedurally. In this study, the investigators reported a sensitivity of c-TCD that was higher for detecting RLS post PFO device closure than ICE, which was performed simultaneously. The study also demonstrated that a significantly lower right atrial pressure is achieved during voluntary Valsalva maneuver, compared with forced expiration into a manometer. While the study numbers were low (n = 38 patients), it is a positive step in attempting to improve c-TCD accuracy as the first diagnostic test of choice for ruling out PFO. This is especially important during c-TCD where the operator may be unaware of the effectiveness of the Valsalva compared with TEE, where the appropriate effect of the Valsalva can be seen by the septum bulging towards the left atrium.
▪ Device selection for PFO closure
There are several companies that manufacture PFO closure devices, all with effective closure rates and low complications reported. Very few of these devices have been compared in studies and even fewer have been evaluated in randomized, comparative trials. More recently, the concept of device-less PFO closure has been explored and various suture-based systems, as well as radiofrequency ablation options, have been developed. None of these devices have produced acceptable closure rates and as such device-based techniques are still the only widely accepted methods of PFO closure.
Amplatzer PFO occluder
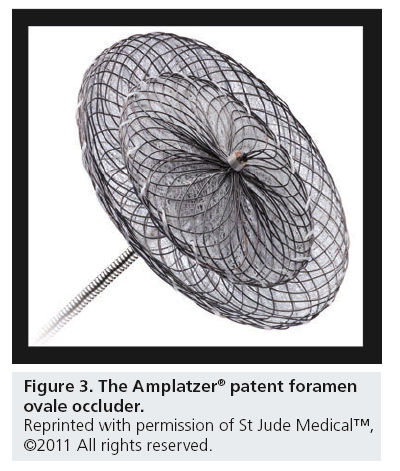
The Amplatzer PFO Occluder (Amplatzer, St Jude–AGA Medical Corporation, MN, USA; Figure 3) is a self-expanding device made of a nitinol wire mesh. It consists of a right atrial and a slightly smaller left atrial disc connected together by a bond bridge made of nitinol wire. It is available in right atrial disc sizes of 18, 25 and 35 mm. It is radio-opaque, simple to use, versatile, fully retrievable and redeployable. The Amplatzer device is the most commonly used device worldwide and as such, many recently developed devices have similar design and implantation characteristics [55–62].
HELEX® septal occluder
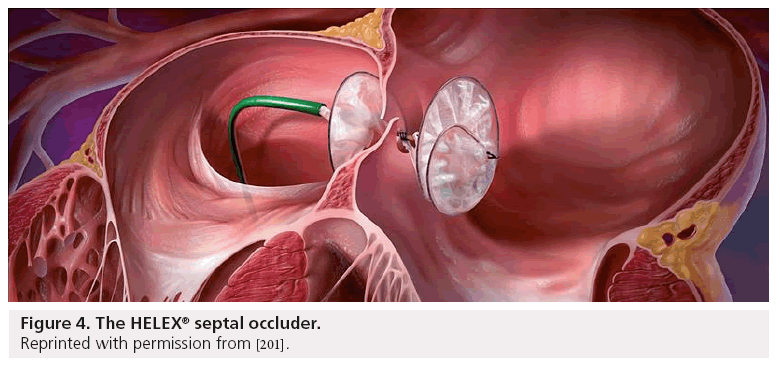
The HELEX septal occluder (HSO; WL Gore & Associates, AZ, USA; Figure 4) is a low-profile, double-disk occluder device designed to close PFOs and secundum ASDs. The device is comprised of an expanded polytetrafluoroethylene membrane, bonded to a single nitinol wire frame and can be delivered though a 9–13 Fr femoral venous sheath. When fully deployed, the occluder assumes a double-disc configuration that bridges the septal defect to prevent shunting of blood between the right and left atria. Unlike the Amplatzer, the HSO is compliant and non-self-centering, making it capable of conforming well to the curvilinear surfaces of the atrial septum. The delivery system allows for repositioning or retrieval of the device after deployment. The device comes in sizes ranging from 15–35 mm and the device is generally sized in a 2:1 ratio compared with the defect size. It is recommended that the maximum balloon stretch size possible to close with this device is 18 mm in the case of large PFOs or ASDs. A safety cord attached to the device provides for removal of the occluder even after device release in the unlikely case of device embolism [57–60].
Figure 4: The HELEX® septal occluder.
Reprinted with permission from [201].
CardioSEAL–STARFlex®
The CardioSEAL–STARFlex (CS–SF; NMT, MA, USA) is a device comprised of two rectangular discs, each consisting of four-wire spring arms covered with a polyester patch. It is a modified version of the original device, with microsprings attached to the opposing arms of each umbrella. The device is available in sizes of 23, 28, 33, 38, 40 and 43 mm. The Biostar® device is a novel bioabsorbable device mounted on a STARFlex occluder design [57–62]. These devices are no longer commercially available.
Premere™ PFO occluder
The Premere PFO occluder (St Jude Medical) is a flexible device with a low profile and independent anchors that make it ideal for long PFO tunnels. It has an adjustable tether connecting the anchor and can conform to different septal anatomies. It has the advantage of minimal left atrial material, theoretically reducing potential thrombus formation [63].
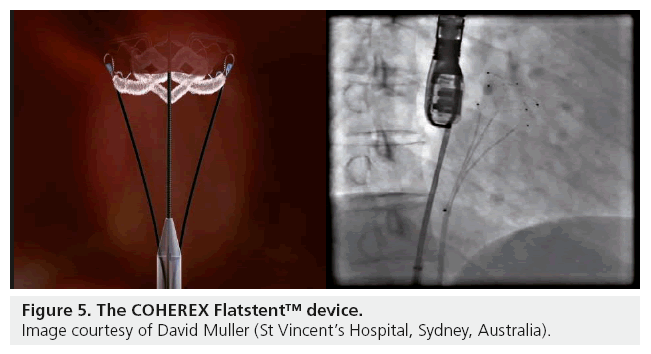
The Coherex Flatstent™
The Coherex Flatstent (Coherex Medical, UT, USA; Figure 5) is the first device with CE mark designed for use only within the PFO tunnel. The device is retrievable and redeployable, has an expandable stent design and is made with a polymer filling to aid endothelialization and includes radio-opaque markers to guide deployment. It has the theoretical advantage of an intunnel design that may reduce rare, but potential, complications of PFO closure including left atrial device surface thrombus formation and device perforation. The disadvantage of the device from the early experience of the Coherex EU study, is that it seems to be more effective (100 vs 58%) in PFO tunnels of 4 mm or longer, limiting its use as a device for all PFO closure anatomy.
There are several new dedicated PFO closure devices with varying numbers of patients treated to date. A full description of each device is beyond the scope of this review. They include the Cierra PFx™ (Cierra, Inc., CA, USA), SeptRx (Stout Medical, PA, USA), Heartstitch (Sutura, CA, USA), Nitocclud (PFM Medical, CA, USA), Edwards Suture (Edwards Lifesciences, IR, CA, USA), Coaptus RF (Coaptus Medical, WA, USA).
▪ Device comparison
There are limited data comparing the commonly used PFO closure devices [64–67]. Taaffe et al. compared PFO closure devices in a randomized trial [57]. A total of 660 patients were randomized to PFO closure with one of the three most widely used devices (Amplatzer, CS–SF and HSO) and follow-up at the time of publishing was 30 days. There were no differences between PFO sizes and the incidence of atrial septal aneurysm between the three groups. The procedural success rate was 100% for all groups, with two device embolizations in the HSO group (retrieved and a larger device implanted) and no embolizations in the Amplatzer or CS–SF groups. There were also two unspecified technical complications related to insertion in the CS–SF group, and these devices had to be removed through an auxiliary vascular access due to unsatisfactory positioning. The procedural and fluoroscopic times were shorter in the Amplatzer group, and the mean sheath size for all three devices was 9 r.
The primary end point for the study was successful PFO closure (i.e., no residual shunt) at 30 days as measured by TEE. The Amplatzer device was superior for this end point, with no residual shunt at 30 days in 65% of patients versus 52.7% in the HSO group (p = 0.0005), with no statistically significant difference versus the CS–SF group (62.3%; p = 0.847). There was a slightly higher incidence of AF in the Amplatzer group with 1.4% of patients affected at 30 days versus 0.9% with HSO and 0.5% with CS–SF (p = 0.02). The investigators of this study also noted 3.6% of patients in the CS–SF group developed thrombus on the device noted on TEE at 30 days. There was no such thrombus noted on the Amplatzer or HSO devices [57]. A study by Krumsdorf et al. of 1000 consecutive patients undergoing PFO or ASD closure also noted a higher rate of device related thrombus on CardioSEAL (7.1%) and STARFlex (5.7%) devices when compared with HSO (0.8%) and Amplatzer (0%) devices on TEE 4 weeks post-procedure [58]. Braun et al. compared the Amplazter, the CS–SF and the PFO-Star and found no significant differences between the devices in 307 patients [62].
▪ Which device for which anatomy?
Atrial septal aneurysm
Atrial septal aneurysms are known to present a higher risk of recurrent neurological events and are usually associated with larger PFOs. Most operators in this situation stabilize the septum by covering a larger area than needed in order to cover the PFO with a stiffer device (e.g., Amplatzer PFO, Occulotech or Nitocclud). Some operators still feel that smaller devices present less risk of device thrombus formation and prefer to use a smaller device that will conform to the anatomy (e.g., HSO).
Long PFO tunnel
Traditional double-disc devices tend to distort the septal anatomy in PFOs with long tunnels and some operators report using transseptal puncture to place the device in these cases. This seems to overcomplicate what should be a simple procedure and, to this end, the Premere™ and the Coherex devices are designed specifically for long PFO tunnels. These devices also have the advantage of minimal left atrial material, meaning thrombosis risk is reduced; however, they are yet to be effectively compared with other devices and the closure rates still remain uncertain. As these devices have not yet come into mainstream use for most centers, most operators still tend to use stronger devices to collapse the PFO tunnel (e.g., Amplatzer), which is often equally effective [55–63,68].
Multiple defects
A septum with multiple defects most likely represents either a fenestrated secundum defect in conjunction with a PFO or an isolated fenestrated secundum defect. In the vast majority of cases, these can be effectively closed with a single device and often the Amplatzer Cribiform occluder is the most effective for this purpose. Some operators advocate using a transseptal puncture and single-device approach; however, for the vast majority of cases a single, larger device through the largest defect is sufficient. A two-device strategy is more appropriate for multiple true secundum ASDs [55–63,68].
ASDs
ASDs account for approximately one-third of all congenital heart disease detected in adults and occur in about four of out 100,000 newborns. Secundum ASDs are by far the most common, accounting for 75% of all ASDs and occur in the region of the fossa ovalis. Primum ASDs, coronary sinus and sinus venosus defects are less common. Secundum defects are the only ASDs truly amenable to percutaneous closure. A predominant left-to-right shunt is the characteristic feature of an ASD, the consequence of which is right ventricular volume overload. Most patients with sizable ASDs still remain asymptomatic for the first two decades of life, however most patients eventually develop symptoms due to cardiac failure, pulmonary hypertension, atrial arrhythmias or paradoxical embolism [5–7,69–72].
▪ Device versus surgical closure
Surgical closure of an ASD was first performed in 1953 and for many years has been considered the standard treatment for patients with a sizable secundum ASD. Surgery usually involves insertion of a pericardial patch or direct suture and does provide good early postoperative and long-term results, and has been shown to improve mortality (vs medical therapy) for hemodynamically significant left-to-right shunts [73,74]. Since the first percutaneous ASD closure described by King and Mills in 1974, transcatheter approaches have become increasingly utilized [75]. The main advantages of the percutaneous approach, besides the avoidance of surgery, include a high sealing rate (97–99%) of the defect, along with short procedural times and length of stay for the patient.
Surgical ASD closure versus transcatheter approaches have been compared. Butera et al. reported a large single-center series while multicenter studies were reported by Jones et al. and Du et al. [76–79]. These retrospective studies showed similar (very low) mortality rates in both groups, with higher complications in the surgical groups. To date, there are no large RCTs of surgical versus percutaneous ASD closure. A recently published large meta-analysis of secundum ASD closure in a total of 3082 patients (1270 treated surgically and 1812 treated percutaneously), showed a higher rate of both total and major complications in the surgical groups. Patients treated with surgery had a 5.4- and 3.8-fold higher risk of total and major complications occurring, respectively. The length of stay was also 2.5-times longer in the surgical group [80]. Kim and Hughes found the cost of ASD closure to be higher for with surgery while Thomson found no cost difference. Vida et al. also showed that the cost of Amplatzer device closure was higher than surgery in a developing country [81–84].
▪ Indications for ASD closure
Patients with small shunts and normal right ventricle (RV) and right atrium size are generally asymptomatic and require no medical therapy. Routine follow-up of the patient with a small ASD, without evidence of RV enlargement or PAH, should include assessment of symptoms, especially arrhythmias and possible paradoxical embolic events. Serial echocardiography should be obtained to assess RV size and function and pulmonary pressure. Reductions in LV compliance related to hypertension, coronary artery disease or acquired valvular disease may increase the degree of le ft-to-right shunt across an existing ASD over time. Transcatheter or surgical closure of an ASD is indicated if there is evidence of right-sided volume overload as seen by echocardiography irrespective of the patient’s symptoms [6,85,87].
Large atrial shunts lead to symptoms caused by excess pulmonary blood flow and right-sided heart failure, including dyspnea, frequent pulmonary infections, fatigue, reduced exercise tolerance and palpitations. Traditionally a significant left-to-right shunt is defined as a pulmonary-tosystemic flow (Qp/Qs) ratio of >1.5/1; however, in reality any patients with symptoms should have their ASD closed, as should any patients with documented right-sided volume overload or platypnea-orthodeoxia syndrome. Atrial arrhythmias – atrial flutter, AF and sick sinus syndrome – are a common result of long-standing right-sided heart volume and pressure overload. If surgery is indicated over a percutaneous approach (e.g., due to very large defect >40 mm or a non-secundum defect) then a MAZE procedure should also be considered. Paradoxical embolism from peripheral venous or pelvic vein thromboses, atrial arrhythmias, unfiltered intravenous infusions or indwelling venous catheters, is a risk for all defects regardless of size and any previous paradoxical embolism is an indication for device closure. Important prerequisites for ASD closure are pulmonary arterial pressures less than two-thirds systemic and a pulmonary vascular resistance <7 Woods units. ASD closure in patients with severe, irreversible pulmonary arterial hypertension is contraindicated, as it may shorten their life-expectancy [6,85–88].
▪ Diagnosis & evaluation of the defect TTE
Most ASDs can be diagnosed by TTE, and should be suspected in the presence of an enlarged right atrium and/or right ventricle. The sensitivity in detecting secundum and primum ASDs with TTE using standard precordial views has been reported to be >90%. However, anatomically the atrial septum is a concave–convex structure and therefore, an ultrasound beam can cut the defect in different planes and may not reflect its true size. Thus, before any device closure is undertaken, another form of anatomical assessment is recommended and TEE is often required only for a more detailed view of the anatomy rather than a diagnosis. The significance of an ASD can be determined during TTE by applying the continuity principle and calculating the intracardiac shunt ratio (Qp:Qs). This method in combination with anatomical information from TEE, has replaced routine right heart catheterization for the diagnosis and assessment of ASD shunts [89,90].
TEE
TEE is a vital component of percutaneous ASD closure, whether it is used for diagnosis and evaluating suitability or for the implantation itself. While some interventionalists are happy to perform PFO closure without intraprocedural TEE or ICE, it is not recommended to perform ASD closure without either TEE or ICE. This is due to the risk of device embolization or missing multiple defects. The rims of a secundum ASD have been well described and are designated as aortic (superoanterior), atrioventricular (inferoanterior), superior venacaval (superoposterior), inferior venacaval (inferoposterior) and posterior [89–93]. By convention, a margin or rim of >5 mm in each direction is considered to be adequate for device closure. There are multiple anatomical variations of ASD morphology described echocardiographically, but the most useful from a structural interventionalist’s perspective are those that include the rim. Podner et al. described ten morphological variations of defects, the most common being the defect with deficient aortic rim (42.1%). The other variants included central defects (24.2%), deficient inferoposterior rim (12.1%), perforated aneurysm of the septum (7.9%), multiple defects (7.3%), combined deficiency of mitral and aortic rims (4.1%), deficient SVC rim (1%) and deficient coronary sinus rim (1%) [92]. Defects with a deficient aortic rim can be closed percutaneously; however, deficiencies of the rim in other planes are not suitable for device closure. With careful TEE evaluation, approximately 80% of secundum ASDs are suitable for device closure [90,92].
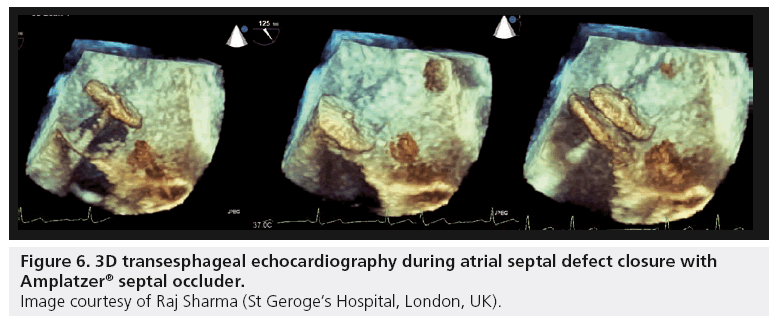
3D TTE and TEE, both in real-time and with image postprocessing, have been shown to be feasible for the qualitative and quantitative assessment of ASDs. While this technique often requires more effort to obtain quality 3D images, there are many ASDs which have complex anatomy that are sometimes underestimated on TEE. The use of 3D imaging during device closure (Figure 6) allows a rapid assessment to ensure the defect has achieved coverage of the defect and is anchored in all directions by a septal rim. Another advantage of the 3D transducers is the ability to perform biplane imaging. During device closure this gives the ability to view two orthogonal planes simultaneously, increasing the operators understanding of the anatomy [91,94]. As with PFO closure, ICE has become the procedural imaging modality of choice for some centers during ASD closure.
Figure 6: 3D transesphageal echocardiography during atrial septal defect closure with Amplatzer® septal occluder.
Image courtesy of Raj Sharma (St Geroge’s Hospital, London, UK).
Cardiac MRI
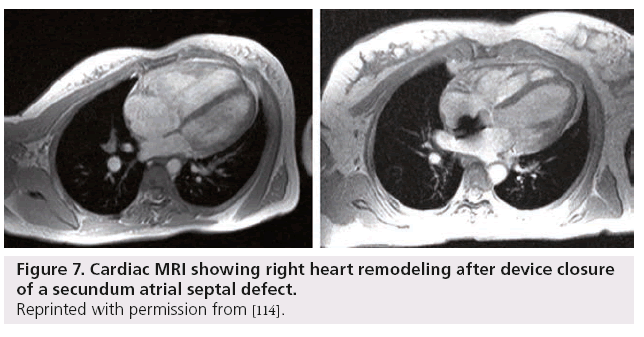
While TEE has long been the gold standard for anatomical assessment of secundum ASD, there are several disadvantages to this imaging modality. TEE is still a semi-invasive procedure and in children and some adults, for various reasons, it can be difficult and possibly unpleasant for the patient. There are also certain patients in whom the posterior inferior margins of the septum cannot be visualized. Cardiac MRI (CMR) is a useful tool, which in some centers has become the pre-assessment imaging modality of choice for ASDs. CMR can perform shunt calculation and can identify anomalous pulmonary venous drainage that might require surgical correction. There are several studies comparing CMR with TEE in both pediatric and adult populations, all with favorable results. While TEE still remains the imaging modality of choice in most centers, CMR may become the new gold standard of ASD imaging to assess suitability for device closure in the future [95–98]. Figure 7 shows a secundum ASD pre- and post-closure with an Amplatzer device.
Figure 7: Cardiac MRI showing right heart remodeling after device closure of a secundum atrial septal defect.
Reprinted with permission from [114].
▪ Device selection for ASD closure Amplatzer septal occluder
The Amplatzer Septal Occluder (ASO; Amplatzer, St Jude–AGA Medical Corporation) is the most frequently used device for ASD closure worldwide. Its design is similar to that of the PFO device – it has a self-expandable nitinolwire mesh double-disc design; however, there is a broad connecting waist that differs from the PFO closure device. The device comes in sizes of 4–40 mm with a 3–4 mm connecting waist. It is deployed through venous sheath sizes of up to 12 Fr for the largest 40 mm device.
The Amplatzer Multi-Fenestrated Septal Occluder or Cribriform Occluder is designed for use in fenestrated ASDs. It is placed exactly like the ASO, but has a narrow waist to place it through one of the central holes in the septal wall, with the discs covering the surrounding holes [99–101].
The HSO
This device has already been described above for PFO closure and it is the same device that is used for ASD closure (Figure 8). The recommended sizing ratio of 2:1 means that it is a useful device for small to medium sized ASDs. The safety cord is of particular advantage for ASDs where device embolization due to unexpected anatomy is possible. Successful delivery of this device was reported by Jones et al. in 88% of patients undergoing ASD closure from the pivotal US trial [77].
Figure 8: The HELEX™ device.
Reprinted with permission from [201].
The CS–SF device
This device, as well as the Biostar device, has been used for small to moderate sized ASD closure. The device is the same as that used for PFO closure with sizes ranging from 23–40 mm. Butera et al. compared the CS–SF device and the ASO in 274 patients who underwent a percutaneous ASD closure. The mean stretch sizes of the defects closed were 13.6 mm in the CS–SF and 15.5 mm in the ASO device subgroups. They reported device embolization in three (2.5%) out of 121 patients who received the CS–SF device and in one (0.7%) out of 153 patients who received an 18 mm ASO device [99]. Post et al. reported four (6.2%) out of 65 patients needing surgical intervention because of an embolization or dislocation of the device; all were CS–SF devices [101]. Again, these devices are no longer commercially available for ASD closure.
Figulla® ASD occluder
The Occlutech Figulla ASD Occluder (FSO; Occlutech GmbH, Jena, Germany) is constructed from 0.082–0.186 mm nitinol wires, tightly woven into two flat, round discs with a 4 mm connecting waist. The size of the device is determined by the diameter of the waist, which ranges from 6–40 mm. The prosthesis is filled with a polyester patch to enhance thrombogenicity. There is only one stainless steel hub (microscrew) at the right atrial disc for cable connection. It is delivered through a 9–14 Fr sheath depending on the size of the device. The device is very similar to the ASO and is delivered in exactly the same manner, meaning there is no learning curve to speak of for those familiar with the ASO. The device is also able to be recaptured and repositioned like the ASO. The FSO device is individually braided, avoiding a distal clamp. Pac et al. reported the rate of residual shunting to be higher with the FSO postprocedure, with 24.3% of patients having a residual shunt immediately post closure as compared with 7.1% in the ASO group. This shunting did improve (3 vs 0% with the ASO at 1 month) and this difference may be explained by the different composition of the polyester patch within the waist of the device [102].
▪ Selecting the appropriate device for ASD closure
There is little data outlining the selection of appropriate devices for ASD closure. Small to medium centrally located ASDs can be treated with either CS–SF, HSO, FSO or ASO devices. While procedural success is similar in this group for most devices, there is a trend towards an increased residual shunt in the CS–SF devices. For medium to large and very large centrally located defects, with balloon stretch sizes of 38–40 mm, the ASO device has the best procedural results reported thus far, with the lowest rate of device embolization [99,102–109]. Evidence also suggests that the ASO is the device of choice for closure of ASDs with deficient anterior-superior rim [99]. While the FSO has a very similar design to the ASO and may perform similarly, in these patients there is not enough published data to support the use of these devices over the ASO for larger ASDs.
Traditionally, multifenestrated defects are preferably treated with a CS–SF device. However, the Cribriform ASD Amplatzer and the HSO devices have now been successfully used and are becoming the devices of choice in this setting [110,112].
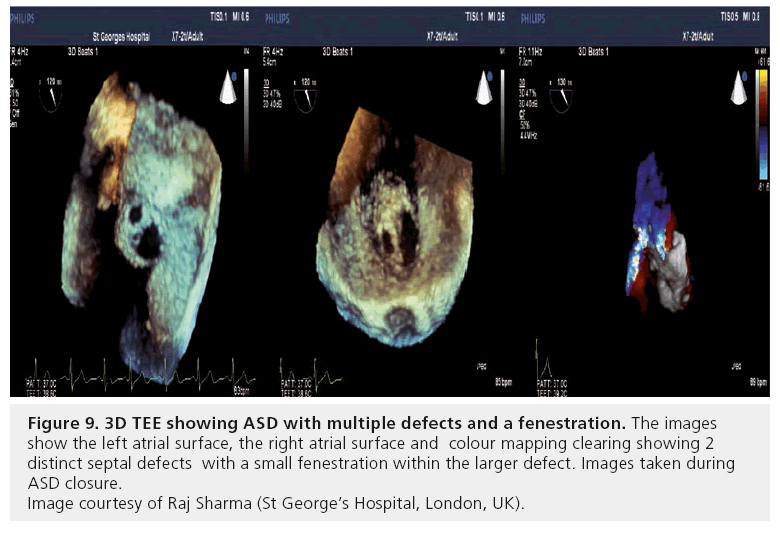
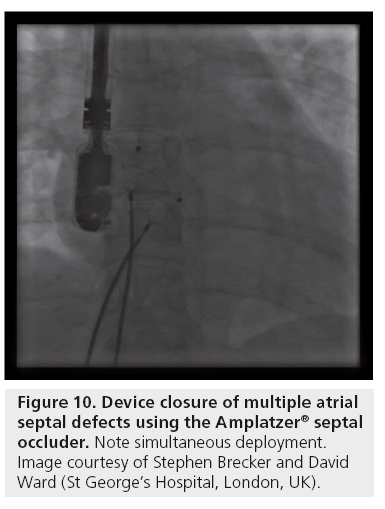
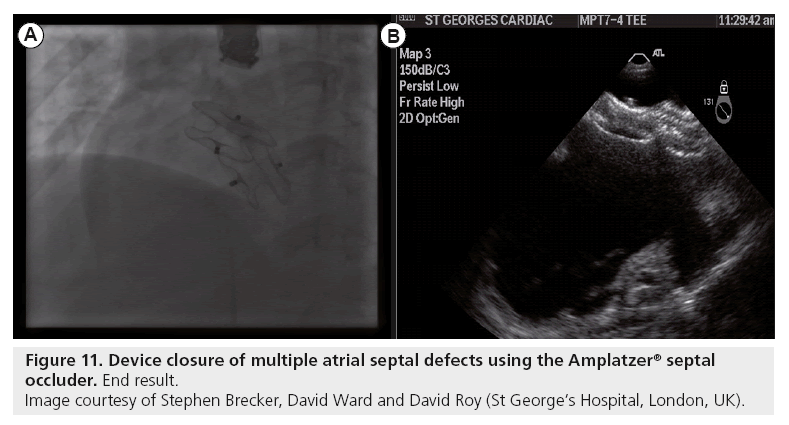
Multiple defects require careful echocardiographic evaluation and planning for device closure. Occasionally, multiple defects with complex anatomy and deficient rims in one or more planes are encountered, and should be referred for surgical closure consideration. With thorough TTE and TEE (preferably 3D; Figure 9) assessment, multiple defects with suitable anatomy are best treated by using two ASO devices, deployed simultaneously (Figures 10 & 11) [113].
Figure 9: 3D TEE showing ASD with multiple defects and a fenestration. The images show the left atrial surface, the right atrial surface and colour mapping clearing showing 2 distinct septal defects with a small fenestration within the larger defect. Images taken during ASD closure.
Image courtesy of Raj Sharma (St George’s Hospital, London, UK).
Conclusion
The presence of a PFO in adults is common and does not require closure in the vast majority of cases. PFO has been associated with certain clinically relevant conditions, the most common being cryptogenic stroke due to presumed paradoxical embolism. There is published evidence from observational studies that PFO closure reduces the incidence of recurrent stroke in patients suffering from cryptogenic stroke, but this has not yet been supported by RCTs. There are many devices available for PFO closure but routine use of any of these should be limited to those with the most clinical data available and to those that the operator feels comfortable using. This is especially important given that the current indications for this procedure remain uncertain.
Secundum ASD is a common congenital cardiac defect that is readily treatable with device closure. Evidence supports device closure over surgery in suitable anatomy and there are many available ASD closure devices. The choice of which device to use remains at the operators discretion and the anatomy of the septum, the clinical data available for the device and the experience of the operator with the specific device should all be considered when deciding which ASD closure device to use.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Executive summary
Indications for patent foramen ovale closure
▪ Patent Foramen Ovale (PFO) is normal fetal atrial communication that persists postpartum in 20–40% of the population.
▪ Cryptogenic Stroke is associated with PFO and these patients have been reported to have a significant incidence of recurrent neurological events. There are case series reports of the benefit of PFO closure following cryptogenic stroke.
▪ The only randomized controlled trial of PFO closure versus medical therapy (CLOSURE 1), failed to demonstrate superiority for closure. However, the trial design has been widely criticized.
▪ Migraine with aura is associated with PFO; however, in the absence of ischemic events, the evidence for PFO closure is poor.
▪ Systemic Arterial Embolization (excluding stroke) is known to occur in patients with PFO and whilst there is no evidence for PFO closure in these patients, many cardiologists still recommend it.
▪ Decompression illness is more likely in scuba divers with PFO. Closure is therefore recomended to prevent cerebral decompression illness.
Diagnosis and imaging for PFO
▪ Bubble contrast transthoracic echocardiography or transesophageal echocardiography (TEE) are the gold standard in PFO screening.
▪ Contrast enhanced transcranial Doppler is emerging as an effective – and in some centers more accessible – investigation in PFO screening.
▪ TEE is the investigation of choice for detailed anatomical assessment prior to or during PFO closure.
▪ Intracardiac echocardiography is routinely used in preference to TEE during PFO closure.
Device selection for PFO
▪ The most common devices for PFO closure include the Amplatzer PFO occluder, HELEX Septal Occluder and the CardioSEAL–STARFlex.
Device comparison
▪ There is limited evidence comparing PFO occlusion devices but the available evidence shows the Amplatzer device to be superior in terms of shunt closure, procedural time and risk of device-related thrombus.
Which device for which anatomy?
▪ For patients with atrial septal aneurysm, most operators choose to stabilize the septum with a device that will cover a larger area (Amplatzer or Nitocclud).
▪ For long PFO tunnels, the Premere™ or Coherex Flatstent™ devices are an ideal choice. However, the Amplatzer device can also be effective by collapsing the PFO tunnel. For multiple defects the Amplatzer cribiform occluder is ideal.
Atrial septal defects
▪ Secundum atrial septal defects (ASDs) make up a third of all congenital heart disease and are the only ASD truly amenable to percutaneous device closure. Surgical ASD closure in appropriately selected patients improves mortality when compared with medical therapy.
Device versus surgical closure
▪ Evidence suggests percutaneous ASD closure is superior to surgery (in anatomically appropriate patients), with a lower incidence of major complications, lower procedure cost and reduced length of stay.
Indications for ASD closure
▪ Any patient with symptoms, evidence of right-sided volume overload or a significant shunt should be considered for ASD closure.
Diagnosis and imaging of an ASD defect
▪ Most ASDs are diagnosed by transthoracic echocardiography and the intracardiac shunt ratio (Qp/Qs) calculated without the need for right heart catheterization.
▪ TEE is vital for detailed anatomical assessment of the interatrial septum and 3-D TEE is particularly useful in the setting of ASD closure.
▪ Cardiac MRI is emerging as a useful pre-assessment ASD closure imaging modality and offers uninvasive, good septal anatomical with shunt calculations.
Device selection for ASD closure
▪ Commonly used devices for ASD closure include the Amplatzer Septal Occluder, The Amplatzer Cribiform Occluder, The HELEX Septal Occluder, The CardioSEAL-STARFlex and the Occlutech Figulla ASD Occluder.
▪ Some evidence suggests that the Amplatzer Septal Occluder is the device of choice for closure of ASDs with a deficient anterior– superior rim. It is also recommended for medium to large centrally located defects and has the lowest reported risk of device embolization.
▪ Multi-fenestrated defects can be successfully closed using a HELEX or Cribiform Amplatzer ASD occluders.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Lechat P, Mas JL, Klimczac M et al. Prevalence of patent foramen ovale in patients with stroke. N. Engl. J. Med. 318, 1148–1152 (1988).
- Mas JL, Arquizan C, Derumeaux G et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N. Engl. J. Med. 345, 1740–1746 (2001).
- Webster MW, Chancellor AM, Bass NM et al. Patent foramen ovale in young stroke patients. Lancet 332, 11–12 (1988).
- Sardesai SH, Marshall RJ, Mourant AJ. Paradoxical systemic embolization through a patent foramen ovale. Lancet 1, 1732–1733 (1989).
- Dickinson DF, Arnold R, Wilkinson JL. Congenital heart disease among 160,480 liveborn children in Liverpool 1960 to 1969. Implications for surgical treatment. Br. Heart J. 46, 55–62 (1981).
- Wolf PS, Vogel JH, Pryor R, Blount SG Jr. Atrial septal defect in patients over 45 years of age. Merits of surgical versus medical therapy. Br. Heart J. 30, 115–124 (1968).
- Thomas JD, Tabakin BS, Ittleman FP. Atrial septal defect with right to left shunt despite normal pulmonary artery pressure. J. Am. Coll. Cardiol. 9, 221–224 (1987).
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 32, 2735–2740 (2001).
- Marnane M, Duggan CA, Curtin D et al. Stroke subtype classification to mechanismspecific and undetermined categories by TOAST, A-S-C-O and causative classification system: direct comparison in the North Dublin population stroke study. Stroke 41, 1579–1586 (2010).
- Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods and baseline characteristics. Stroke 19, 547–554 (1988).
- Calvert PA, Rana BS, Kydd AC, Shapiro LM. Patent foramen ovale: anatomy, outcomes, and closure. Nat. Rev. Cardiol. 8, 148–160 (2011).
- Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 55, 1172–1179 (2000).
- Windecker S, Wahl A, Seiler C et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J. Am. Coll. Cardiol. 44, 750–758 (2004).
- Furlan AJ, Reisman M, Albers GW et al. Study design of the CLOSURE I trial: a prospective, multicenter, randomized, controlled trial to evaluate the safety and efficacy of the STARFlex septal closure system versus best medical therapy in patients with stroke or transient ischemic attack due to presumed paradoxical embolism through a patent foramen ovale. Stroke 41, 2872–2883 (2010).
- Furlan AJ, Reisman M, Massaro J et al. for the CLOSURE I Investigators. A prospective, multicenter, randomized, controlled trial to evaluate the safety and efficacy of the STARFlex septal closure system versus best medical therapy in patients with stroke or transient ischemic attack due to presumed paradoxical embolism through a patent foramen ovale. Presented at: The American Heart Association Meeting. Chicago, IL, USA, 13–17 November 2010.
- Rigatelli G, Aggio S, Dell’avvocata F et al. Left atrial dysfunction in patients with patent foramen ovale and atrial septal aneurysm: an alternative concurrent mechanism for arterial embolism? J. Am. Coll. Cardiol. Interv. 2, 655–662 (2009).
- Sharma R, Curzen NP. Caught in the act: paradoxical pulmonary embolus captured in transit. Eur. J. Neurol. 16, e111 (2009).
- Nelson CW, Snow FR, Barnett M, McRoy L, Wechsler AS, Nixon JV. Impending paradoxical embolism: echocardiographic diagnosis of an intracardiac thrombus crossing a patent foramen ovale. Am. Heart J. 122, 859–862 (1991).
- Missault L, Trouerbach J, Vanmeerhaeghe X, Caes F, Van Nooten G, Clement DL. Trapped venous embolus in a patent foramen ovale causing recurrent paradoxical embolism. Cardiology 86, 86–89 (1995).
- Balli E, Alfieri A, Del Citerna F. Direct evidence of patent foramen ovale as a route for paradoxical embolism. Br. Heart J. 74, 470 (1995).
- Gersony DR, Kim SH, Di Tullio M, Fard A, Rabbani L, Homma S. Acute myocardial infarction caused by paradoxical coronary embolization in a patient with a patent foramen ovale. J. Am. Soc. Echo. 14, 1227–1229 (2001).
- Mehan VK, Wahl A, Walpoth N, Meier B. Instant percutaneous closure of patent foramen ovale in patients with acute myocardial infarction and normal coronary arteries. Catheter. Cardiovasc. Interv. 67, 279–282 (2006).
- Garachemani A, Eshtehardi P, Meier B. Paradoxical emboli through the patent foramen ovale as the suspected cause of myocardial and renal infarction in a 48-year old woman. Catheter. Cardiovasc. Interv. 70, 1010–1012 (2007).
- Cuculi F, Togni M, Meier B. Myocardial infarction due to paradoxical embolism in a patient with large atrial septal defect. J. Invas. Cardiol. 21, E184–E186 (2009).
- Sztajzel R, Genoud D, Roth S, Mermillod B, Le Floch-Rohr J. Patent foramen ovale, a possible cause of symptomatic migraine: a study of 74 patients with acute ischemic stroke. Cerebrovasc. Dis. 13, 102–106 (2002).
- Morandi E, Anzola GP, Angeli S, Melzi G, Onorato E. Transcatheter closure of patent foramen ovale: a new migraine treatment? J. Interv. Cardiol. 16, 39–42 (2003).
- Schwerzmann M, Wiher S, Nedeltchev K et al. Percutaneous closure of patent foramen ovale reduces the frequency of migraine attacks. Neurology 62, 1399–1401 (2004).
- Post MC, Thijs V, Herroelen L, Budts WI. Closure of a patent foramen ovale is associated with a decrease in prevalence of migraine. Neurology 62, 1439–1440 (2004).
- Schwerzmann M, Windecker S, Meier B et al. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology 65, 1415–1418 (2005).
- Giardini A, Donti A, Guidetti D et al. Long-term efficacy of transcatheter patent foramen ovale closure on migraine headache with aura and recurrent stroke. Catheter. Cardiovasc. Interv. 67, 625–629 (2006).
- Spies C, Schrader R. Transcatheter closure of patent foramen ovale in patients with migraine headache. J. Interv. Cardiol. 19, 552–557 (2006).
- Rodes-Cabau J, Mineau S, Cote JM et al. Incidence, timing, and predictive factors of new-onset migraine headache attack after transcatheter closure of atrial septal defect or patent foramen ovale. Am. J. Cardiol. 101, 688–692 (2008).
- Dowson A, Mullen MJ, Wells C et al. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 117, 1397–1404 (2008).
- Trabattoni D, Fabbiocchi F, Grancini L et al. Sustained long-term benefit of patent foramen ovale closure on migraine. Catheter. Cardiovasc. Interv. 77, 570–574 (2011).
- Garg P, Servoss SJ, Dineen A et al. Lack of association between migraine headache and patent foramen ovale: results of a case-control study. Circulation 121, 1406–1412 (2010).
- Rigatelli G, Dell’Avvocata F, Braggion G et al. Primary transcatheter patent foramen ovale closure is effective in improving migraine in patients with high-risk anatomic and functional characteristics for paradoxical embolism. J. Am. Coll. Cardiol. Interv. 3, 282–287 (2010).
- Vigna C, Marchese N, Di Viesti P et al. Improvement of migraine after patent foramen ovale percutaneous closure in patients with subclinical brain lesions: a case-control study. J. Am. Coll. Cardiol. Interv. 2, 107–113 (2009).
- Cheng TO. Platypnea-orthodeoxia syndrome: etiology, differential diagnosis and management. Catheter. Cardiovasc. Interv. 47, 64–66 (1999).
- Medina A, de Lezo JS, Caballero E et al. Platypnea-orthodeoxia due to aortic elongation. Circulation 104, 741 (2001).
- Wilmshurst PT, Byrne JC, Webb-Peploe MM. Relationship between interatrial shunts and decompression illness in divers. Lancet 2, 1302–1305 (1989).
- Torti SR, Billinger M, Schwerzmann M, Vogel R, Zbinden R, Windecker S et al. Risk of decompression illness among 230 divers in relation to the presence and size of patent foramen ovale. Eur. Heart J. 25, 1014–1020 (2004).
- Moon RE, Bove AA. Transcatheter occlusion of patent foramen ovale: a prevention for decompression illness? Undersea Hyperbar. Med. 31, 271–274 (2004).
- Abreu TT, Mateus S, Correia J. Therapy implications of transthoracic echo in acute ischemic stroke patients. Stroke 36, 1565–1566 (2005).
- Dubourg O, Bourdarias JP, Ferrier A et al. Contrast echocardiographic visualization of cough-induced right to left shunt through a patent foramen ovale. J. Am. Coll. Cardiol. 14, 587–594 (1984).
- Buttignoni SC, Khorsand A, Zehetgruber M et al. Agitated saline versus polygelatine for the echocardiographic assessment of patent foramen ovale. J. Am. Soc. Echo. 17, 1059–1065 (2004).
- Langholz D, Louie EK, Konstadt SN, Rao TL, Scanlon PJ. Transesophageal echocardiographic demonstration of distinct mechanisms for right to left shunting across a patent foramen ovale in the absence of pulmonary hypertension. J. Am. Coll. Cardiol. 18, 1112–1117 (1991).
- Hildick-Smith D, Behan M, Haworth P, Rana B, Thomas M. Patent foramen ovale closure without echocardiographic control: use of ‘standby’ intracardiac ultrasound. J. Am. Coll. Cardiol. Interv. 1, 387–391 (2008).
- Wahl A, Tai T, Praz F et al. Late results after percutaneous closure of patent foramen ovale for secondary prevention of paradoxical embolism using the amplatzer PFO occluder without intraprocedural echocardiography: effect of device size. J. Am. Coll. Cardiol. Interv. 2, 116–123 (2009).
- Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. J. Am. Coll. Cardiol. Imag. 3, 749–760 (2010).
- Koenig P, Cao QL, Heitschmidt M, Waight DJ, Hijazi ZM. Role of intracardiac echocardiographic guidance in transcatheter closure of atrial septal defects and patent foramen ovale using the Amplatzer device. J. Interv. Cardiol. 16, 51–62 (2003).
- Teague SM, Sharma MK. Detection of paradoxical cerebral echo contrast embolization by transcranial Doppler ultrasound. Stroke 22, 740–745 (1991).
- Spencer MP, Moehring MA, Jesurum J, Gray WA, Olsen JV, Reisman M. Power M-mode transcranial Doppler for diagnosis of patent foramen ovale and assessing transcatheter closure. J. Neuroimaging 14, 342–349 (2004).
- Caputi L, Carriero MR, Anzola GP. Transcranial Doppler and transesophageal echocardiography: comparison of both techniques and prospective clinical relevance of transcranial Doppler in patent foramen ovale detection. J. Stroke Cerebrovasc. Dis. 18, 343–348 (2009).
- Van H, Poommipanit P, Shalaby M, Gevorgyan R, Tseng CH, Tobis J. Sensitivity of transcranial Doppler versus intracardiac echocardiography in the detection of right-to-left shunt. J. Am. Coll. Cardiol. Imaging 3, 343–348 (2010).
- Azarbal B, Tobis J, Suh W, Chan V, Dao C, Gaster R. Association of interatrial shunts and migraine headaches: impact of transcatheter closure. J. Am. Coll. Cardiol. 45, 489–492 (2005).
- Fischer D, Haentjes J, Meyer GP et al. Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism: procedural and follow-up results after implantation of the Amplatzer® occluder device. J. Interv. Cardiol. 24, 85–91 (2011).
- Taaffe M, Fischer E, Leetz M et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARFlex versus HELEX occluder). Am. J. Cardiol. 101, 1353–1358 (2008).
- Krumsdorf U, Ostermayer M, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defects and patent foramen ovale closure devices in 1000 consecutive patients. J. Am. Coll. Cardiol. 43, 302–309 (2004).
- Herrmann HC, Silvestry FE, Bradbury D et al. Percutaneous patent foramen ovale and atrial septal defect closure in adults: results and device comparison in 100 consecutive implants at a single center. Catheter. Cardiovasc. Interv. 64, 197–203 (2005).
- Schwerzmann M, Windecker S, Mattle H et al. Percutaneous closure of patent foramen ovale: impact of device design on safety and efficacy. Heart 90, 186–190 (2004).
- Braun M, Gliech V, Reichmann H et al. Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism. Periprocedural safety and mid-term follow-up results of three different device occluder systems. Eur. Heart J. 25, 424–430 (2004).
- El Said HG, McMahon CJ, Nihill MR et al. Patent foramen ovale morphology and impact on percutaneous device closure. Ped. Cardiol. 26, 62–65 (2005).
- Buscheck F, Sievert H, Windecker S et al. Patent foramen ovale using the Premere device: the results of the CLOSEUP trial. J. Interv. Cardiol. 19, 328–333 (2006).
- Somberg J. Patent foramen ovale closure devices: thoughts from the circulatory device advisory panel. Am. J. Cardiol. 100, 905–906 (2007).
- Van den Branden BJ, Luermans JG, Suttorp MJ. The BioSTAR® device versus the CardioSEAL® device in patent foramen ovale closure: comparison of mid-term efficacy and safety. EuroIntervention 6, 498–504 (2010).
- Saguner AM, Wahl A, Praz F, de Marchi SF, Windecker S, Meier B. Figulla PFO occluder versus Amplatzer PFO occluder for percutaneous closure of patent foramen ovale. Catheter. Cardiovasc. Interv. 77, 709–714 (2011).
- Kimmelstiel C. Device closure of patent foramen ovale – do similar devices achieve similar results? Catheter. Cardiovasc. Interv. 77, 715 (2011).
- Luermans JG, Post MC, Vermeersch P et al. Outcome after percutaneous closure of a patent foramen ovale using the Intrasept device: a multicenter study Catheter. Cardiovasc. Interv. 71, 822–828 (2008).
- McMahon CJ, El Said HG, Mullins CE. Use of the transseptal puncture in transcatheter closure of long tunnel-type patent foramen ovale. Heart 88, E3 (2002).
- Campbell M. Natural history of atrial septal defect. Br. Heart J. 32, 820–826 (1970).
- Christensen DD, Vincent RN, Campbell RM. Presentation of atrial septal defect in the pediatric population. Ped. Cardiol. 26, 812–814 (2005).
- Craig RJ, Selzer A. Natural history and prognosis of atrial septal defect. Circulation 37, 805–815 (1968).
- Lewis FJ, Taufic M. Closure of atrial septal defects with the aid of hypothermia: experimental accomplishments and the report of one successful case. Surgery 33, 52–59 (1953).
- Konstantinides S, Geibel A, Spillner G et al. A comparison of surgical and medical therapy for atrial septal defect in adults. N. Engl. J. Med. 333, 469–473 (1995).
- King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 235, 2506–2509 (1976).
- Butera G, Carminati M, Giamberti A et al. Percutaneous versus surgical closure of secundum atrial septal defect: comparison of early results and complications. Am. Heart J. 151, 228–234 (2006).
- Jones TK, Latson LA, Vincent R et al. Results of the US multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. J. Am. Coll. Cardiol. 49, 2215–2221 (2007).
- Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J. Am. Coll. Cardiol. 39, 1836–1844 (2002).
- Cowley CG, Lloyd TR, Bove EL, Gaffney D, Dietrich M, Rocchini AP. Comparison of results of closure of secundum atrial septal defect by surgery versus Amplatzer septal occluder. Am. J. Cardiol. 88, 589–591 (2001).
- Butera G, Biondi-Zoccai G, Bussadori C et al. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention 7, 77–385 (2011).
- Kim JJ, Hijazi ZM. Clinical outcomes and costs of Amplatzer transcatheter closure as compared with surgical closure of ostium secundum atrial septal defects. Med. Sci. Mon. 8, CR787–CR791 (2002).
- Hughes ML, Maskell G, Goh TH, Wilkinson JL. Prospective comparison of costs and short term health outcomes of surgical versus device closure of atrial septal defect in children. Heart 86, 67–70 (2002).
- Thomson JD, Aburawi EH, Watterson KG, Van Doorn C, Gibbs JL. Surgical and transcatheter (Amplatzer) closure of atrial septal defects: a prospective comparison of results and cost. Heart 87, 466–469 (2002).
- Vida VL, Barnoya J, O’Connell M, Leon-Wyss J, Larrazabal LA, Castaneda AR. Surgical versus percutaneous occlusion of ostium secundum atrial septal defects: results and cost–effective considerations in a low-income country. J. Am. Coll. Cardiol. 47, 326–331 (2006).
- Warnes CA, Williams RG, Dearani JA et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 52, e1–e121 (2008).
- Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 115, 163–172 (2007).
- De Lezo JS, Medina A, Caballero E et al. Effectiveness of percutaneous device occlusion for atrial septal defect in adult patients with pulmonary hypertension. Am. Heart J. 44, 877–880 (2002).
- Krasuski RA. When and how to fix a ‘hole in the heart’: approach to ASD and PFO. Cleve. Clin. J. Med. 74, 137–147 (2007).
- Lange A, Walayat M, Sutherland GR et al. Assessment of atrial septal defect morphology by transthoracic three dimensional echocardiography using standard grey scale and Doppler myocardial imaging techniques: comparison with magnetic resonance imaging and intraoperative findings. Heart 8, 382–389 (1997).
- Qureshi SA. Selection of patients with secundum atrial septal defects for transcatheter device closure. Eur. Heart J. 21, 510–511 (2000).
- Maeno YV, Benson LN, McLaughlin PR, Boutin C. Dynamic morphology of the secundum atrial septal defect evaluated by three dimensional transesophageal echocardiography. Heart 83, 673–677 (2000).
- Podnar T, Martanovic P, Gavora P, Masura J. Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders. Catheter. Cardiovasc. Interv. 53, 386–391 (2001).
- Chan KC, Godman MJ. Morphological variations of fossa ovalis atrial septal defects (secundum): feasibility for transcutaneous closure with the clam-shell device. Br. Heart J. 69, 52–55 (1993).
- Lang RM, Mor-Avi V, Sugeng L, Nieman PS, Sahn DJ. Three-dimensional echocardiography: the benefits of the additional dimension. J. Am. Coll. Cardiol. 48, 2053–2069 (2006).
- Beerbaum P, Korperich H, Hartmann J et al. Atrial septal defects in pediatric patients: noninvasive sizing with cardiovascular MR imaging. Radiology 22, 361–369 (2003).
- Holmvang G, Palacios IF, Liberthson RR et al. Imaging and sizing of atrial septal defects by magnetic resonance. Circulation 92(12), 3473–3480 (1995).
- Rickers C, Jerosch-Herold M, Kong H et al. Magnetic resonance image-guided transcatheter closure of atrial septal defects. Circulation 107, 132–138 (2003).
- Teo KS, Disney PJ, Sanders P et al. Assessment of atrial septal defects in adults comparing cardiovascular magnetic resonance with transesophageal echocardiography. J. Cardio. MR 12, 44 (2010).
- Butera G, Romagnoli E, Negura D et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1013 consecutive patients. Am. Heart J. 156, 706–712 (2008).
- Chessa M, Butera G, Carminati M. Risk of thrombus formation on devices used to.close transcatheter atrial septal defect and patent foramen ovale. J. Am. Coll. Cardiol. 44, 1712 (2004).
- Post MC, Suttorp MJ, Jaarsma W, Plokker HW. Comparison of outcome and complications using different types of devices for percutaneous closure of a secundum atrial septal defect in adults: a single-center experience. Catheter. Cardiovasc. Interv. 67, 438–443 (2006).
- Pac A, Polat TB, Cetin I, Oflaz MB, Balli S. Figulla ASD occluder versus Amplatzer Septal Occluder: a comparative study on validation of a novel device for percutaneous closure of atrial septal defects. J. Interv. Cardiol. 22, 489–495 (2009).
- Krizanic F, Sievert H, Hijazi Z et al. The Occlutech Figulla PFO and ASD occluder: a new nitinol wire mesh device for closure of atrial septal defects. J. Invas. Cardiol. 22, 182–187 (2010).
- Herrmann HC, Silvestry FE, Bradbury D et al. Percutaneous patent foramen ovale and atrial septal defect closure in adults: results and device comparison in 100 consecutive implants at a single center. Catheter. Cardiovasc. Interv. 64, 197–203 (2005).
- Becker M, Frings D, Hoffmann R et al. Impact of occluder device type on success of percutaneous closure of atrial septal defects: a medium-term follow-up study. J. Interv. Cardiol. 22, 503–510 (2009).
- Law MA, Josey J, Justino H, Mullins CE, Ing FF, Nugent AW. Long-term follow-up of the STARFlex device for closure of secundum atrial septal defect. Catheter. Cardiovasc. Interv. 73, 190–195 (2009).
- Delaney JW, Li JS, Rhodes JF. Major complications associated with transcatheter atrial septal occluder implantation: a review of the medical literature and the Manufacturer And User Facility Device Experience (MAUDE) database. Congen. Heart Dis. 2, 256–264 (2007).
- Swan L, Varma C, Benson L et al. Transcatheter device closure of atrial septal defects in the elderly: technical considerations and short-term outcomes. Inter. J. Cardiol. 107, 207–210 (2006).
- Dalvi BV, Pinto RJ, Gupta A. New technique for device closure of large atrial septal defects. Catheter. Cardiovasc. Interv. 64, 102–107 (2005).
- Tchana B, Hagler DJ, Carano N, Agnetti A, Squarcia U. Device closure of fenestrated atrial septal aneurysm: difficulties and complications with implantation of two devices. J. Invas. Cardiol. 16, 532–534 (2004).
- Hildick-Smith DJ, O’Sullivan M, Wisbey CR, Mackay JH, Lee EM, Shapiro LM. Amplatzer device closure of atrial septal defects in mature adults: analysis of 76 cases. Heart 90, 334–335 (2004).
- Varma C, Benson LN, Webb G et al. Outcomes and alternative techniques for device closure of the large secundum atrial septal defect. Catheter. Cardiovasc. Interv. 61, 131–139 (2004).
- Szkutnik M, Masura J, Kusa J et al. Transcatheter closure of double atrial septal defects with a single Amplatzer device. Catheter. Cardiovasc. Interv. 61, 237–241 (2004).
- Gatzoulis MA, Swan L, Therrien J, Pantely GA. Atrial septal defects and anomalous pulmonary venous drainage. In: Adult Congenital Heart Disease: A Practical Guide. (Eds). Blackwell Publishing Ltd, Malden, MA, USA (2007).
▪ Important study that lends weight to the argument for patent foramen ovale (PFO) closure.
▪ Very comprehensive review of PFO closure devices.
▪▪ First randomized controlled trial of PFO closure versus medical therapy for stroke.
▪ First study to describe this relationship.
▪▪ Excellent review of the anatomical essentials for PFO closure.
▪▪ This is the only randomized trial comparing PFO closure devices.
▪▪ This important study showed that the incidence of thrombus formation on these devices is low but there was a difference between the devices.
▪▪ Very comprehensive meta-analysis of atrial septal defect closure.
▪ Website
201 W. L. Gore & Associates, Inc. www.gore.com/en_gb/index.html