Review Article - Imaging in Medicine (2011) Volume 3, Issue 1
Role of imaging in bariatric procedures: Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding
Laura R Carucci*
VCU Medical Center, 1250 East Marshall St., Main Hospital 3rd Floor, Room 3–417, PO Box 980615, Richmond, VA 23298–0615, USA
- *Corresponding Author:
- Laura R Carucci
VCU Medical Center 1250
East Marshall St., Main Hospital
3rd Floor Room 3–417, PO Box 980615
Richmond VA 23298–0615, USA
Tel.: +1 804 828 0554
Fax: +1 804 628 1132
E-mail: Lcarucci@vcu.edu
Abstract
Morbid obesity is an increasing health problem in western countries and as a consequence bariatric procedures are increasingly performed in both private practice and academic centers. At present, the two most commonly performed procedures, laparoscopic adjustable gastric band and Roux-en-Y gastric bypass, are effective treatment options for morbid obesity with sustained weight loss, decreased morbidity, reversal of comorbidities and prolonged life expectancy. However, many complications may occur following these procedures and patients are often assessed with CT or fluoroscopy examinations. It is important to be aware of the expected postsurgical anatomy and potential complications that may be identified on imaging studies in order to avoid misdiagnosis.
Keywords
bariatric surgery; computed tomography; fluoroscopy; laparoscopic adjustable gastric banding; morbid obesity; postoperative complications; Roux-en-Y gastric bypass
Obesity is an increasingly prevalent health problem in western countries. More than 50% of the US population is overweight or obese with a BMI of greater than 25 kg/m2, and as many as 7% of US adults are considered morbidly obese (BMI >40 kg/m2) [1,2]. Nonsurgical weight loss measures have had limited long-term success for morbid obesity, and bariatric surgery overall has proven an effective treatment in terms of sustained weight loss, decreased morbidity, reversal of comorbidities and prolonged life expectancy [3–5]. Bariatric surgery may be considered following failed conservative treatment in patients with a BMI of >40 kg/m2 or a BMI of greater than 35 kg/m2 with associated high-risk, obesity-related comorbidities [1,3,4].
Bariatric procedures may be restrictive (limiting the intake of solid food), malabsorptive, or a combination of restrictive and malabsorptive. The first malabsorptive procedure was the jejunoileal bypass. This procedure bypassed a long segment of small intestine to induce malabsorption and subsequent weight loss. Although very successful, it has been abandoned due to problems from severe malabsorption, including liver and renal failure. The early restrictive procedures included the horizontal gastroplasty and the vertical banded gastroplasty. These procedures partitioned a small gastric pouch from the remainder of the stomach via a fixed stoma and have been essentially replaced in the USA by the widely successful Roux-en-Y gastric bypass (RYGB; restrictive and malabsorptive) and the adjustable restrictive gastric band.
At present, the two most commonly performed procedures in the USA are RYGB and laparoscopic adjustable gastric band (LAGB), and this article will primarily discuss these procedures. RYGB has been the most commonly performed bariatric procedure in the USA in recent years, representing an estimated 88% of procedures in 2002 [2,6]. However, LAGB is now increasingly performed due to its relative ease of placement and decreased morbidity as compared with RYGB. Despite the success of these procedures, many complications may occur and are often diagnosed with imaging. It is important to recognize the expected postoperative anatomy on fluoroscopic and CT examinations and the potential complications.
Roux-en-Y gastric bypass
The highest long-term success rates have been demonstrated with RYGB in comparison with other surgical weight loss procedures [3,5,7–10]. With RYGB, a small gastric pouch is created from the proximal stomach and anastomozed to a Roux jejunal limb, most often with an end-to-side anastomosis. This creates a narrow stoma between the gastric pouch and the jejunum. The remainder of the stomach, duodenum and proximal jejunum (biliopancreatic limb) are excluded from the path of food. The small pouch and narrow stoma restrict food intake and cause early and prolonged satiety. The bypassed biliopancreatic limb contributes a malabsorptive component of weight loss [5,11]. The Roux limb often has a short, blind-ending limb and an antegrade-flowing (alimentary) limb. The alimentary Roux limb may be retrocolic (through a defect in the transverse mesocolon) or antecolic (anterior to the transverse colon) as it is brought to the gastric pouch. There is a jejunojejunal (JJ) side-to-side anastomosis between the alimentary limb and the excluded biliopancreatic limb followed by a common channel of small bowel.
Imaging techniques following RYGB Upper gastrointestinal examination
Following RYGB, patients are often evaluated with upper gastrointestinal (UGI) examination in the early postoperative period to assess for leak or obstruction. In the late postoperative course, patients may be evaluated for pain, dysphagia, failed weight loss or actual weight gain and possible obstruction.
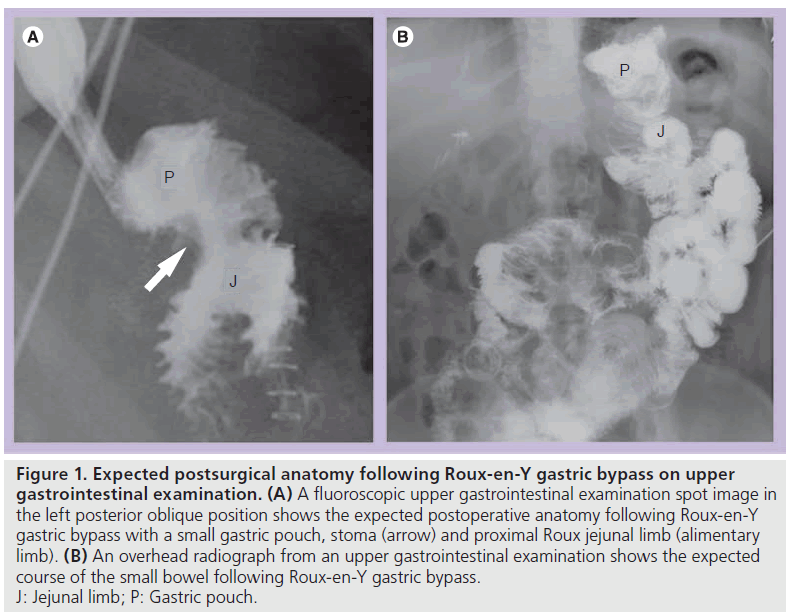
In the early postoperative period (less then 1 month following surgery), UGI examination is initially performed with oral water-soluble, iodinated contrast material. If no leak is identified, barium may be administered. Late postoperative UGI examination (more then 1 month following surgery) may be performed similarly; however, only oral barium is administered. At UGI examination, the patient is initially evaluated in the supine left posterior oblique position. This position allows for optimal assessment of postsurgical anatomy, including the gastric pouch and the stoma (Figure 1). Adequate distension of the pouch and stoma are essential to assess for leaks [12]. Additional fluoroscopic views are obtained as necessary. Overhead radiographs should be obtained until contrast material passes the JJ anastomosis. Late postoperatively, the study should continue until the terminal ileum is opacified, as obstruction or internal hernia (IH) may not become evident until the entire small bowel is opacified.
Figure 1. Expected postsurgical anatomy following Roux-en-Y gastric bypass on upper gastrointestinal examination. (A) A fluoroscopic upper gastrointestinal examination spot image in the left posterior oblique position shows the expected postoperative anatomy following Roux-en-Y gastric bypass with a small gastric pouch, stoma (arrow) and proximal Roux jejunal limb (alimentary limb). (B) An overhead radiograph from an upper gastrointestinal examination shows the expected course of the small bowel following Roux-en-Y gastric bypass. J: Jejunal limb; P: Gastric pouch.
Computed tomography
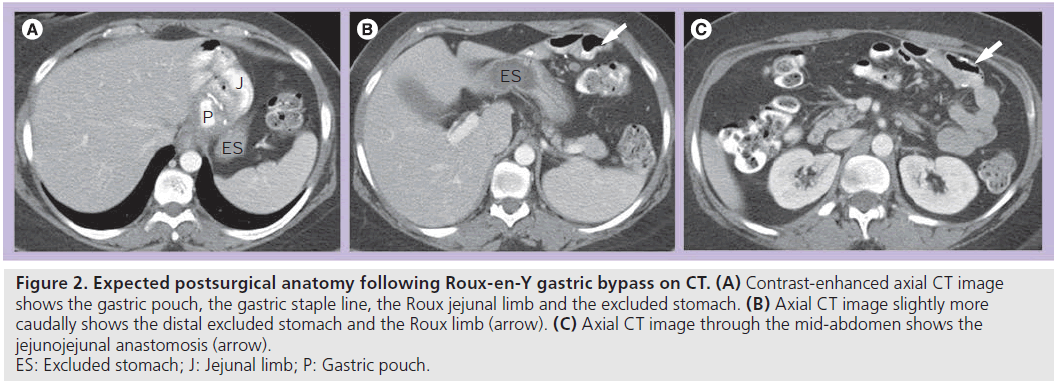
Patients following RYGB may also be assessed with CT for unexplained pain or for possible obstruction or IH. Knowledge of the expected postsurgical anatomy is crucial for the diagnosis of potential complications following the procedure. CT is ideally performed with positive luminal contrast to help distinguish the alimentary limb from the excluded limb, as well as intravenous contrast. The gastric pouch, excluded stomach, Roux limb and JJ anastomosis should be identified (Figure 2).
Figure 2.Expected postsurgical anatomy following Roux-en-Y gastric bypass on CT. (A) Contrast-enhanced axial CT image shows the gastric pouch, the gastric staple line, the Roux jejunal limb and the excluded stomach. (B) Axial CT image slightly more caudally shows the distal excluded stomach and the Roux limb (arrow). (C) Axial CT image through the mid-abdomen shows the jejunojejunal anastomosis (arrow). ES: Excluded stomach; J: Jejunal limb; P: Gastric pouch.
Complications after RYGB
Despite the success of RYGB, many complications may occur in the early (<1 month) and late (>1 month) postoperative course. Early complications may include leak, stomal edema or hematoma, obstruction, staple line disruption or gastrogastric fistula. Late complications may include staple line dehiscence or disruption, marginal ulcers, stomal stenosis, obstruction, internal or abdominal wall hernia, and intussusception.
Postoperative leaks
Leaks may occur in up to 6% of patients and are most often diagnosed within 10 days postoperatively [7,9,12,13]. Leaks increase the morbidity and mortality following RYGB and additional surgery is required in up to 80% of patients [11–13]. Early diagnosis and treatment are essential and UGI examination should be the imaging study of choice to detect leaks [12,14].
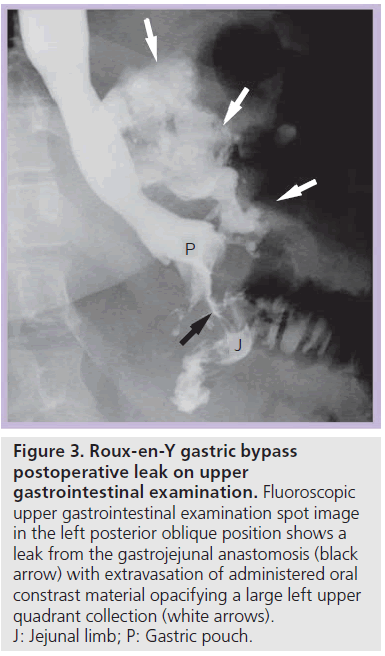
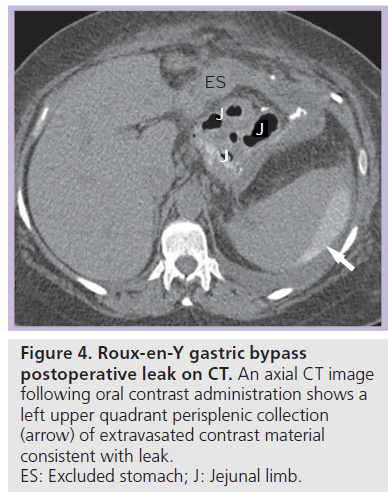
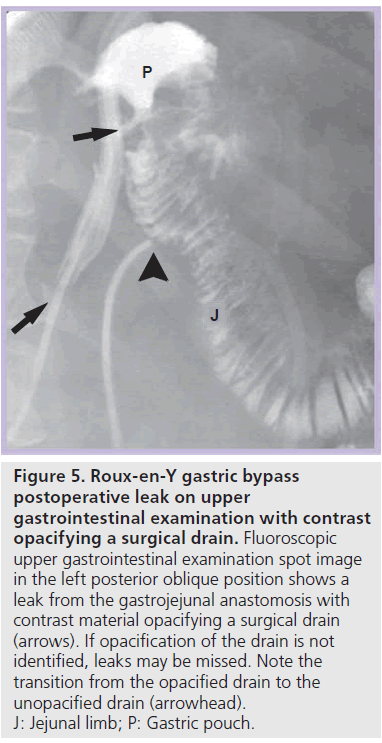
Most postoperative leaks arise from the gastrojejunal anastomosis (77%) and extend to the left of the stoma (75%), often producing a left upper quadrant fluid collection (Figures 3 & 4) [12,14]. Other leak locations include the distal esophagus, gastric pouch, blind-ending jejunal limb and rarely the JJ anastomosis [12]. On UGI examination, extravasated contrast material is identified, most often extending to the left of the anastomosis (Figure 3). Leakage of contrast material to opacify a surgical drain may be the only indication of leaks (Figure 5).
Figure 3.Roux-en-Y gastric bypass postoperative leak on upper gastrointestinal examination. Fluoroscopic upper gastrointestinal examination spot image in the left posterior oblique position shows a leak from the gastrojejunal anastomosis (black arrow) with extravasation of administered oral constrast material opacifying a large left upper quadrant collection (white arrows). J: Jejunal limb; P: Gastric pouch.
Figure 4.Roux-en-Y gastric bypass postoperative leak on CT. An axial CT image following oral contrast administration shows a left upper quadrant perisplenic collection (arrow) of extravasated contrast material consistent with leak. ES: Excluded stomach; J: Jejunal limb.
Figure 5.Roux-en-Y gastric bypass postoperative leak on upper gastrointestinal examination with contrast opacifying a surgical drain. Fluoroscopic upper gastrointestinal examination spot image in the left posterior oblique position shows a leak from the gastrojejunal anastomosis with contrast material opacifying a surgical drain (arrows). If opacification of the drain is not identified, leaks may be missed. Note the transition from the opacified drain to the unopacified drain (arrowhead). J: Jejunal limb; P: Gastric pouch.
Communication with the excluded stomach
A leak across the gastric staple line into the excluded stomach may be an early or late postoperative complication. This allows communication between the gastric pouch and the remainder to the stomach, and may occur in up to 4% of patients [8,14,15]. Ingested material may enter the excluded stomach from inadequate surgical division of the pouch, dehiscence of the staple line (often due to overdistention of the pouch with food), as a consequence of free leak or gastrogastric fistula [15]. This complication can result in inadequate weight loss and surgical revision may be required for a more optimal clinical outcome [14,15].
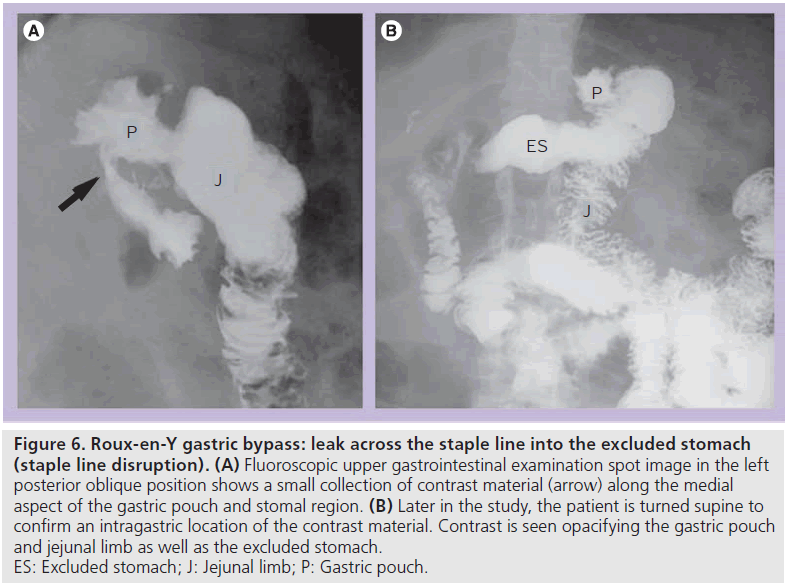
At UGI examination, contrast material may enter the excluded stomach via a leak across the gastric staple line or gastrogastric fistula [14,15]. Contrast material opacifies the gastric pouch and the excluded stomach (Figure 6). Depending on the severity, contrast material may preferentially enter the excluded stomach or the Roux jejunal limb. This diagnosis should be made at initial fluoroscopy because later on in the study contrast can enter the excluded limb via retrograde flow (often with ileus or obstruction). On CT, it may be difficult to distinguish communication with the excluded stomach from retrograde flow. Contrast material in the excluded fundus and not in the duodenum on CT may suggest communication with the excluded stomach. UGI examination should easily make this distinction.
Figure 6.Roux-en-Y gastric bypass: leak across the staple line into the excluded stomach (staple line disruption). (A) Fluoroscopic upper gastrointestinal examination spot image in the left posterior oblique position shows a small collection of contrast material (arrow) along the medial aspect of the gastric pouch and stomal region. (B) Later in the study, the patient is turned supine to confirm an intragastric location of the contrast material. Contrast is seen opacifying the gastric pouch and jejunal limb as well as the excluded stomach. ES: Excluded stomach; J: Jejunal limb; P: Gastric pouch.
Stomal edema or stenosis
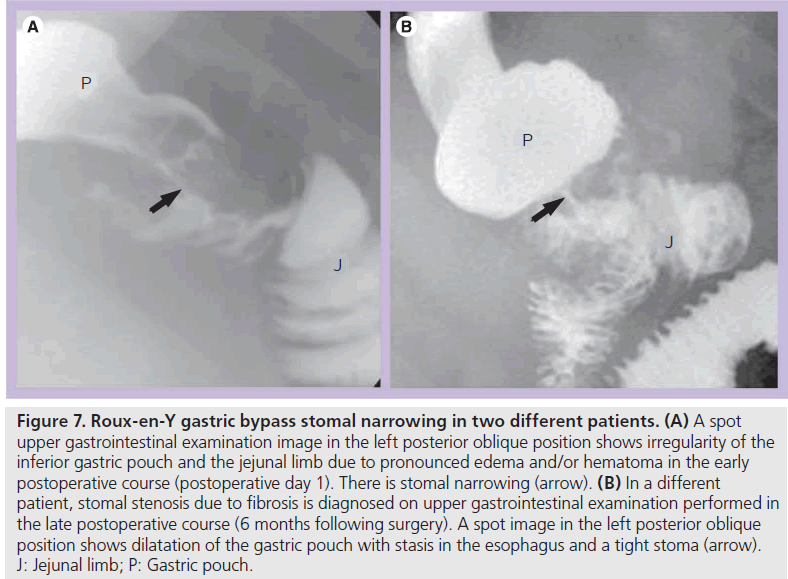
Early postoperative obstruction most often involves an anastomosis due to postoperative edema and/or hematoma (Figure 7A). This can cause mild-to-severe obstruction, and often resolves spontaneously with delayed initiation of diet. However, narrowing at the JJ anastomosis can cause a severe acute distention of the excluded stomach, a serious complication that can result in perforation or necrosis. This may be temporarily relieved by percutaneous decompression until edema and/or hematoma resolve.
Figure 7.Roux-en-Y gastric bypass stomal narrowing in two different patients. (A) A spot upper gastrointestinal examination image in the left posterior oblique position shows irregularity of the inferior gastric pouch and the jejunal limb due to pronounced edema and/or hematoma in the early postoperative course (postoperative day 1). There is stomal narrowing (arrow). (B) In a different patient, stomal stenosis due to fibrosis is diagnosed on upper gastrointestinal examination performed in the late postoperative course (6 months following surgery). A spot image in the left posterior oblique position shows dilatation of the gastric pouch with stasis in the esophagus and a tight stoma (arrow). J: Jejunal limb; P: Gastric pouch.
In the late postoperative course, stomal stenosis may occur with anastomotic fibrosis. Stenosis occurs most often at the gastrojejunal anastomosis (Figure 7B) (up to 10% of patients) with esophageal and pouch dilatation and delayed emptying [7,8,16,17]. Gastrojejunal stenosis usually responds well to endoscopic dilatation. JJ stomal stenosis occurs in less than 0.9% of patients and may require surgical revision [16].
Late small bowel obstruction
Small bowel obstruction (SBO) following RYGB occurs in up to 5% of patients and may be due to adhesions, internal hernia (IH), abdominal wall hernia, stomal stenosis and rarely intussusception [2,18,19]. Adhesions are the most common cause of SBO following open surgery and IH is more common following laparoscopic surgery [8,18–21].
There are three patterns of SBO that can be seen on UGI examination or CT following RYGB due to the altered anatomy of the GI tract [14]. The patterns of obstruction can be classified according to an ABC taxonomic system based on the location of obstruction [22,23]:
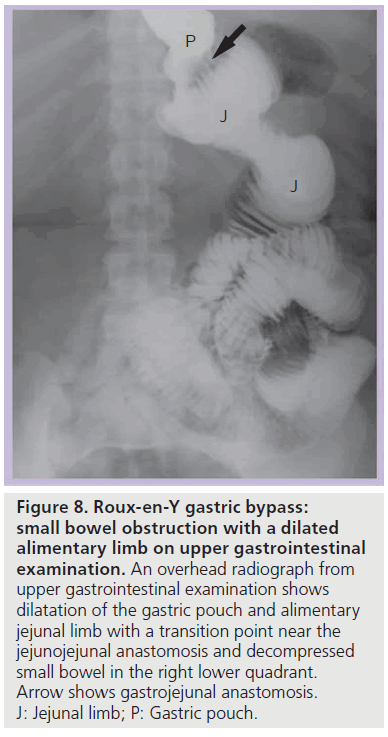
• (A) SBO with a dilated alimentary (Roux) limb and a decompressed biliopancreatic (excluded) limb (Figure 8). The collapsed excluded stomach and duodenum may cause confusion on CT and identification of a dilated Roux limb is necessary to make this diagnosis;
Figure 8.Roux-en-Y gastric bypass: small bowel obstruction with a dilated alimentary limb on upper gastrointestinal examination. An overhead radiograph from upper gastrointestinal examination shows dilatation of the gastric pouch and alimentary jejunal limb with a transition point near the jejunojejunal anastomosis and decompressed small bowel in the right lower quadrant. Arrow shows gastrojejunal anastomosis. J: Jejunal limb; P: Gastric pouch.
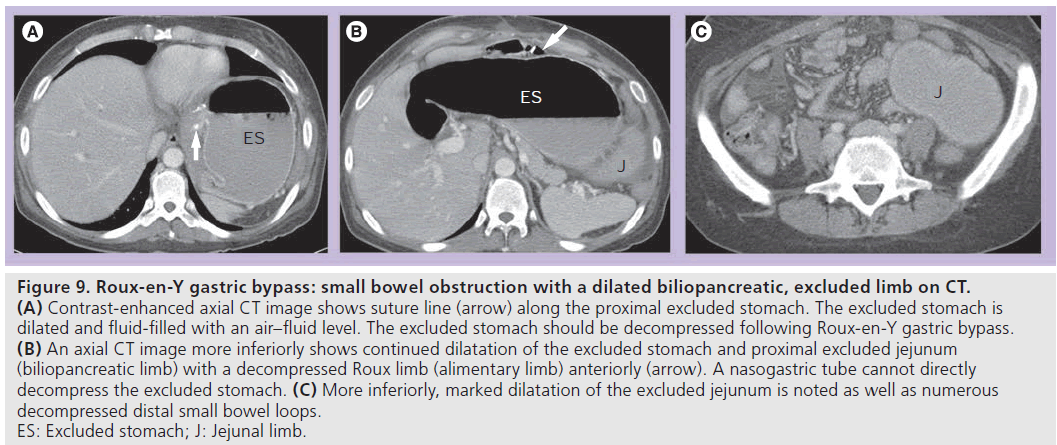
• (B) SBO with a dilated biliopancreatic (excluded) limb only (Figure 9). This is a type of closed loop obstruction that exerts pressure on the excluded stomach and can cause perforation if not treated promptly. On UGI examination, there may be mass effect with compression of an opacified, decompressed alimentary limb due to a dilated fluid-filled biliopancreatic limb. This should be readily recognized on CT; however, the significance of the finding may not be realized without recognition of RYGB anatomy and a decompressed Roux limb (Figure 9). The excluded stomach should be decompressed in patients following RYGB;
Figure 9.Roux-en-Y gastric bypass: small bowel obstruction with a dilated biliopancreatic, excluded limb on CT. (A) Contrast-enhanced axial CT image shows suture line (arrow) along the proximal excluded stomach. The excluded stomach is dilated and fluid-filled with an air–fluid level. The excluded stomach should be decompressed following Roux-en-Y gastric bypass. (B) An axial CT image more inferiorly shows continued dilatation of the excluded stomach and proximal excluded jejunum (biliopancreatic limb) with a decompressed Roux limb (alimentary limb) anteriorly (arrow). A nasogastric tube cannot directly decompress the excluded stomach. (C) More inferiorly, marked dilatation of the excluded jejunum is noted as well as numerous decompressed distal small bowel loops. ES: Excluded stomach; J: Jejunal limb.
• (C) SBO with obstruction of the common channel and dilated alimentary and biliopancreatic limbs (via retrograde flow).
Internal hernia
Internal hernia is a potentially fatal complication following RYGB that may occur in up to 3% of patients [7,8,17,20,24]. IH is most often a late complication, but it can occur at any time following RYGB and may occur on more than one occasion. With IH, bowel herniates through a mesenteric defect, most often a defect in the transverse mesocolon for a retrocolic Roux limb, a small bowel mesenteric defect, or posterior to the Roux limb (Petersen’s defect) [16,17,20,21,24,25]. IH can lead to obstruction, ischemia, infarction and perforation [8,16,17,20,21]. IH may be difficult to diagnose clinically as symptoms may be intermittent and nonspecific and the imaging findings of IH may be difficult to identify [19–21]. Diagnosis of IH with UGI examination or CT requires knowledge of the expected postoperative anatomy and detection of changes in bowel configuration. A high index of suspicion is also necessary to make the proper diagnosis.
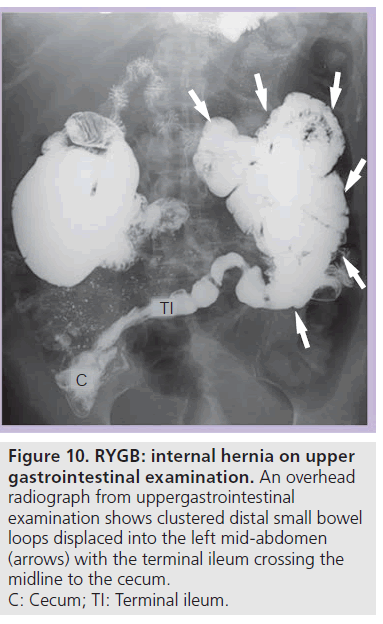
On UGI examination and CT there is an altered bowel configuration, often associated with migration of an anastomotic suture line. The small bowel anastomotic suture line is most often displaced into the left upper quadrant [24]. Small bowel loops appear clustered and displaced, often displacing other bowel (Figure 10). On UGI examination, clustered small bowel is most often seen in the left abdomen (90%), but can be located anywhere in the abdomen and pelvis [14,24]. UGI examination may also demonstrate small bowel limbs entering and exiting the clustered segment and stasis in the clustered bowel [17,24]. UGI examination can show changes in the bowel configuration over time during the study. CT can identify the associated changes in the mesentery with stretching and swirling of vessels and mesenteric engorgement [14,25–27].
Figure 10.RYGB: internal hernia on upper gastrointestinal examination. An overhead radiograph from uppergastrointestinal examination shows clustered distal small bowel loops displaced into the left mid-abdomen (arrows) with the terminal ileum crossing the midline to the cecum. C: Cecum; TI: Terminal ileum.
Laparoscopic adjustable gastric banding
Laparoscopic adjustable gastric banding is now an increasingly popular bariatric procedure in the USA [28,29]. Although LAGB has gained worldwide popularity since the early 1990s and has been the leading procedure performed internationally for several years, the US FDA did not approve the first adjustable gastric band until 2001, delaying its widespread use in the USA [30,31]. LAGB is the least invasive bariatric surgical procedure, and involves no cutting or bypassing of portions of the GI tract [31–33]. It is a restrictive procedure that limits the volume of food consumed [28]. The adjustable gastric band allows for stomal adjustments based on the patient’s individual weight loss curve or symptoms [34,35]. Weight loss results are similar to other restrictive procedures, but may be less than RYGB, especially in the super-obese (BMI >50 kg/m2) [28,32,33,36–38]. However, LAGB is an effective bariatric procedure with less overall significant morbidity than RYGB [28,32,33,37].
A silicone band with an inflatable balloon cuff is placed around the proximal stomach to create a small gastric pouch and a narrow stoma communicating with the remainder of the stomach [32,39]. The inflatable cuff is connected via tubing to a subcutaneous port along the anterior rectus sheath. Accessing the port percutaneously allows for band adjustment. Injection of saline into the port will inflate the cuff and narrow the stoma and aspiration of saline will deflate the cuff and widen the stoma [29,35].
Imaging techniques following LAGB Conventional radiography
A conventional radiograph can be used to assess the position of the band, continuity of the tubing and position of the port. The Phi angle is the angle of the long axis of the band with the vertical, and should be 4–58° in the anteroposterior projection [2,39].
UGI examination
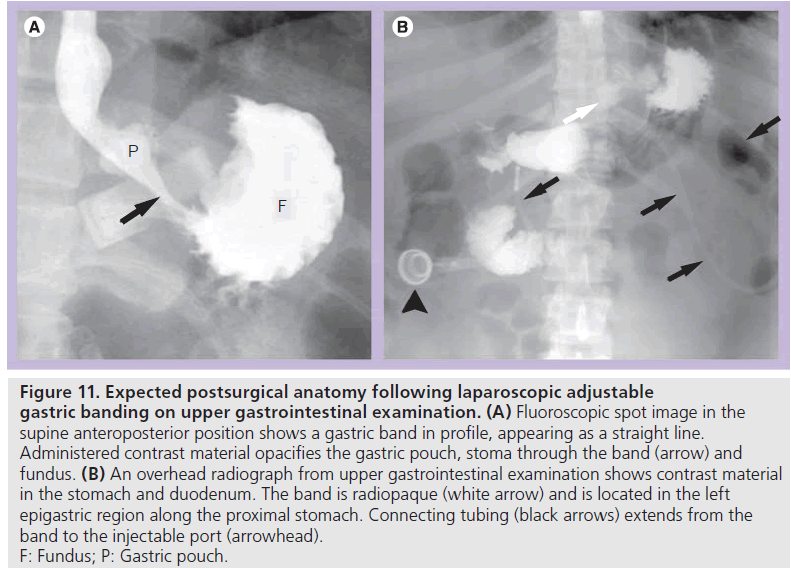
Early postoperative UGI examination is useful to assess device position and to detect leak or obstruction. Late postoperative UGI examination may be performed for vomiting, food intolerance, failed or excessive weight loss, epigastric pain or planned adjustment. At fluoroscopy, the patient should be positioned prior to administering contrast material such that the band is visualized in profile rather than as a ring shape. This allows for optimal evaluation of the stoma and pouch and is most often in the supine anteroposterior or slight right posterior oblique position. If the patient is improperly positioned, the opacified fundus will obscure the stoma [35,40,41]. In the early postoperative period or with concern for perforation, water-soluble contrast material is administered. If no leak is demonstrated, barium may be administered. Ingested contrast material flows from the pouch through the stoma created by the band and into the remainder of the stomach (Figure 11). Pouch configuration and stomal diameter should be assessed. Fluoroscopy allows for assessment of esophageal motility, esophageal or pouch distention, and pouch emptying.
Figure 11.Expected postsurgical anatomy following laparoscopic adjustable gastric banding on upper gastrointestinal examination. (A) Fluoroscopic spot image in the supine anteroposterior position shows a gastric band in profile, appearing as a straight line. Administered contrast material opacifies the gastric pouch, stoma through the band (arrow) and fundus. (B) An overhead radiograph from upper gastrointestinal examination shows contrast material in the stomach and duodenum. The band is radiopaque (white arrow) and is located in the left epigastric region along the proximal stomach. Connecting tubing (black arrows) extends from the band to the injectable port (arrowhead). F: Fundus; P: Gastric pouch.
Computed tomography
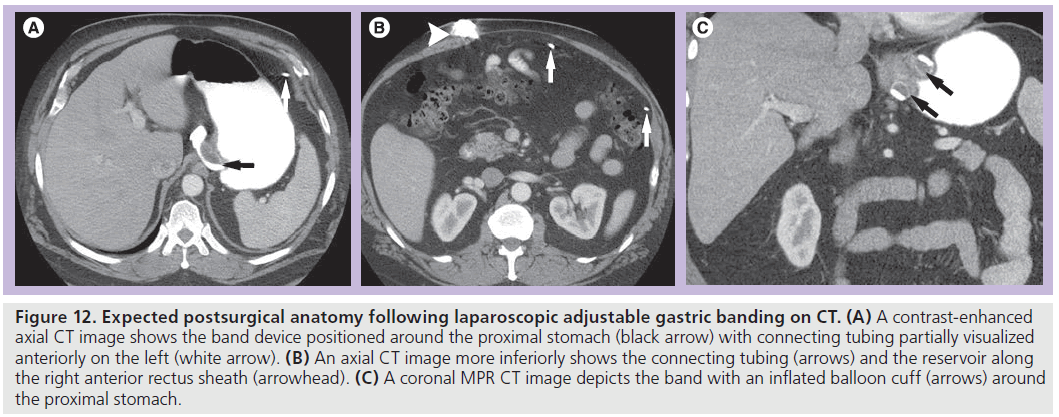
On CT, the radiopaque band can be identified around the proximal stomach with the attached connecting tubing extending through the peritoneal space and then traversing the rectus muscles to connect to the port along the anterior rectus sheath (Figure 12). CT may be helpful to evaluate for a source of infection and to assess soft tissue changes related to the tubing and reservoir.
Figure 12.Expected postsurgical anatomy following laparoscopic adjustable gastric banding on CT. (A) A contrast-enhanced axial CT image shows the band device positioned around the proximal stomach (black arrow) with connecting tubing partially visualized anteriorly on the left (white arrow). (B) An axial CT image more inferiorly shows the connecting tubing (arrows) and the reservoir along the right anterior rectus sheath (arrowhead). (C) A coronal MPR CT image depicts the band with an inflated balloon cuff (arrows) around the proximal stomach.
Band adjustment
Band adjustments can be performed with fluoroscopy, ideally with UGI evaluation before and after adjustment. At fluoroscopy, the subcutaneous port is accessed with a 20–22 gauge noncoring, deflected-tip needle. Improper technique may cause damage and leakage from the system [35,40]. A designated volume of saline can then be injected or withdrawn to narrow or widen the stoma, respectively. Contrast material is then administered orally to confirm adequate narrowing of the stoma without obstruction [35,40]. The use of fluoroscopy may reduce complications from a tight stoma, including obstruction, motility disorders, pouch enlargement, band slippage and band migration [42]. Optimal stomal diameter is 3–5mm and several band adjustments may be necessary to achieve adequate weight loss [29,36,37,40,42].
Complications after LAGB
Laparoscopic adjustable gastric band is a relatively safe procedure with minimal perioperative mortality [28,32,35,36]. However, many patients may experience some degree of morbidity, with additional surgery required in up to 11% of patients [28,31–33,37,43]. Early complications of LAGB are very rare and may include perforation, improper band positioning, early band slippage and acute stomal obstruction [28,29,31–33,37,43]. Early dysphagia and reflux may occur until dietary habits change [29,36]. Late complications are much more common and may include pouch dilatation, band slippage, band migration, obstruction and device-related complications, including device failure [28–30,32,33,36,37,41,44]. Very rarely (<0.3%), gastric necrosis may occur, most often due to band slippage with strangulation [28,32,33]. Many of these complications are best diagnosed with UGI examination [29,31,36,37].
Pouch dilatation
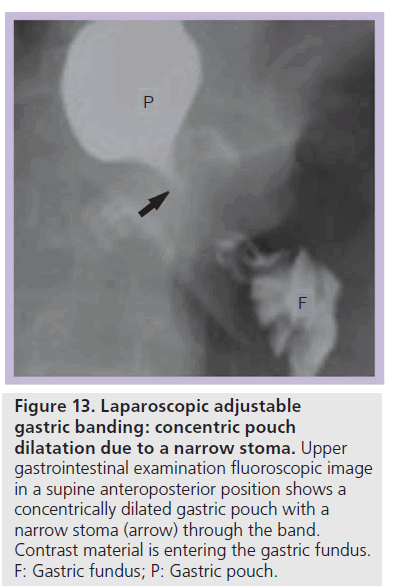
The incidence of pouch dilatation varies with surgical modifications, but may occur in up to 25% of patients and can lead to failed weight loss [45]. Pouch dilatation can occur with a normal or widened stoma, a narrow stoma, or due to band slippage. Pouch dilatation may be concentric or eccentric. On UGI examination a concentrically dilated pouch may be seen with a normal caliber or widened stoma. This type of pouch dilatation is due to chronic overfilling of the pouch with food and requires nutritional counseling [29,36,40]. The pouch may also become dilated due to a narrow stoma, most often caused by band over inflation at adjustment. On UGI examination, pouch dilatation also appears concentric, but the stoma is tight (Figure 13). This presents more acutely with vomiting, dysphagia, esophageal dysmotility or obstruction [29,36]. Once recognized, the band should be deflated to attempt to reverse pouch dilatation and additional complications.
Figure 13.Laparoscopic adjustable gastric banding: concentric pouch dilatation due to a narrow stoma. Upper gastrointestinal examination fluoroscopic image in a supine anteroposterior position shows a concentrically dilated gastric pouch with a narrow stoma (arrow) through the band. Contrast material is entering the gastric fundus. F: Gastric fundus; P: Gastric pouch.
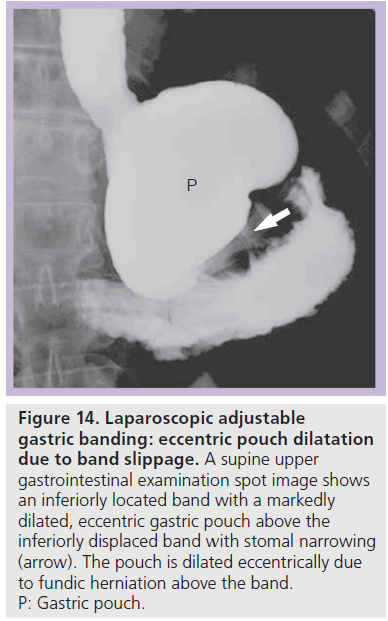
Figure 14.Laparoscopic adjustable gastric banding: eccentric pouch dilatation due to band slippage. A supine upper gastrointestinal examination spot image shows an inferiorly located band with a markedly dilated, eccentric gastric pouch above the inferiorly displaced band with stomal narrowing (arrow). The pouch is dilated eccentrically due to fundic herniation above the band. P: Gastric pouch.
Pouch dilatation & band slippage
With band slippage, the band is dislocated and the fundus herniates above the band. This causes eccentric dilatation of the gastric pouch (Figure 14) [35,39]. Three types of band slippage have been described, including anterior, posterior and concentric slippage. Band slippage may occur in up to 24% of patients; however, the incidence may be decreased with surgical modifications and with modification of eating behaviors [29,32,36,43–48]. Risk factors for band slippage include pouch overdistention, band overinflation and excessive vomiting. Patients may present with acute food intolerance, pain, vomiting, progressive reflux, early satiety and/ or aspiration pneumonia [33,48,49]. Rarely, band slippage may produce sudden severe abdominal pain and acute gastric obstruction [35,49].
On a conventional radiograph, the band is displaced (most often inferiorly) and in a more vertical or horizontal configuration with an abnormal Phi angle. Gas may be seen within a dilated gastric pouch above or below the band [29,32,36]. Contrast material ingestion demonstrates eccentric pouch dilatation, often with a tight stoma and with some degree of pouch obstruction (Figure 14). The band should be deflated immediately to prevent further complications [35,42], and band repositioning or replacement is often necessary [29,35,43,48,49]. If band slippage is not diagnosed and treated, progressive herniation of the stomach above the band may occur and this can lead to acute severe obstruction, gastric volvulus, ischemia, infarction, perforation and hemorrhage [29,39,40].
Band erosion & migration
Intragastric erosion or migration of the band occurs in approximately 2% of LAGB patients [30,32,44,45,49,50]. The gastric band may gradually erode through the gastric wall into the gastrics lumen and can even migrate distally [29,30]. This may be related to the use of nonsteroidal anti-inflammatory medicine, excessive vomiting or pressure from band overinflation [29,32]. Patients may present with pain, gastrointestinal bleeding, weight gain, lack of satiety, abdominal abscess, peritonitis, port site infection, obstruction or perforation [29,30,50]. At UGI examination, contrast material is seen within the stoma as well as surrounding the intragastric portion of the band so that the band appears as a filling defect [29,30]. Band migration may require urgent band removal and repair of the stomach [29,30,50]. Band removal may be attempted endoscopically prior to surgical intervention.
Device-related complications
Device-related complications may involve the port, connecting tubing or band, and are reported in up to 26% of patients. These complications often require surgical repair, but the procedure may be relatively minor [31,37,51]. Devicerelated infection occurs in up to 6% of patients [28,29,32,33,36,51]. The port may migrate or invert, precluding band adjustment in up to 3% of patients [30,32,35,51]. As the port is typically radiopaque, the location and configuration of the port can be readily assessed with conventional radiography or at fluoroscopy. Leakage of saline from the system with band deflation may occur in up to 5% of patients and may be suspected in the setting of failed weight loss despite seemingly appropriate adjustment procedures [29,30,32,41,51]. Acute band deflation widens the stoma and patients experience a change in dietary habits [29,41,51,52]. Fluid leakage may occur from the port, tubing or inflatable cuff. If device leakage is suspected, a plain film should first be obtained to assess the device and to assess for discontinuity of the connecting tubing. Injection of water-soluble contrast into the port at fluoroscopy can identify the location of the leak to direct surgical repair [40,52].
Future perspective
As a reversal of the obesity epidemic does not seem likely in the near future, bariatric procedures will continue to increase in prevalence. There will likely be a trend towards less invasive procedures, including gastric banding devices that may be adjusted electronically and endoscopic bariatric procedures. Laparoscopically implanted leads to block the vagus nerve may prove useful as well. Any procedure that may be utilized to produce weight loss will likely require imaging evaluation to assess for potential complications.
Conclusion
Obesity remains a serious health problem in the USA and European countries. With the limitations of conservative treatment for morbid obesity and the proven effectiveness of bariatric surgery, procedures for weight loss are increasingly performed. Symptomatic patients following bariatric procedures are often evaluated with UGI examination and/or CT. Understanding the types of procedures that may be performed, the expected postsurgical anatomy, the appropriate examination techniques and the potential complications following bariatric surgery is essential to avoid misdiagnosis.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Buchwald H: Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg. Obes. Relat. Dis. 1(3), 371–381 (2005).
- Chandler RC, Srinivas G, Chintapalli KN, Schwesinger WH, Prasad SR: Imaging in bariatric surgery: a guide to postsurgical anatomy and common complications. AJR Am. J. Roentgenol. 190(1), 122–135 (2008).
- Gastrointestinal surgery for severe obesity: National Institutes of Health consensus development conference statement. Am. J. Clin. Nutr. 55(2 Suppl.), 615S–619S (1992).
- Brolin RE: Update: NIH consensus conference. Gastrointestinal surgery for severe obesity. Nutrition 12(6), 403–404 (1996).
- Fisher BL, Schauer P: Medical and surgical options in the treatment of severe obesity. Am. J. Surg. 184(6B), 9S–16S (2002).
- Santry HP, Gillen DL, Lauderdale DS: Trends in bariatric surgical procedures. JAMA 294(15), 1909–1917 (2005).
- Demaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG: Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann. Surg. 235(5), 640–645; discussion 645–647 (2002).
- Higa KD, Boone KB, Ho T: Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients – what have we learned? Obes. Surg. 10(6), 509–513 (2000).
- Schauer PR, Ikramuddin S, Gourash W, Ramanathan R, Luketich J: Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann. Surg. 232(4), 515–529 (2000).
- Sugerman HJ: Gastric bypass surgery for severe obesity. Semin. Laparosc. Surg. 9(2), 79–85 (2002).
- Fobi Ma, Lee H, Holness R, Cabinda D: Gastric bypass operation for obesity. World J. Surg. 22(9), 925–935 (1998).
- Carucci LR, Turner MA, Conklin RC, Demaria EJ, Kellum JM, Sugerman HJ: Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of postoperative extraluminal leaks with upper gastrointestinal series. Radiology 238(1), 119–127 (2006).
- Buckwalter JA, Herbst CA Jr: Leaks occurring after gastric bariatric operations. Surgery 103(2), 156–160 (1988).
- Carucci LR, Turner MA, Yu J: Imaging evaluation following Roux-en-Y gastric bypass surgery for morbid obesity. Radiol. Clin. North Am. 45(2), 247–260 (2007).
- Carucci LR, Conklin RC, Turner MA: Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of leak into excluded stomach with upper gastrointestinal examination. Radiology 248(2), 504–510 (2008).
- Blachar A, Federle MP: Gastrointestinal complications of laparoscopic Roux-en-Y gastric bypass surgery in patients who are morbidly obese: findings on radiography and CT. AJR Am. J. Roentgenol. 179(6), 1437–1442 (2002).
- Blachar A, Federle MP, Pealer KM, Ikramuddin S, Schauer PR: Gastrointestinal complications of laparoscopic Roux-en-Y gastric bypass surgery: clinical and imaging findings. Radiology 223(3), 625–632 (2002).
- Champion JK, Williams M: Small bowel obstruction and internal hernias after laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 13(4), 596–600 (2003).
- Filip JE, Mattar SG, Bowers SP, Smith CD: Internal hernia formation after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Am. Surg. 68(7), 640–643 (2002).
- Higa KD, Ho T, Boone KB: Internal hernias after laparoscopic Roux-en-Y gastric bypass: incidence, treatment and prevention. Obes. Surg. 13(3), 350–354 (2003).
- Iannelli A, Buratti MS, Novellas S et al.: Internal hernia as a complication of laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 17(10), 1283–1286 (2007).
- Sunnapwar A, Sandrasegaran K, Menias CO, Lockhart M, Chintapalli KN, Prasad SR: Taxonomy and imaging spectrum of small bowel obstruction after Roux-en-Y gastric bypass surgery. AJR Am. J. Roentgenol. 194(1), 120–128 (2010).
- Tucker ON, Escalante-Tattersfield T, Szomstein S, Rosenthal RJ: The ABC system: a simplified classification system for small bowel obstruction after laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 17(12), 1549–1554 (2007).
- Carucci LR, Turner MA, Shaylor SD: Internal hernia following Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of radiographic findings at small-bowel examination. Radiology 251(3), 762–770 (2009).
- Blachar A, Federle MP, Dodson SF: Internal hernia: clinical and imaging findings in 17 patients with emphasis on CT criteria. Radiology 218(1), 68–74 (2001).
- Lockhart ME, Tessler FN, Canon CL et al.: Internal hernia after gastric bypass: sensitivity and specificity of seven CT signs with surgical correlation and controls. AJR Am. J. Roentgenol. 188(3), 745–750 (2007).
- Reddy SA, Yang C, Mcginnis LA, Seggerman RE, Garza E, Ford KL 3rd: Diagnosis of transmesocolic internal hernia as a complication of retrocolic gastric bypass: CT imaging criteria. AJR Am. J. Roentgenol. 189(1), 52–55 (2007).
- Demaria EJ, Jamal MK: Laparoscopic adjustable gastric banding: evolving clinical experience. Surg. Clin. North Am. 85(4), 773–787, VII (2005).
- Wiesner W, Schob O, Hauser RS, Hauser M: Adjustable laparoscopic gastric banding in patients with morbid obesity: radiographic management, results, and postoperative complications. Radiology 216(2), 389–394 (2000).
- Hainaux B, Agneessens E, Rubesova E et al.: Intragastric band erosion after laparoscopic adjustable gastric banding for morbid obesity: imaging characteristics of an underreported complication. AJR Am. J. Roentgenol. 184(1), 109–112 (2005).
- Suter M, Giusti V, Worreth M, Heraief E, Calmes JM: Laparoscopic gastric banding: a prospective, randomized study comparing the lapband and the SAGB: early results. Ann. Surg. 241(1), 55–62 (2005).
- Chevallier Jm, Zinzindohoue F, Douard R et al.: Complications after laparoscopic adjustable gastric banding for morbid obesity: experience with 1,000 patients over 7 years. Obes. Surg. 14(3), 407–414 (2004).
- Zinzindohoue F, Chevallier Jm, Douard R et al.: Laparoscopic gastric banding: a minimally invasive surgical treatment for morbid obesity: prospective study of 500 consecutive patients. Ann. Surg. 237(1), 1–9 (2003).
- Kuzmak Li: A review of seven years’ experience with silicone gastric banding. Obes. Surg. 1(4), 403–408 (1991).
- Szucs RA, Turner MA, Kellum JM, Demaria EJ, Sugerman HJ: Adjustable laparoscopic gastric band for the treatment of morbid obesity: radiologic evaluation. AJR Am. J. Roentgenol. 170(4), 993–996 (1998).
- Zacharoulis D, Roy-Chadhury SH, Dobbins B et al.: Laparoscopic adjustable gastric banding: surgical and radiological approach. Obes. Surg. 12(2), 280–284 (2002).
- Favretti F, Cadiere GB, Segato G et al.: Laparoscopic banding: selection and technique in 830 patients. Obes. Surg. 12(3), 385–390 (2002).
- Mognol P, Chosidow D, Marmuse JP: Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding in the super-obese: a comparative study of 290 patients. Obes. Surg. 15(1), 76–81 (2005).
- Mehanna Mj, Birjawi G, Moukaddam HA, Khoury G, Hussein M, Al-Kutoubi A: Complications of adjustable gastric banding, a radiological pictorial review. AJR Am. J. Roentgenol. 186(2), 522–534 (2006).
- Carucci LR, Turner MA, Szucs RA: Adjustable laparoscopic gastric banding for morbid obesity: imaging assessment and complications. Radiol. Clin. North Am. 45(2), 261–274 (2007).
- Pomerri F, De Marchi F, Barbiero G, Di Maggio A, Zavarella C: Radiology for laparoscopic adjustable gastric banding: a simplified follow-up examination method. Obes. Surg. 13(6), 901–908 (2003).
- Frigg A, Peterli R, Zynamon A, Lang C, Tondelli P: Radiologic and endoscopic evaluation for laparoscopic adjustable gastric banding: preoperative and follow-up. Obes. Surg. 11(5), 594–599 (2001).
- Weiner R, Blanco-Engert R, Weiner S, Matkowitz R, Schaefer L, Pomhoff I: Outcome after laparoscopic adjustable gastric banding – 8 years experience. Obes. Surg. 13(3), 427–434 (2003).
- Ponce J, Paynter S, Fromm R: Laparoscopic adjustable gastric banding: 1,014 consecutive cases. J. Am. Coll. Surg. 201(4), 529–535 (2005).
- Zappa Ma, Micheletto G, Lattuada E et al.: Prevention of pouch dilatation after laparoscopic adjustable gastric banding. Obes. Surg. 16(2), 132–136 (2006).
- Iannelli A, Facchiano E, Sejor E, Baque P, Piche T, Gugenheim J: Gastric necrosis: a rare complication of gastric banding. Obes. Surg. 15(8), 1211–1214 (2005).
- Weiner R, Bockhorn H, Rosenthal R, Wagner D: A prospective randomized trial of different laparoscopic gastric banding techniques for morbid obesity. Surg. Endosc. 15(1), 63–68 (2001).
- Wolnerhanssen B, Kern B, Peters T, Ackermann C, Von Flue M, Peterli R: Reduction in slippage with 11-cm lap-band and change of gastric banding technique. Obes. Surg. 15(7), 1050–1054 (2005).
- Kriwanek S, Schermann M, Ali Abdullah S, Roka R: Band slippage – a potentially life-threatening complication after laparoscopic adjustable gastric banding. Obes. Surg. 15(1), 133–136 (2005).
- Abu-Abeid S, Bar Zohar D, Sagie B, Klausner J: Treatment of intra-gastric band migration following laparoscopic banding: safety and feasibility of simultaneous laparoscopic band removal and replacement. Obes. Surg. 15(6), 849–852 (2005).
- Keidar A, Carmon E, Szold A, Abu-Abeid S: Port complications following laparoscopic adjustable gastric banding for morbid obesity. Obes. Surg. 15(3), 361–365 (2005).
- Mittermair RP, Weiss HG, Nehoda H et al.: Band leakage after laparoscopic adjustable gastric banding. Obes. Surg. 13(6), 913–917 (2003).