Perspective - Interventional Cardiology (2014) Volume 6, Issue 6
Resistant hypertension and renal denervation: what do the guidelines say? A nephrologist perspective
- Corresponding Author:
- Orfeas Liangos
Klinikum Coburg, III. Med. Klinik, Ketschendorfer Str. 33, 96450 Coburg, Germany
Tel: +49 9561 223 3304
Fax: +49 9561 249 612
E-mail: liangos_o@hotmail.com
Abstract
Arterial hypertension continues to be a major risk factor for premature cardiovascular morbidity and mortality worldwide. Blood pressure control rates remain stubbornly low, typically not exceeding 60%. As a response, an increasing number of diverse management guidelines have been published by various governmental and nongovernmental organizations around the world. While this kaleidoscope of guidelines provides a host of information, the multitude of partially contrasting data may also contribute to therapeutic confusion and inertia. The present perspective attempts to summarize the most important guidelines for resistant hypertension with a special focus on catheter-based renal denervation. Summary tables facilitate direct comparison of individual guidelines. In addition, the latest trial results on renal denervation for resistant hypertension are critically reviewed.
Arterial hypertension continues to be a major risk factor for premature cardiovascular morbidity and mortality worldwide. Blood pressure control rates remain stubbornly low, typically not exceeding 60%. As a response, an increasing number of diverse management guidelines have been published by various governmental and nongovernmental organizations around the world. While this kaleidoscope of guidelines provides a host of information, the multitude of partially contrasting data may also contribute to therapeutic confusion and inertia. The present perspective attempts to summarize the most important guidelines for resistant hypertension with a special focus on catheter-based renal denervation. Summary tables facilitate direct comparison of individual guidelines. In addition, the latest trial results on renal denervation for resistant hypertension are critically reviewed.
Keywords
cardiovascular disease, guidelines, hypertension, renal denervation, treatment resistance
Arterial hypertension remains one of the most important major cardiovascular risk factors and, as such is one of the most important causes for premature morbidity and mortality worldwide [1,2]. Despite increasing rates of detection, improvements in management and implementation of therapies, its prevalence remains on the increase, due to an increasing life expectancy and changes in lifestyle and nutrition in populations around the world [1,2]. In addition, despite detectable progress, rates of therapeutic control of hypertension remain stubbornly low, with the highest achieving only around 60% control to normotensive values among recognized hypertensives [3].
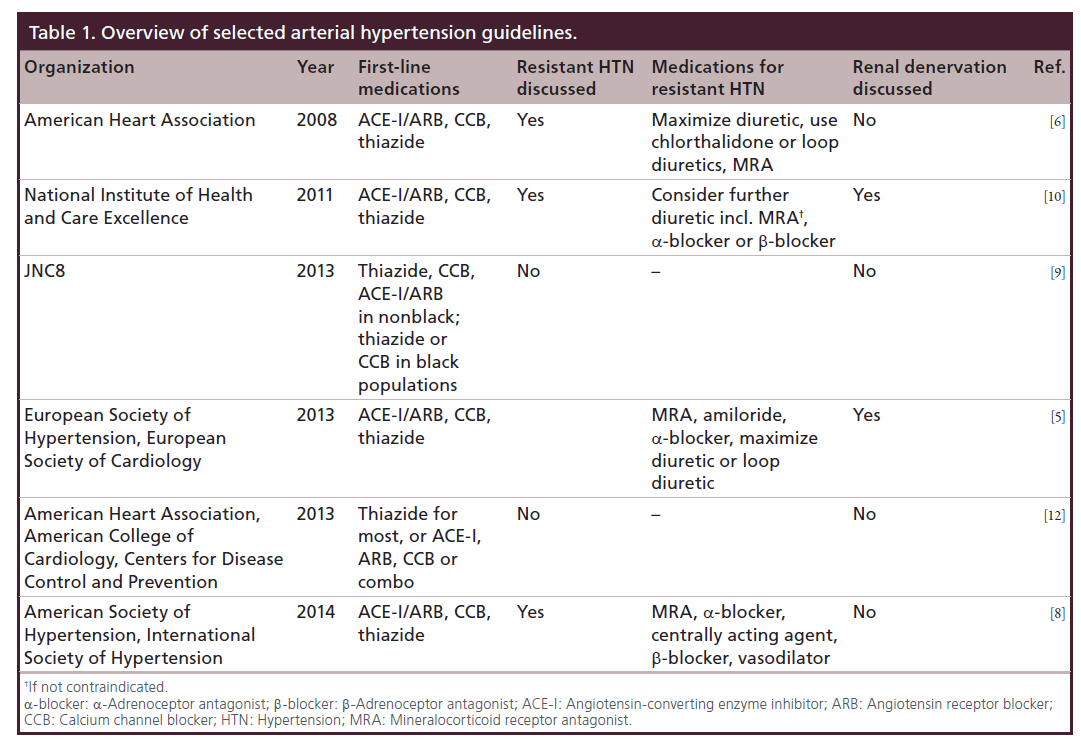
In recent years, an increasing number of management and treatment guidelines were developed and published [4–10]. The initial goal of such guidelines was to extract the most important results from the multitude of existing research studies in hypertension and create useful and effective guidance for practicing clinicians. Table 1 summarizes the clinical hypertension guidelines discussed in this perspective. Unfortunately, there has been an increasing number of individual guidelines from a multitude of organizations, national, regional, specialist and generalist, private/professional and public/governmental, some of which are in agreement and others in contradiction to each other. This increasing atomization of guidelines might discourage clinicians from their consistent implementation and contribute to therapeutic confusion and intertia, rather than alleviate it [11].
Table 1. Overview of selected arterial hypertension guidelines.
Definition of resistant hypertension
With regard to resistant hypertension and to demonstrate the difficulty created by the existence of multiple guidelines, follows a brief summary of definitions for this condition from the most important organizations and guideline-issuing bodies:
• The European Society of Cardiology (ESC) defined resistant hypertension in 2013 as a condition, when a therapeutic strategy that includes lifestyle measures plus a diuretic and two other antihypertensive drugs belonging to different classes at adequate doses (not necessarily including mineralocorticoid receptor antagonist [MRA]) fails to lower blood pressure (BP) <140/90 mmHg [5];
• The American Heart Association (AHA) published a landmark article on this topic already in 2008 and defined resistant hypertension as a BP that remains above goal in spite of the concurrent use of three antihypertensive agents of different classes. Ideally, one of the three agents should be a diuretic and all agents should be prescribed at optimal dose amounts [6];
• The governmentally sponsored US Joint National Committee (JNC) on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure, in their seventh-issued guideline (JNC7), already over 10 years ago in 2004, defined resistant hypertension as the failure to achieve goal BP in patients who are adhering to full doses of an appropriate three-drug regimen that includes a diuretic [4];
• Surprisingly, the long-awaited eighth JNC statement, which was not issued as a full guideline but was entitled Report From the Panel Members Appointed to the Eighth JNC (JNC8) and published in late 2013 included no statement on resistant hypertension but instead focused on early and mild hypertension and when to start pharmacologic treatment [9];
• The UK National Institute for Health and Care Excellence, formerly known as National Institute for Clinical Excellence (NICE) in 2011, defined resistant hypertension as a clinic BP that remains higher than 140/90 mmHg after treatment with the optimal or best tolerated doses of an angiotensin- converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) plus a calcium channel blocker (CCB) plus a diuretic [10].
Resistant hypertension management guidelines
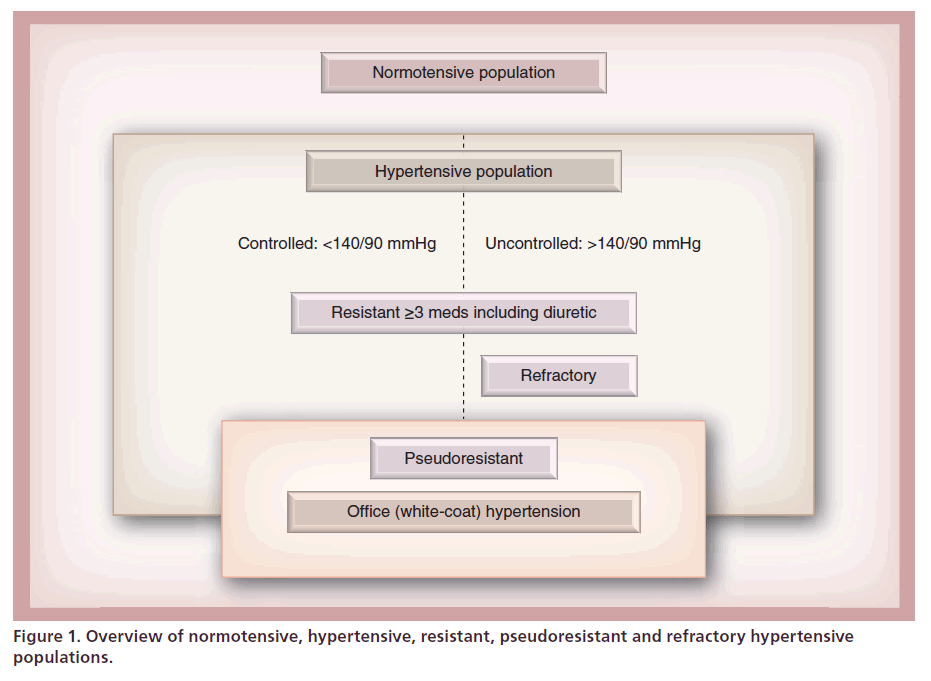
Presence of true resistant hypertension needs to be confirmed by repeated office and ambulatory or home measurements in order to exclude pseudoresistance. In addition, patient adherence to therapy needs to be confirmed prior to making the diagnosis of treatment resistance [3,13]. The latter is not easy as patients might be reluctant to reveal their nonadhering to prescribed medications [2,14]. A nonauthoritarian approach and seeking reasons for nonadherence, for example, side effects or fear of potential side effects, might be most helpful in this situation [15]. In this context, it is important to note that normotensive patients requiring four or more antihypertensives, always including at least one diuretic to lower their BP to the goal should also be considered resistant to treatment, whereas patients who are still not at goal at this point, are considered to be refractory to treatment [4–6]. Figure 1 attempts to illustrate the complex and rather confusing nomenclature, indicating partially overlapping populations with pseudoresistant, resistant, controlled or refractory hypertension (Figure 1).
Diagnostics & screening
In their clinical diagnostic guidance, most societies agree on the following algorithm, in order to identify true resistant hypertension and then render appropriate treatment options.
First, pseudoresistance needs to be ruled out. This includes confirmation of treatment adherence of the prescribed regimen and exclusion of office or so-called white coat hypertension.
Next, modifiable lifestyle factors that can contribute to treatment resistance need to be identified and reversed. Among those, obesity, physical inactivity, excessive alcohol ingestion and a high-sodium, low-fiber diet are among the most important [12].
Once this has been accomplished, the clinician should search for any use of substances interfering with antihypertensive treatment. Among those substances are legal over-the-counter drugs such as nonsteroidal anti-inflammatory medications [16] and sympathomimetics such as ephedra-derivatives commonly used as decongestants or diet pills [17]. In addition, certain prescription drugs, for example, oral contraceptives can play a role as well [12]. European licorice, containing glycyrrhizic acid, if consumed on a regular basis may also lead to hypertension and treatment resistance [18]. Finally, illegal recreational drugs, among them psychostimulants such as the amphetamine derivatives N-methylamphetamine (‘Crystal’) or 3,4-methylendioxy-N-methylamphetamin (‘Extasy’) among many others, play an important but underreported causative or aggravating role in uncontrolled or treatment resistant hypertension [17,19].
As a next step, screening for so-called secondary or identifiable causes of hypertension is recommended. Here, the emphasis should be on obtaining a detailed and focused history and physical examination and perform a limited set of simple, straightforward tests to look for clues that such a condition might be present [20]. Not all patients with hypertension require screening, but those with resistant hypertension, unusually young age or an abrupt onset of severe hypertension might be considered. If there is increased suspicion, confirmatory tests can be performed. For example, excessive snoring, witnessed apnea or excessive daytime sleepiness may point to obstructive sleep apnea. Typical physical exam findings are small anterior nares, a crowded oropharynx and a short, thick neck. A polysomnographic examination in a sleep laboratory could be performed for confirmation [21]. In primary aldosteronism, inappropriate or severe hypokalemia paired with metabolic alkalosis might alert the clinician, who could then measure a plasma aldosterone to renin ratio [22]. Screening for chronic kidney disease is straightforward, evidenced by a reduced estimated glomerular filtration rate derived from a routine serum creatinine or cystatin C measurement and/or evidence of kidney damage by proteinuria, glomerular microhematuria or the finding of multiple cysts on imaging studies [23]. In renal artery stenosis, the issue is more complex. While in fibromuscular dysplasia one rather typically finds a scenario of an abrupt onset of severe hypertension and evidence of secondary (hyper-reninemic) hyperaldosteronism in an otherwise healthy young female [24], elderly patients with known atherosclerotic disease and worsening renal function may have atherosclerotic renal artery stenosis [25]. The management options here are, however, far from clear. Current evidence from two large prospective randomized trials, however, points strongly against routine revascularization, both for improvement of BP control as well as preservation of renal function alike [26,27]. In pheochromocytoma, one should encounter severe episodic hypertension with palpitations, diaphoresis and headaches [28]. Cushing’s syndrome often presents with the typical moon facies, central obesity, abdominal striae rubrae and inter-scapular fat deposition [29]. Finally aortic coarctation with a differential in brachial or femoral pulses and vascular systolic bruit often becomes evident already during physical examination. Once secondary hypertension is identified or strongly suspected, specialist referral for further evaluation and treatment is justified [10].
Pharmacologic treatment
Parallel to above work-up, the clinician should not lose focus on optimizing pharmacologic therapy. In fact, studies from hypertension referral clinics have demonstrated repeatedly, that in most instances, resistant hypertension is due to nonoptimal therapeutic regimens. One crucial aspect, one might be tempted to call it the ‘Achilles heel’ of antihypertensive pharmacotherapy is the correct and appropriate use of diuretics [30].
It is well known and supported by a wealth of experimental and clinical investigations over many years that absolute or relative volume expansion in relationship to the present arterial BP plays an important role in the pathogenesis of hypertension [31,32]. In addition, occult volume expansion detected by thoracic bioimpedance techniques can be demonstrated in a substantial number of patients with resistant hypertension [33]. A comprehensive review of the literature about the role of volume and/or sodium excess in hypertension would be beyond the scope of this perspective, but the interested reader may readily find several excellent review articles on this topic [34,35]. In a recent study, it was shown that intensified diuretic therapy had measurable effects on changes in leg fluid volume, neck circumference and improved nocturnal apnea–hypopnea indices in patients with resistant hypertension and obstructive sleep apnea, which was thought to be due to the relief of congestion in naso- and oro-pharyngeal tissues [36], and may serve as an illustration of further, previously unrecognized pathomechanisms in volume-induced resistant hypertension. However, treatment with diuretics carries a negative image due to adverse metabolic effects including hyperuricemia, lipid abnormalities [37] and impaired glucose tolerance [38]. However, these effects were observed in an era, when especially thiazide diuretics were given at much higher doses compared with today. As an example, while 50 mg two-times a day of hydrochlorothiazide might have been a usual regimen in the 1970s, today’s dosing is usually not escalated beyond 25 mg daily [39]. In this low-dose range, metabolic adverse effects are much milder. Another important point is the choice of diuretic, while hydrochlorothiazide is the most popular antihypertensive diuretic in the USA and bendroflumethiazide in the UK, the thiazide used in the ALLHAT study was in fact chlorthalidone [40]. Albeit closely related to the other thiazides, it appears to be more potent and more importantly longer acting, which increases its BP lowering potential. Furthermore, in patients with concomitant conditions or use of medications causing significant salt and water (i.e., volume) retention, for example, congestive heart failure, chronic kidney disease or nephrosis, or who are using direct vasodilators such as minoxidil, one might need to resort to loop diuretics to counter volume retention [30]. The latter will otherwise blunt the BP response of most other antihypertensives that might have been added previously. In this regard, it is important to note that especially short acting loop diuretics, like furosemide, need to be given two- or three-times daily to prevent rebound sodium retention after their effect wears off [6]. MRAs are per-definition diuretics, but have additional putative effects. Their use in addition to a pre-existing antihypertensive combination regimen has been repeatedly shown to substantially reduce BP [41–43]. Potential mechanisms, outside the known primary hyperaldosteronism, might be summarized under the so-called aldosterone-escape phenomenon, where plasma aldosterone is not frankly elevated, but remains at a physiologically effective level despite suppressed renin levels [44]. Such a situation limits the effects of ACE inhibitors or ARBs. On the other hand, since the use of aldosterone receptor antagonists has been on the rise, so have its complications, among them severe episodes of hyperkalemia and prerenal acute renal failure requiring hospitalization. For that reason, for example, NICE recommends the addition of MRAs only if serum potassium is below 4.5 mmol/l and renal function is preserved [10].
When combining agents, those with different mechanisms of action should be chosen that would complement each other and/or block compensatory mechanisms or adverse effects. One example is a ratecontrolling medication in combination with a diuretic and a direct vasodilator, counteracting potential reflex tachycardia and sodium retention due to the latter [3,5–6]. Within this realm, the use of single pill preparations, formerly known as fixed-dose combinations, can be discussed. In the past, these were not supported due to limited choice for the titration of dose of individual antihypertensive drugs contained in these combinations. In recent years, however, especially in stages II and III and in resistant hypertension, emerging evidence from clinical studies suggests increasingly that use of single pill combinations is faster in lowering BP to goal and treatment adherence is enhanced [5,12,14,45].
Catheter-based renal denervation guidelines in resistant hypertension
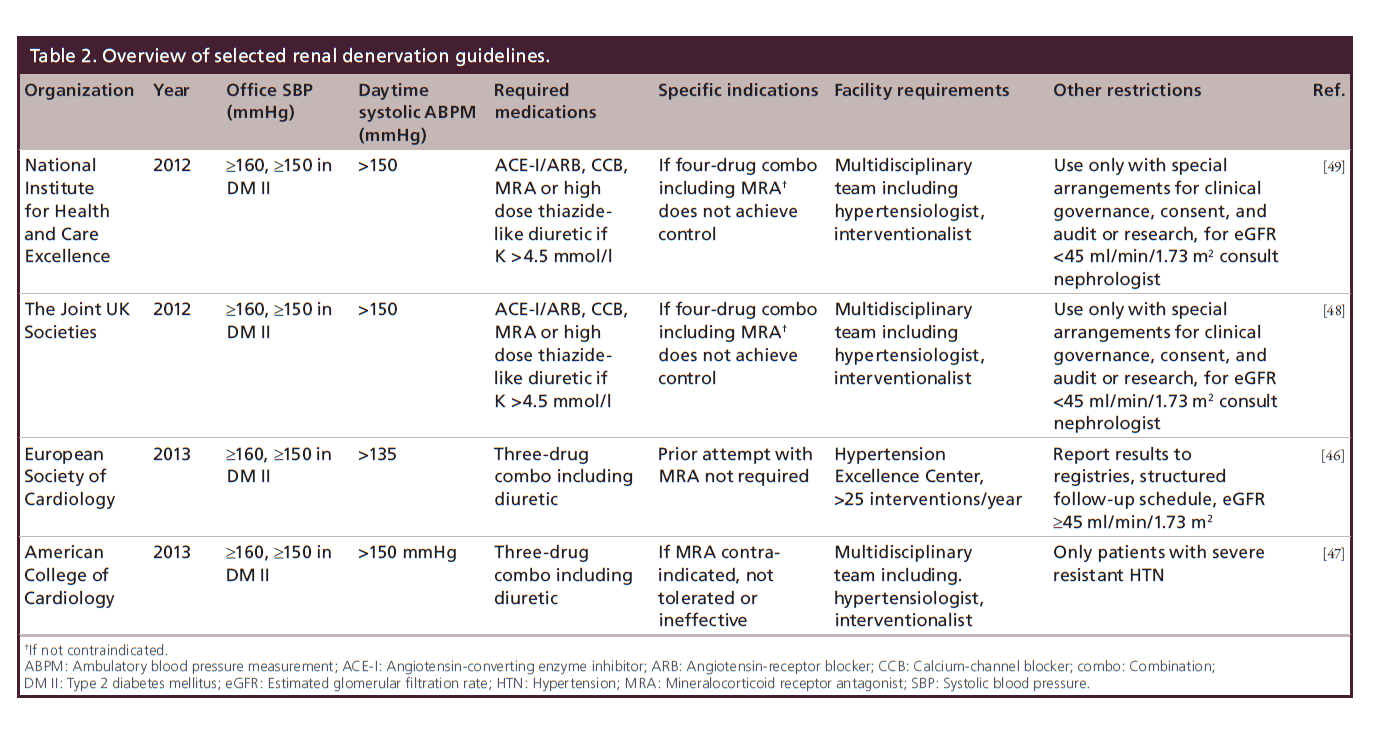
In contrast to the multitude of differing guidance documents in hypertension, there is surprising agreement between the ESC [46], the American Society of Cardiology (ACC) [47], the Joint UK Societies (a collaboration of cardiology, interventional radiology, hypertension and renal societies) [48] and NICE [49]. Also see Table 2 for a synopsis of the renal denervation guidelines discussed below. All essentially recommend the same algorithm leading up to renal denervation.
First, a sustained elevated clinic systolic BP (SBP) ≥160 mmHg (with a stricter cut-off of ≥150 mmHg in Type 2 diabetes) under confirmed use of a minimum of three antihypertensive medications including one diuretic is required. As an exception, the UK organizations specify four drugs including specific drug classes such as an ACE inhibitor or ARB, a CCB, a thiazide diuretic and/or spironolactone as a requirement. All organizations also ask for confirmation of treatment resistance by performing an ambulatory BP measurement (ABPM), showing a minimum of 150 mmHg as the daytime average ambulatory BP as a mandatory precondition. This is required in order to confirm true treatment resistance and exclude office- or ‘white coat’ hypertension. As an exception, ESC only requires that the average daytime ABPM be elevated, in other words, ≥135 mmHg [46]. In addition, as discussed above, presence of secondary forms of hypertension needs to be excluded and potential contributing lifestyle factors carefully explored, identified and corrected. This also includes identification and discontinuation of substances that can increase BP. Further preconditions mentioned by all societies are a preserved renal function, i.e., an estimated GFR of greater than 45 ml/min/1.73 m2. Following these guidelines and a long-enough follow-up period prior to referral with confirmed continued treatment resistance are best to ensure that only those patients are referred to undergo a procedure, the long-term efficacy and safety of which has not yet been established [46–49].
Procedural requirements
As in above diagnostic guidelines, existing recommendations for technical and procedural requirements for renal denervation are similar across the board [46–49]. In Europe, currently available CE-certified systems for catheter-based renal denervation are the Medtronic Symplicity, St Jude EnligHTN, Vessix V2 and Covidien One Shot systems, all of which utilize radiofrequency, as well as the Recor Paradise system, that uses ultrasound as an energy source. The guidelines also agree on certain anatomical requirements to perform the procedure, which are a renal artery length greater than 20 mm with a minimum diameter of 4 mm and no evidence of significant stenosis or plaque. Latter requires performing prior vascular imaging, for example, aortogram or renal arteriography, which can even be performed immediately prior to denervation. All guidelines agree on anticoagulation, usually a pretreatment with acetyl–salicylic acid 250 mg intravenously, followed by oral 75–100 mg daily for 4 weeks. In addition, intraprocedural activated clotting time-guided anticoagulation with heparin with a goal of 200–250 s is also recommended. The percutaneous femoral access under fluoroscopy is the preferred approach, although radial catheter devices have already been developed. Since during the procedure, due to destruction of pain conducting nerve fibers, severe visceral pain can develop, an effective intravenous analgesia and conscious sedation is unanimously and strongly recommended. Consequently, the denervation center needs to be equipped with vital sign monitoring capabilities and a recovery room infrastructure as well [46–49].
New study results on renal denervation
The ‘elephant in the room’, although not a guideline in itself, are the recently published results of the so-far largest and best-designed Controlled Trial of Renal Denervation for Resistant Hypertension (Simplicity HTN-3; ClinicalTrials.gov: NCT01418261) [50]. Therefore, a discussion of this study must be included in the present perspective. Bhatt and colleagues undertook a prospective, single-blind, randomized, sham-controlled multicenter trial of renal denervation in North America with a total of 535 patients randomized in a 2:1 ratio to undergo percutaneous transluminal radiofrequency ablation for treatment of resistant hypertension. Earlier studies were smaller and designed as single-arm nonrandomized or randomized but not sham-controlled trials and have shown substantial BP lowering of up to -32/14 mmHg change in office measurements [51,52]. The goal of the Simplicity HTN-3 trial was to demonstrate efficacy of renal denervation with a more rigorous study design, including ABPM, blinding of the participants and ultimately achieving US FDA approval for clinical use in the USA [53]. The study was funded by Medtronic, the manufacturer of the renal denervation device employed. The study was negative with a mean difference in office SBP between the intervention and the sham group of only -2.39 mmHg (p = 0.26) and a mean difference in ABPM of only -1.96 mmHg (p = 0.98) after 6 months [50]. All the results of prespecified subgroup analyses were also nonsignificant. Nonetheless and in addition to the already voiced concerns about operator proficiency with the device [54], one can find several points for question, both in the results as well as the design of the study. For instance, it is the opinion of this author that the results were nonsignificant not because patients in the renal denervation group did not experience lowering of their BP (average -14.13 mmHg office SBP; p < 0.001), but because the sham procedure also leads to a substantial and sustained reduction in both office and ambulatory daytime BP by -11.74 (p < 0.001) and -4.79 mmHg (p < 0.001), respectively. The authors interpreted this result as an example of the placebo effect as well as the Hawthorne effect [50]. While generally, placebo effects are well recognized and can be detected in a multitude of situations and after various interventions, one may wonder, how sustained such an effect can be. Would the placebo effect of a sham renal artery catheterization remain in effect for 6 months? Or, if renal artery catheterization alone would cause such a significant and sustained BP reduction, then why are we not catheterizing all our hypertensive patients? A more convincing hypothesis would be, that the control group experienced a reduction in BP by the so-called Hawthorne effect, a phenomenon, by which study participants performance improves merely by inclusion into a study and not by the intervention employed in the study [55]. This is an interesting point, since long-term adherence to therapy is a key component of successful antihypertensive treatment and nonadherence a common cause for treatment failure in this condition. One could argue that the Hawthorne effect caused reductions in BP in both the intervention as well as the control groups of the study and therefore biased its results toward the null hypothesis. One could envision an alternative study design, where randomization does not occur during the first renal angiography, like in this study, but where BP responses are observed following an initial sham procedure (diagnostic renal angiography) for all participants and only those, whose BP does not improve following this procedure are ultimately enrolled in the trial. But despite this speculation, currently it seems unlikely that a similar trial will be conducted again.
Another important point of potential critique is the run-in phase, in which study participants were required to remain on a stable regimen of a minimum of three antihypertensive drugs including a diuretic, all in appropriate doses, for a minimum of 2 weeks and have a systolic office BP of 160 mmHg or higher in order to qualify for enrollment [53]. However, this might seem like a rather short period of time allowed for stabilization and it might take longer than 2 weeks for a new medication or dose change to fully take effect. This point can be illustrated by data from the ALLHAT, the results of which indicate a continued decrease in systolic and diastolic office BP measurements even without a step-up in medications well beyond 6 months into the study [40,56]. One might speculate, that in the control group of the Simplicity HTN-3 study, decrements in BP after randomization might have therefore been, perhaps at least in part, a reflection of medication adjustments that were made prior to randomization. Another point might be the relatively low margin for the average daytime SBP determined by ABPM. It was set at 135 mmHg, which is the upper limit of normal for this measurement and stands in contrast to the relatively high minimum required office SBP of at least 160 mmHg to qualify for the study (20 mmHg higher than the upper limit of normal). For comparison, the UK National Institute of Health and Care Excellence, in their guideline for renal denervation, requires an average daytime ABPM of greater than 150 mmHg to recommend referral for renal denervation [49]. This discrepancy between a substantially elevated office BP required and an only marginally elevated ABPM might have led to enrollment of patients with a greater component of office hypertension into the study. Accordingly, average office measurements were approximately 20 mmHg above the ABPM averages, taken from Figures 1 and 2 of the original article [50]. One might speculate, that enrollment of patients with such a substantial BP peak in response to an office measurement, compared with their ABPM daytime average, might also be more responsive to placebo treatments, for example, a sham renal denervation procedure.
But let’s make no mistake, the Simplicity HTN-3 trial has been so far the largest and most carefully designed study of the effects of catheter-based renal denervation on hypertension and its results were negative, despite the above concerns, that are not more than an opinionated viewpoint of the author of this perspective. Therefore, it should go without question that renal denervation should, until further data emerge, not be used routinely in all cases of resistant hypertension but at best only in well-selected cases and if possible within the realm of controlled research studies or registries. This notion is backed by several expert reviews and statements following publication of the results of Simplicity HTN-3 [57,58], whereas the existing management guidelines have not been revised so far in response to these results.
Future perspective
Despite the negative results of the Simplicity HTN-3 randomized trial, device-based therapies are an appealing concept for resistant hypertension, a lifelong condition that requires life-long management and is subject to a high degree of nonadherence of prescribed drug treatments and health-promoting lifestyles and diets. A one-shot approach, a procedure that could effectively lower BP for a sustained period of time would complement an otherwise chronic, continuous treatment with combinations of multiple drugs, associated with sometimes uncomfortable side effects and mounting costs. The current setback may allow other approaches than catheter-based radiofrequency ablation of renal nerves to have an opportunity to emerge or re-emerge, for example, carotid body stimulation therapy, among other concepts.
One more provocative thought at the end: Industrialized societies tend to provide industrial solutions to problems that might be caused by lifestyle and societal problems. In the case of hypertension, which is ubiquitous in industrialized societies and is surging in countries with emerging economies, nonpharmacologic factors such as lifestyle and diet, for example, sodium, potassium, animal protein and fat intake, energy density of foods and physical inactivity all appear to be playing a much more important role in the pathogenesis of this condition. Broad-scale interventions in this context might hold the promise to be more successful in preventing the condition.
But it might be the inability or lack of resolve of the societies that will prevent such low-tech approaches to take effect on a broad scale.
Financial & competing interests disclosure
O Liangos provided the conception, design and acquisition of data, and wrote the manuscript. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Definition of resistant hypertension
• A sustained elevation in the office blood pressure ≥140/90 mmHg despite adherence to three or more antihypertensive drugs at appropriate doses including one diuretic.
Resistant hypertension management guidelines
• A structured approach is recommended, where true treatment resistance is confirmed by excluding pseudoresistance, lack of adherence and office hypertension.
Diagnostics & screening
• Identify and reverse lifestyle factors including obesity, physical inactivity, excessive alcohol ingestion and a high-sodium, low-fiber diet.
• Search for interfering drug treatments or illicit drug abuse.
• Screen for and treat secondary forms of hypertension.
Pharmacologic treatment
• Choose drugs according to underlying conditions. Review compelling indications and contra-indications for each individual patient.
• Combination of several drugs is frequently required and should be chosen to complement each other’s pharmacodynamics and side-effect profiles.
• In general, appropriate and effective diuretic therapy is paramount to a successful management of resistant hypertension.
• Consider adding-on mineralocorticoid receptor antagonist if no hyperkalemia or chronic kidney disease.
• Consider single-pill combination therapies to improve adherence.
Catheter-based renal denervation guidelines in resistant hypertension
• General agreement among guideline-issuing organizations on patient identification and preparation for renal denervation.
• Work-up for resistant hypertension and daytime ambulatory blood pressure measurement average greater than 150 mmHg systolic are required.
New study results on renal denervation
• The Simplicity HTN-3 trial, a well-designed multicenter sham-controlled and randomized study, has been published in April 2014 with negative results, which weigh heavily on the future role of catheter-based renal denervation.
• Investigators and clinicians need to carefully evaluate all existing information on this procedure and also await potential future results.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Borzecki AM, Kader B, Berlowitz DR. The epidemiology and management of severe hypertension. J. Hum. Hypertens. 24(1), 9–18 (2010).
- Redon J, Brunner HR, Ferri C et al. Practical solutions to the challenges of uncontrolled hypertension: a white paper. J. Hypertens. 26(Suppl. 4), S1–S14 (2008).
- Moser M, Setaro JF. Clinical practice. Resistant or difficultto- control hypertension. N. Engl. J. Med. 355(4), 385–392 (2006).
- Chobanian AV, Bakris GL, Black HR et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289(19), 2560–2572 (2003).
- Mancia G, Fagard R, Narkiewicz K et al. 2013 ESH/ ESC Practice guidelines for the management of arterial hypertension. Blood Press. 23(1), 3–16 (2014).
- Calhoun DA, Jones D, Textor S et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 117(25), e510–e526 (2008).
- McCormack T, Arden C, Begg A et al. Optimising hypertension treatment: NICE/BHS guideline implementation and audit for best practice. Br. J. Cardiol. 20(Suppl. 1), S1–S16 (2013).
- Weber MA, Schiffrin EL, White WB et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J. Clin. Hypertens. (Greenwich) 16(1), 14–26 (2014).
- James PA, Oparil S, Carter BL et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311(5), 507–520 (2014).
- Clinical management of primary hypertension in adults National Institute for Health and Care Excellence (2011).
- www.nice.org.uk/guidance/cg127 11 Sisson SD, Rastegar D, Rice TN, Prokopowicz G, Hughes MT. Physician familiarity with diagnosis and management of hypertension according to JNC 7 guidelines. J. Clin. Hypertens. (Greenwich) 8(5), 344–350 (2006).
- Go AS, Bauman MA, Coleman King SM et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 63(4), 878–885 (2014).
- Cameron C. Patient compliance: recognition of factors involved and suggestions for promoting compliance with therapeutic regimens. J. Adv. Nurs. 24(2), 244–250 (1996).
- Abdellatif AA. Role of single-pill combination therapy in optimizing blood pressure control in high-risk hypertension patients and management of treatment-related adverse events. J. Clin. Hypertens. (Greenwich) 14(10), 718–726 (2012).
- Lukoschek P. African Americans’ beliefs and attitudes regarding hypertension and its treatment: a qualitative study. J. Health Care Poor Underserved 14(4), 566–587 (2003).
- Roth SH, Anderson S. The NSAID dilemma: managing osteoarthritis in high-risk patients. Phys. Sports Med. 39(3), 62–74 (2011).
- Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 125(3), 363–375 (2010).
- Walker BR, Edwards CR. Licorice-induced hypertension and syndromes of apparent mineralocorticoid excess. Endocrinol. Metab. Clin. North Am. 23(2), 359–377 (1994).
- Devlin RJ, Henry JA. Clinical review: major consequences of illicit drug consumption. Crit. Care 12(1), 202 (2008).
- Onusko E. Diagnosing secondary hypertension. Am. Fam. Physician 67(1), 67–74 (2003).
- Pedrosa RP, Drager LF, Gonzaga CC et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 58(5), 811–817 (2011).
- Mosso L, Carvajal C, Gonzalez A et al. Primary aldosteronism and hypertensive disease. Hypertension 42(2), 161–165 (2003).
- Levey AS, Eckardt KU, Tsukamoto Y et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: Improving Global Outcomes (KDIGO). Kidney Int. 67(6), 2089–2100 (2005).
- Slovut DP, Olin JW. Fibromuscular dysplasia. N. Engl. J. Med. 350(18), 1862–1871 (2004).
- Dworkin LD, Cooper CJ. Clinical practice. Renal-artery stenosis. N. Engl. J. Med. 361(20), 1972–1978 (2009).
- Investigators A, Wheatley K, Ives N et al. Revascularization versus medical therapy for renal-artery stenosis. N. Engl. J. Med. 361(20), 1953–1962 (2009).
- Cooper CJ, Murphy TP, Cutlip DE et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N. Engl. J. Med. 370(1), 13–22 (2014).
- Pacak K, Lenders JWM, Eisenhofer G. Clinical presentation of pheochromocytoma. In: Pheochromocytoma: Diagnosis, Localization, and Treatment. Blackwell Publishing Ltd, Oxford, UK (2008).
- Nieman LK, Biller BM, Findling JW et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 93(5), 1526–1540 (2008).
- Garg JP, Elliott WJ, Folker A et al. Resistant hypertension revisited: a comparison of two university-based cohorts. Am. J. Hypertens. 18(5 Pt 1), 619–626 (2005).
- Wilson IM, Freis ED. Relationship between plasma and extracellular fluid volume depletion and the antihypertensive effect of chlorothiazide. Circulation 20, 1028–1036 (1959).
- Hamlyn JM, Blaustein MP. Sodium chloride, extracellular fluid volume, and blood pressure regulation. Am. J. Physiol. 251(4), F563–F575 (1986).
- Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension 39(5), 982–988 (2002).
- Adrogué H, Madias N. Sodium surfeit and potassium deficit: keys to the pathogenesis of hypertension. J. Am. Soc. Hypertens. 8(3), 203–213 (2013).
- Coffman TM. The inextricable role of the kidney in hypertension. J. Clin. Invest. 124(6), 2341–2347 (2014).
- Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J. Hypertens. 32(3), 673–680 (2014).
- Grimm RH, Jr., Leon AS, Hunninghake DB et al. Effects of thiazide diuretics on plasma lipids and lipoproteins in mildly hypertensive patients: a double-blind controlled trial. Ann. Intern. Med. 94(1), 7–11 (1981).
- Murphy MB, Lewis PJ, Kohner E, Schumer B, Dollery CT. Glucose intolerance in hypertensive patients treated with diuretics; a fourteen-year follow-up. Lancet 2(8311), 1293–1295 (1982).
- Ames RP. A comparison of blood lipid and blood pressure responses during the treatment of systemic hypertension with indapamide and with thiazides. Am. J. Cardiol. 77(6), b12–b16 (1996).
- ALLHAT Officers - Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive Lipid- Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288(23), 2981–2997 (2002).
- Chapman N, Dobson J, Wilson S et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 49(4), 839–845 (2007).
- Marrs JC. Spironolactone management of resistant hypertension. Ann. Pharmacother. 44(11), 1762–1769 (2010).
- Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann. Intern. Med. 150(11), 776–783 (2009).
- Neves MF, Virdis A, Schiffrin EL. Resistance artery mechanics and composition in angiotensin II-infused rats: effects of aldosterone antagonism. J. Hypertens. 21(1), 189–198 (2003).
- Bahl VK, Jadhav UM, Thacker HP. Management of hypertension with the fixed combination of perindopril and amlodipine in daily clinical practice: results from the STRONG prospective, observational, multicenter study. Am. J. Cardiovasc. Drugs 9(3), 135–142 (2009).
- Mahfoud F, Luscher TF, Andersson B et al. Expert consensus document from the European Society of Cardiol. on catheterbased renal denervation. Eur. Heart J. 34(28), 2149–2157 (2013).
- Schlaich MP, Schmieder RE, Bakris G et al. International Expert Consensus Statement. Percutaneous transluminal renal denervation for the treatment of resistant hypertension. J. Am. Coll. Cardiol. 62(22), 2031–2045 (2013).
- Caulfield M, de Belder M, Cleveland T et al. The Joint UK Societies’ consensus statement on renal denervation for resistant hypertension. The British Hypertension Society, the British Cardiovascular Intervention Society, the British Society for Interventional Radiology, National Institute for Clinical Outcomes Research, the British Cardiovascular Society, and the Renal Association (2012).
- www.bcs.com/documents/The_Joint_UK_Societies_ Consensus_on_Renal_Denervation_for_resistant_ hypertension.pdf 49 Percutaneous transluminal radiofrequency sympathetic denervation of the renal artery for resistant hypertension National Institute for Health and Care Excellence (2012).
- www.nice.org.uk/guidance/ipg418 50 Bhatt DL, Kandzari DE, O’Neill WW et al. A controlled trial of renal denervation for resistant hypertension. N. Engl. J. Med. 370(15), 1393–1401 (2014).
- Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 57(5), 911–917 (2011).
- Esler MD, Krum H, Schlaich M et al. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 126(25), 2976–2982 (2012).
- Kandzari DE, Bhatt DL, Sobotka PA et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin. Cardiol. 35(9), 528–535 (2012).
- Neale T. Cardiologists Respond to SYMPLICITY HTN-3 MedPage Today, LLC and the Perelman School of Medicine at the University of Pennsylvania, PA, USA (2014).
- www.medpagetoday.com/Cardiology/Hypertension/43726 55 McCarney R, Warner J, Iliffe S et al. The Hawthorne effect: a randomised, controlled trial. BMC Med. Res. Methodol. 7, 30 (2007).
- Cushman WC, Ford CE, Cutler JA et al. Success and predictors of blood pressures. control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J. Clin. Hypertens. (Greenwich) 4(6), 393–404 (2002).
- Pathak A, Ewen S, Fajadet J et al. From SYMPLICITY HTN-3 to the Renal Denervation Global Registry: where do we stand and where should we go? EuroIntervention 10, 21–23 (2014).
- Blankestijn PJ, Alings M, Voskuil M, Grobbee DE. The complexity after simplicity: how to proceed with renal denervation in hypertension? Eur. J. Prev. Cardiol. pii:2047487314538859 (2014) (Epub ahead of print).
• Landmark review article and management guidance.
• Up-to-date practice-relevant guidance.
•• Landmark trial of renal denervation.
• Earlier trial of renal denervation.
• Earlier trial of renal denervation.
•• Methodology paper for the SYMPLICITY HTN-3 trial.