Review Article - Imaging in Medicine (2010) Volume 2, Issue 3
Radiation dose in interventional cardiology
Virginia Tsapaki*
Medical Physics Unit, Konstantopoulio Hospital, 1 Ifaistou Str., 14569 Anixi, Athens, Greece
- *Corresponding Author:
- Virginia Tsapaki
Medical Physics Unit, Konstantopoulio Hospital
1 Ifaistou Str., 14569 Anixi, Athens, Greece
Tel.: +30 210 813 1052
Fax: +30 210 813 1052
E-mail: virginia@otenet.gr
Abstract
Interventional procedures are becoming routine around the world, owing to their successful clinical outcome and improved patient safety. However, they can be difficult and demanding, since the physician must simultaneously consider many technical and clinical aspects. Although the patient’s condition carries a more substantial risk compared with radiation dose, the increasing number of reports of adult radiation injuries has highlighted various issues of patient radiation safety. Unfortunately, most operators are unaware of the high doses delivered and possible radiation injuries. This article summarizes current, available information regarding benefits and risks of radiation exposure during interventional cardiology procedures, clarifies dose descriptors and presents current levels of patient radiation doses for various interventional cardiology procedures.
Keywords
catheterization; coronary intervention; interventional cardiology ; radiation dose; radiation injury; radiation protection; radiation safety
The last 10 years have witnessed substantial improvements in medical imaging technology, leading to an increase in interventional cardiology (IC) procedures [1,2]. New digital detectors coupled with sophisticated, dedicated software and large advances in computing techniques that extract valuable clinical information within medical images have facilitated the expansion of applications of IC procedures [3]. Furthermore, the introduction of digital imaging has improved workflow within the department and final clinical outcome. The ability to easily view and store digital medical images using the picture archiving and communications system, and modify them at request at any time, at any site or at multiple sites at the same time, have boosted patient throughput in medical departments. Diagnostic and therapeutic IC techniques are becoming routine in numerous centers around the world, promising a successful clinical outcome and better patient safety, owing to the fact that they are catheter based and do not require open surgery, extracorporeal circulation or a lengthy hospital stay. For example, percutaneous coronary intervention (PCI) was considered a complex procedure in the past, and was only performed in highly specialized laboratories for cardiovascular research, but is now routine in many hospitals [4]. Previously, PCI was only performed in one occluded vessel; it is now carried out in multivessel disease, multiple substenosis in the same vessel or even complete occlusions in acute myocardial infarction. The technique has evolved so as to include more urgent comorbidity cases, achieving high success rates with significantly reduced need for repeat revascularization [5]. Furthermore, catheter- guided radiofrequency ablation of cardiac arrhythmias is generally accepted as an effective and safe procedure for the treatment of most supraventricular tachycardias, thus, exhibiting a rapid increase in frequency in the last decade [6]. Pediatric interventional procedures have also recently expanded owing to the successful treatment of many congenital and structural heart problems. With evolving stent technology, they are currently applied in multiple areas, including pulmonary arteries, vena cava, aortic and arch, and descending aorta, for coarctation. A recent concept, hybrid surgery, involves the close collaboration of the interventional cardiologist and the cardiac surgeon, combining catheter intervention and surgery in the surgical theater, in procedures such as pulmonary artery stent implantation associated with pulmonary valve replacement [7]. Furthermore, in selected cases, pulmonary valve device implantation is an accepted approach to a surgical problem [7]. According to a recent European report, which was based on the data submitted by 29 countries, the number of IC procedures in Europe is increasing at a typical rate of 6.7% (range: 3.8–11.8%) with over 3 million coronary angiographies (CAs), 910,000 PCIs and 690,000 pacemaker insertions in the year 2007 [2]. This increase was more prominent in developing countries, where the rate of increase over the last 4 years was approximately 6–196% (median: 76%), as shown in the initial results of an International Atomic Energy Agency (IAEA) project [8]. This was not only found in adults, but also in pediatric patients, where the workloads reached the levels of the adult techniques in some of the hospitals [8].
Unfortunately, the use of x‑ray equipment is inevitable in these procedures. Despite the advantages of IC procedures, the reports of patient radiation dose studies have raised concern regarding radiation-dose levels in IC [8–16]. This is extremely important in pediatric cardiology, since children have a higher radiosensitivity than adults and a longer lifetime expectancy [17]. Furthermore, the increasing number of reports on adult radiation injuries during these procedures has raised various issues of patient radiation safety [18–24]. Most operators are unaware of the high patient radiation doses and risk of radiation injuries, which are possible even when using the latest equipment [25]. Occasionally, these radiation burns can be chronically and severely painful, not to mention that both operators and hospitals may be subject to legal action [22]. Despite all the publications on radiation dose and harmful radiation effects, the major cardiological conferences lack presentations covering radiation protection issues [26]. Most operators use a ‘ learn-as-you-go’ approach instead of formal instruction concerning radiological equipment or radiation safety [27]. The consequences of no formal training in radiation protection are that operators are unaware of possible dose descriptors (some of which are currently displayed on modern x‑ray equipment), not familiar with the level of radiation dose and associated risk imparted to the patient or what are acceptable values of radiation dose. To date, review articles have mostly focused on radiation dose reduction techniques or are not recent enough to cover current radiation dose issues [28–31]. This article summarizes currently available information regarding benefits and risks of radiation exposure during IC procedures, clarifies dose descriptors, especially those currently shown on the control unit of the x‑ray systems, and presents the levels of patient radiation doses in various IC procedures.
Radiation-dose parameters
Generally speaking, the use of ionizing radiation could lead to an increased probability of cancer induction, this probability increases as the radiation dose increases (also called stochastic effect). Furthermore, the high radiation dose imparted during IC procedures may cause varying radiation injuries, including simple erythema, desquamation, cataracts, decreased white blood cell count, organ atrophy, fibrosis and sterility. The onset of any of these somatic effects depends on the absorbed dose, dose rate and extent of the body area exposed. Each factor has a dose threshold and the intensity of the effect increases with increasing dose (deterministic effects). For these reasons, interventional procedures have been recognised as a special case in the recent European Medical Exposure Directive [32]. The purpose of radiation protection in cardiology is to minimize the risk of cancer induction and to avoid, wherever possible, deterministic effects. Thus, radiological equipment must provide dosimetry information in a form that relates to both stochastic and deterministic effects. Unfortunately, no single quantity can provide unequivocal information on the induction of both types of effect. Cardiologists are presented with a suitable dose display, one that will provide an indication of whether skin effects may occur and another to warn about the risk of cancer induction [33]. Therefore, it is appropriate that kerma area product (KAP) is the dose display that correlates with the stochastic risk and peak skin dose (PSD) with deterministic skin injuries [33]. Both dose displays are usually shown on the TV monitor inside the interventional room and also in the control room.
Kerma area product
Kerma area product was previously called dosearea product and it is the integral of the air kerma free-in-air over the area, A, of the x‑ray beam in a plane perpendicular to the beam axis (integration of air kerma over the area, A):
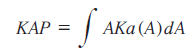
The unit Gy cm2 is the most commonly used. Unfortunately, other units are also used, such as mGy cm2, μGy m2 or cGy cm2, which causes unnecessary confusion, especially amongst inexperienced operators. The manufacturers should agree on a standard unit, such as Gy cm2, rather than using their preference, since the existence of so many units can create misunderstanding.
Kerma area product can be measured using a large area ionization chamber placed in or by the tube housing. Most modern x‑ray systems have KAP meters installed inside the x‑ray tube housing. In older machines, KAP ionization chambers are placed on the exit of the x‑ray beam and arrangements are made to hold it in place. A KAP meter is situated close to the exit window of an x‑ray tube of the machine for practical reasons, as well as to eliminate the contribution of backscattered radiation from the patient to the meter readings. KAP measurement does not interfere with the interventional procedure. KAP can also be calculated using technical data from the generator. KAP is a parameter that correlates reasonably well with the probability of stochastic effects. KAP is currently shown on the control unit of modern x‑ray machines, not only as a dose value at the end of the procedure, but also as a real-time dose quantity. In this way, the operator knows at any time the radiation dose imparted to the patient and how this is changing according to the x‑ray projection or phase of procedure.
Peak skin dose
Kerma area product is a poor indicator of the onset of deterministic effects. A much better indicator is skin entrance dose and, specifically, PSD. PSD is the highest dose on any part of the patient’s skin during any interventional procedure and is usually measured in Gy or mGy. Modern x‑ray machines provide a quantity that correlates with patient skin dose and PSD, the reference point air kerma (RPAK). RPAK is a measure of the radiation level in a specific point relative to the fluoroscopic gantry of the system, named the interventional reference point. This point, which is along the central axis of the x‑ray beam and 15 cm from the isocenter, is close to the patient’s skin surface and is provided by the manufacturer. RPAK was recently introduced by the International Electrotechnical Commission [34] for standardization purposes and is similar to the ‘reference dose’ and ‘cumulative dose’ used in the past. These three terms can be found in the control units of x‑ray machines together with KAP. It does not take into account the scattered radiation and, therefore, is not the actual dose to the patient’s skin. For this reason, the latest guidelines of the Society of Interventional Radiology (SIR) Safety and Health Committee presented a conversion formula to help operators estimate the PSD from the RPAK shown on the x‑ray machine at the end of interventional procedure [35]:
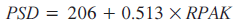
However, it should be noted that this is a broad approximation and not an actual measurement; it cannot be used when RPAK values are below 500 mGy [35].
According to the SIR guidelines, the operator should be notified at specific radiation trigger levels as follows [35]:
• For units with RPAK capability, initial notification should be given at 3000 mGy and then every 1000 mGy thereafter (this corresponds to an initial PSD of ~1800 mGy with increments of ~500 mGy);
• For units with KAP capability, the notification level is based on a procedure-dependent nominal x‑ray field size at the patient’s skin. So, with use of a 100 cm2 field, the initial trigger would be at 300 Gy cm2 and, subsequently, at increments of 100 Gy cm2.
For x‑ray machines that do not have a RPAK meter and when there are no other means of measuring PSD, the SIR guidelines report that the following formula can be used for a very crude estimation of PSD from KAP value if this is above 50 Gy cm2 [35]:
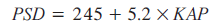
At present, the dose is only displayed on the screen. However, the dose displays could be supplemented by appropriate alarms, which would trigger at various premeditated levels prior to the onset of deterministic effects. This, in turn, will assist in ensuring the appropriate follow-up of interventional patients. Various international standards will need to be developed to implement these initiatives.
If any of these threshold values are reached or passed during an interventional procedure, any subsequent examination or procedure involving ionizing radiation performed within the next 60 days should be closely monitored. Furthermore, given the need to follow-up patients who have received high doses, it is important that the patient’s notes contain the previously mentioned relevant dosimetry data. The dose thresholds for patient follow-up are given in Table 1 according to the SIR guidelines [35] and according to the IAEA recommendations [101].
Peak skin dose can be directly measured using small detectors, such as thermoluminescent, scintillation or diode detectors, as well as large area radiotherapy verification films (slow or radiochromic films), which measure not only the skin dose at various anatomical positions, but also determine the location and actual value of PSD [36]. Film dosimetry provides a real-time skin dose map, which is a valuable tool for assisting the operator in minimizing skin dose, albeit not in real time. The skin-dose map could be added to the medical record at the end of the procedure, thereby indicating not only the magnitude, but also the location of the skin dose, for future patient management.
Diagnostic reference levels
Most patient-dose survey results indicate that there is a wide range of values for nominally the same interventional procedure [8–17]. For this reason, the concept of diagnostic reference levels (DRLs) or guidance levels was included in the latest Medical Exposure Directive as a basic requirement for interventional techniques [32]. The concept of DRLs was first introduced by the International Commission on Radiological Protection in Publication 60 [37] and further expanded in Publication 73 [38]. The results of patient dose surveys could then be compared with DRL, to discover which IC departments have doses above the reference values. An audit process could then be initiated to determine the underlying cause of higher doses and an action plan developed to improve radiological techniques for dose reduction purposes. In practice, the DRL may be regarded as an optimization tool for the reduction of patient doses in IC.
European DRL were recently set by the Safety and Efficacy for New Techniques and Imaging using New Equipment to Support European Legislation) (SENTINEL) research subgroup, which was part of the European SENTINEL coordination action consortium comprising of 22 members from 19 member states, complemented by partners from candidate member states and international organizations [39]. The DRLs were estimated using approximately 2000 procedures collected in cardiac centers by nine European partners [40]. DRLs were given for CA, percutaneous transluminal coronary angioplasty (PTCA) and electrophysiology procedures in terms of KAP (45, 85 and 35 Gy cm2, respectively) and cumulative dose (650 mGy for CA and 1500 mGy for PTCA).
The IAEA launched a coordinated research program to investigate the feasibility of adopting guidance levels in IC procedures together with the concept of action levels [41]. Since a too low dose may also indicate an incomplete procedure or inadequate image quality, centers with mean dose values below action levels should investigate the quality of their procedures. During the project, data on over 4000 techniques were collected from hospitals in Chile, Italy, Spain, Uruguay and the USA. Guidance levels of 50 Gy cm2 for CA and 125 Gy cm2 for PCI (medium complexity) were suggested, providing DRLs for simple, medium and complex PCI techniques, as well as action levels of 15 Gy cm2 for CA and 25 Gy cm2 for PCI [42].
It should be noted that DRLs are not yet set for pediatric patients. There is only one study that reports that instead of KAP, KAP/body weight is appropriate to describe pediatric DRLs and is recommended instead of using mean KAP values for age groups [43].
Radiation risk
Radiation involves a probability of carcinogenic effects, the actual probability increasing with the magnitude of the dose. Such effects may take years to develop and we are currently unable to distinguish between cancers that are radiationinduced and those that are not [26,44]. Effective dose (E) is the only dose quantity that enables medical examinations or techniques to be compared in terms of radiation dose. E is estimated by adding the products of the dose in an organ or tissue and the specific weighting factor for that tissue. The weighting factors are values that express the sensitivity of each particular tissue or organ to radiation. Each weighting factor relates to the risk associated with stochastic effects and has specific value for every organ. E is expressed in millisieverts (mSv) and can be compared with the E from other sources of ionizing radiation (e.g., the radiation received by radon, cosmic radiation or other medical examinations, such as CT or nuclear medicine). It is also used to estimate the detriment from cancer and hereditary effects.
In order to understand the difference in radiation dose between various radiological procedures and where interventional procedures stand between them, various E values are presented in Table 2. As shown in Table 2, chest x‑ray imparts the lowest radiation dose (0.02 mSv) to the patient, and is approximately 15‑times lower than natural background radiation (3 mSv per year) [45]. The SENTINEL group has proposed reference E values for CA (8 mSv), PTCA (15 mSv) and electrophysiology procedures (6 mSv), also shown in Table 2 [40]. Multislice CT (MSCT) CA is currently considered to be a promising, noninvasive alternative to conventional angiography, radiation dose is a major concern, especially for repeated examinations [46]. The organs receiving the highest equivalent doses in MSCT CA are the female breasts, lungs, liver and esophagus [47].
In practice, it is very difficult to determine E, since the radiation doses in 12 organs would have to be measured during a cardiological procedure. Therefore, the use of a special conversion factor provides a practical way to estimate E. The most recent studies determine E from KAP using a conversion factor of 0.185 mSv/Gy cm2 [48,49]. Similar factors are available for pediatric cardiology procedures in the order of 3.7 (neonate), 1.9 (1 year), 1.0 (5 years), 0.6 (10 years) and, finally, 0.4 mSv/Gy cm2 (15 years) [50].
In 1991, the International Commission on Radiological Protection report estimated radiation risk to cause 50 additional fatal cancers per 1 million individuals that are exposed to 1 mSv [37]. E is intended for use as a radiation protection quantity and not for epidemiological evaluations [37]. Accurate analysis of radiation risk requires detailed knowledge of organ doses, age and sex of patients. Risk estimations are subject to several sources of uncertainty owing to limitations in epidemiological data and in understanding how radiation exposure increases the risk of cancer. Furthermore, the population for which the risk estimation is required is different to the one for which epidemiological data exist. Assumptions that introduce uncertainties to the estimation are required. The National Research Council committee in its Biological Effects of Ionizing Radiation (BEIR) VII report has developed various models for risk estimation for the US population [51]. According to these updated risk estimates, the attributable risk of cancer is one in 750 for 15 mSv exposure, corresponding to the dose estimate of a PTCA [51].
In order to have an approximate estimate of radiation burden received by an asymptomatic subject ‘at risk’ during screening, an E of over 100 mSv (which corresponds to 5000 chest x‑rays) can be reached following a combination of a MSCT scan, a thallium nuclear medicine scan, a CA examination, a coronary stenting procedure, a repeat MSCT for follow-up and a thallium scan. This cumulative dose poses an extra risk of cancer of one in 100 exposed patients [52]. Therefore, as the American College of Radiology white paper on radiation dose in medicine states, physicians have the duty to know the risks of what they do. Consequently, the present generation of cardiologists should be aware of the risks related to medical radiation exposure and patients have the right to know what the risks are [53,54].
As far as pediatric patients are concerned, they are generally amore susceptible to the risk of radiation-induced carcinogenesis compared with adults. Children with complex cardiac anomalies are at even greater risk as a result of frequent catheterization and long interventional procedures. In children, some radiosensitive tissues, such as the eyes, thyroid and gonads, are closer to the heart than in adults as the organs are closer to the scattered and primary beam [55]. As seen in Table 2, pediatric radiation doses in interventional procedures reach the values of adults and, therefore, children are at a greater risk for stochastic effects [56].
Radiation-induced injuries
Deterministic radiation injuries following angioplasty have been reported since the early 1990s [44]. The threshold for the first radiationinduced skin injury (erythema) is 2 Gy, whereas permanent hair loss, desquamation and necrosis occur at doses of 3–10, 7–10 and 12–25 Gy, respectively. The exposure necessary to cause chronic radiation dermatitis is 10–12 Gy. Recent literature reports doses of up to 59 Gy in four interventional procedures [25]. Experience has demonstrated that, generally, patients do not seek consultation for their skin lesions from the cardiologist who performed the fluoroscopyguided procedure, and these physicians do not routinely screen their patients prospectively for long-term dermatologic adverse effects [57,58]. Skin injuries resulting from prolonged exposure to ionizing radiation during interventional procedures has been documented in the radiology and cardiology literature, but it has rarely been reported in the dermatologic literature [59]. As reported by a recently published study, only 42 cases of fluoroscopic-induced radiation skin injuries have been reported in the dermatology literature. Of these 42 cases, 31 were defined as chronic radiation dermatitis, three were subacute and nine were acute dermatitis mainly following PCI and/or CA (30 cases) [59]. Fluoroscopyinduced chronic radiation dermatitis has also been associated with breast cancer. The delay between the interventional procedure and occurrence of symptoms, coupled with the lack of instruction to the patient by the interventionalist to report any skin irritation on ports of entry of the x‑ray beam (typically the patient’s back) are responsible for misdiagnosis. There have been instances of misdiagnosis of fluoroscopy-induced chronic radiation dermatitis as insect bites, electrical burns, chemical burns or contact dermatitis. In severe skin injuries, in addition to pain, it is possible that surgical grafting is required. This results in permanent disfigurement and compromised mobility. In some cases, the family’s lifestyle is radically altered owing to the need for daily changes of wound dressings, limited ability to perform simple tasks, inability to work, loss of income and debt caused by high medical costs [58]. In some cases, patients must learn to sleep in awkward positions because their wounds prevent them from sleeping in a normal way, whereas in others, the pain is permanent, requiring a lifetime of medication and treatment [58].
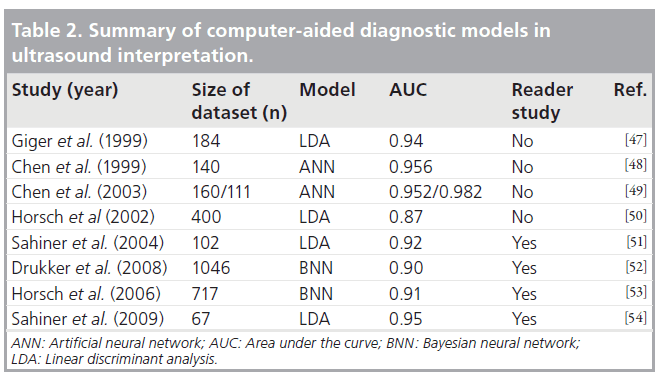
The most common sites of skin injury associated with IC procedures include the right scapular or subscapular area, left scapular or subscapular area, right lateral trunk below the axilla, midback and the right anterolateral chest [60]. In a few cases where doses are very high, erythema can be observed a few hours after irradiation. This timing makes the recognition of the possible link between the irradiation and skin symptoms easier, but this situation is rare. In most cases, symptoms take approximately 2–3 weeks to emerge, and it is 3–4 weeks before the symptoms are sufficiently irritating for the patient to see a doctor. For IC procedures, the patient should be advised about the areas on the skin of the back where erythema might develop. The patient should be asked to examine him- or herself until approximately 2–3 weeks after the procedure for any skin changes in those areas. Some facilities make a follow-up call to the patient during this period to check for any skin irritation, and this has been reported to be effective in ensuring that a patient who develops skin irritation does not seek medical help at a place where there may be a chance of misdiagnosis.
Although the reported threshold for skin erythema is 2 Gy, there are a number of factors that may cause the individual patient to be more or less sensitive to radiation exposure. Biologic factors, such as diabetes mellitus, systemic lupus erythematosus, scleroderma or mixed connective tissue disease, and homozygosity for ataxia telangiectasia, increase the sensitivity and, hence, potential for severe skin reactions [61]. The reason why some patients with collagen vascular disease are more sensitive to radiation is unknown. Furthermore, having the disease does not automatically predispose patients to heightened sensitivity. Only a few patients with collagen vascular disease have been identified to have greater radiation sensitivity. Whether or not the skin type of an individual is correlated with sensitivity for radiation-induced erythema is still a matter of discussion. First-line therapies for chronic dermatitis include topical and intralesional corticosteroid administration, whereas ulcerated or sclerotic lesions limiting mobility may require surgical excision with musculoskeletal skin flap repair [61].
No studies report radiation skin injury in pediatric patients. This can be expected, since maximum PSD reported was 481 mGy for children younger than 10 years, which is much lower than the dose threshold of 2 Gy [62]. However, since these patients often undergo a substantial number of interventional procedures, PSD should be monitored.
Advice on dose optimization
Numerous studies describing radiation dose optimization strategies in interventional procedures have been published, and the reader is referred to these for more detail [28–31,35,46,52,53,55]. The skills of the interventionalist, knowledge of the angiographic machine, the medical history of the patient, the complexity of procedure, the type of procedure and the size of the patient are some of the factors that affect radiation dose [46]. However, some simple and cost-free dosereduction techniques, can be implemented without any loss of diagnostic information during intervention, these include the following:
• Planning the angiographic projections in advance
• Keeping the x‑ray detector (image intensifier or flat panel) as close to the patient as possible and the x‑ray tube as far away as possible
• Not using fluoroscopy to make changes to the patient/table position or collimators/shields
• Removing unnecessary body parts or instruments from the field
• Decreasing beam-on time (fluoroscopy, cine recording and number of runs)
• Using the lowest acceptable fluoroscopy pulse and cine frame rate
• Using the lowest acceptable magnification mode
• Using collimators and an additional copper filter
• Using less angulated projections whenever possible (left anterior oblique projections are the most radiation intensive)
• Using the last image hold (it is the last fluoroscopic frame and it remains displayed on the monitor once the x‑rays are turned off)
• In very new x‑ray systems, rather than one frame, the fluoroscopy of approximately the last 20 s are stored in the memory and are available for viewing
Creating awareness
Interventional cardiology procedures are often difficult and stressful, since the physician must keep in mind many technical and clinical aspects simultaneously. Additionally, the patient’s cardiological condition carries a substantial risk compared with the radiation dose and operators are reluctant to discontinue a procedure [63]. Despite the fact that cardiologists use radiology rather intensively and they should know radiobiology and radioprotection essentials, this issue is usually absent from the curriculum, meetings, textbooks and scientific journals. In most cases, the radiation information is presented in an esoteric, clinically irrelevant way and, as the American College of Radiology white paper suggests, a profound remodelling of radiation protection teaching is needed [53]. Therefore, it is essential that the medical community create data-driven methodologies to quantify risk in objective terms, develop radiation standards and best practice guidelines (evidence-based medicine), develop new technologies and applications to proactively minimize radiation dose while maintaining quality, and create accountability measures for all pertinent stakeholders [64]. The IAEA has taken the lead by organizing training courses in radiation protection, specifically targeted to interventional cardiologists [65,66]. Many cardiologists who participated in IAEA training courses have, subsequently, taken the lead in organizing training courses in their respective countries. The IAEA has also launched a number of projects focused on IC. During one of these projects, the idea to create a network of Asian Cardiologists in Radiation Protection was proposed. The mission of this network was to enhance cooperation among cardiologists on radiation safety in cardiac catheterization procedures. This was followed by the production of a newsletter for strengthening communication on radiation protection. Five issues of this newsletter have been issued so far and are available online [102]. This is the first ever newsletter on radiation protection devoted to IC and being maintained by cardiologists themselves.
Future perspective
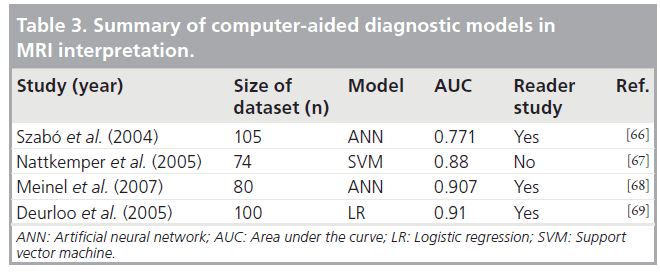
Review of the literature has revealed certain shortcomings and possible items for future research regarding radiation dose optimization and avoidance of radiation-induced skin injuries in IC.
Real-time display of PSD is not currently required but has been demonstrated to be a very helpful tool for patient management. As shown by the results of the IAEA coordinated research program, such methods should be developed to facilitate the optimization of interventional procedures, especially in patients requiring multiple catheterizations [41].
Current dose displays could be supplemented by appropriate alarms, which would trigger at various predetermined levels prior to the onset of deterministic effects.
Despite the fact that children are more radiosensitive, publications on pediatric doses are scarce and DRLs have not been proposed. With the rapidly increasing frequency of these types of procedures, reported not only in western societies, but also in developing countries, it is a matter of urgency to determine specific DRL for pediatric patients. This will help to create a baseline on radiation dose and facilitate a dose optimization program in these procedures.
Conclusion
For a long time, radiation exposure has generally been underestimated by most interventional cardiologists, but it is currently a big concern. Owing to the fact that, in many occasions, the complex clinical problems are more important than radiation protection of patients, it is difficult to develop a strategy for achieving improved radiation protection in IC. It is likely that a large number of operators do not know how to use the x‑ray machine with optimal radiation protection. Furthermore, the medical community has found fluoroscopically induced injuries difficult to diagnose, owing to the lack of experience with radiation- induced skin injuries. Profound remodeling of radioprotection teaching, data-driven methodologies to quantify risk, community-wide radiation standards and best practice guidelines constantly updated to incorporate new technologies and applications can facilitate implementation of radiation protection, minimize cancer induction and avoid radiation-induced injuries while simultaneously using x‑ray equipment for successful clinical outcome.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
* of interest
References
- Rotter M, Pfiffner D, Maier W, Zeiher AM, Meier B; Working Group Interventional Cardiology and Coronary Pathophysiology, European Society of Cardiology: Interventional cardiology in Europe 1999. Eur. Heart J. 24(12), 1164–1170 (2003).
- Faulkner K, Werduch A: Analysis of the frequency of interventional cardiology in various European countries. Radiat. Prot. Dosim. 129(1–3), 74–76 (2008).
- Tsapaki V, Kottou S, Kollaros N, Dafnomili P, Koutelou M, Neofotistou V: Comparison of a conventional and a flat panel digital system in interventional cardiology. Br. J. Radiol. 77(919), 562–567 (2004).
- Dawkins KD, Gershlick T, de Belder M et al.: Percutaneous coronary intervention: recommendations for good practice and training. Heart 91(Suppl. 6), 1–7 (2005).
- Venkitachalam L, Kip KE, Selzer F et al.: Twenty-year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the National Heart, Lung, and Blood Institute-sponsored, multicenter 1985–1986 PTCA and 1997–2006 dynamic registries. Circ. Cardiovasc. Interv. 2(1), 6–13 (2009).
- Scheinman MM: Patterns of catheter ablation practice in the United States: results of the 1992 NASPE Survey. Pacing Clin. Electrophysiol. 17, 873–875 (1994).
- Leblanc JG: New surgery for better outcomes: shaping the field of congenital heart disease. World J. Pediatr. 5(3), 165–168 (2009).
- Tsapaki V, Ahmed N, Salem J et al.: Radiation exposure to patients during interventional procedures in 20 countries: initial IAEA project results. AJR Am. J. Roetgenol. 193, 559–569 (2009).
- Mc Fadden S, Mooney RB, Shepherd PH: X-ray dose and associated risks from radiofrequency catheter ablation procedures. Br. J. Radiol. 75, 253–265 (2002).
- Smith IR, Rivers JT: Measures of radiation exposure in cardiac imaging and the impact of case complexity. Heart Lung Circ. 17(3), 224–231 (2008).
- Morrish OW, Goldstone KE: An investigation into patient and staff doses from x-ray angiography during coronary interventional procedures. Br. J. Radiol. 81(961), 35–45 (2008).
- Balter S, Miller D, Vano E et al.: A pilot study exploring the possibility of establishing guidance levels in x-ray directed internvetional procedures. Med. Phys. 35(2), 673–680 (2008).
- Neofotistou V, Vano E, Padovani R et al.: Preliminary reference levels in interventional cardiology. Eur. Radiol. 13, 2259–2263 (2003).
- Padovani R, Bernardi G, Malisan MR, Vano E, Morocutti G, Fioretti PM: Patient dose related to the complexity of interventional cardiology procedures. Rad. Prot. Dosim. 94(1–2), 189–192 (2001).
- Bar O, Maccia C, Pagès P, Blanchard D: A multicentre survey of patient exposure to ionising radiation during interventional cardiology procedures in France. Eur. Interv. 3, 1–7 (2008).
- Martinez LC, Vano E, Gutierrez F, Rodriguez C, Gilarranz R, Manzanas MJ: Patient doses from fluoroscopically guided cardiac procedures in pediatrics. Phys. Med. Biol. 52, 4749–4759 (2007).
- Roebuck DJ: Risk and benefit in paediatric radiology. Pediatr. Radiol. 29, 637–640 (1999).
- Dehen L, Vilmer C, Humilière C et al.: Chroninc radiodermatitis following cardiac catheterization: a report of two cases and a brief review of the literature. Heart 81, 308–312 (1999).
- Finkelstein NM: Is brain cancer an occupational disease of cardiologists? Can. J. Cardiol. 14(11), 1385–1388 (1998).
- Wiper A, Katira R, Roberts DH: Images in cardiology. Interventional cardiology: it’s a hairy business. Heart 91, 1432 (2005).
- Whitby M, Martin CJ: Radiation dose to the legs of radiologists performing interventional procedures: are they a cause of concern? Br. J. Radiol. 76, 321–327 (2003).
- Vlietstra RE, Wagner LK, Koenig T, Mettler F: Radiation burns as a severe complication of fluoroscopically guided cardiological interventions. J. Inter. Cardiol. 17(3), 131–142 (2004).
- Vano E, Goicolea J, Galvan C et al.: Skin radiation injuries in patients following repeated coronary angioplasty procedures. Br. J. Radiol. 4, 1023–1031 (2001).
- Padovani R, Bernardi G, Quai E et al.: Retrospective evaluation of occurrence of skin injuries in interventional cardiac procedures. Radiat. Prot. Dosim. 117(1–3), 247–250 (2006).
- Ukisu R, Kushihashi T, Inketsou S: Skin injuries caused by fluoroscopically guided interventional procedures: case-based review and self assessment module. AJR Am. J. Roetgen. 193, 59–69 (2009). & A series of multiple-choice questions on radiation-induced injuries caused by fluoroscopically guided interventional procedures with solutions and a discussion following each case.
- Rehani M, Ortiz-Lopez P: Radiation effects in fluoroscopically guided cardiac interventions – keeping them under control. Inter. J. Cardiol. 109(2), 147–151 (2006). & Addresses issues such as radiation safety, proper equipment performance, use of proper techniques, monitoring of patient doses and severe skin injuries.
- Baim D, Grossman W: Cardiac Catheterization, Angiography and Intervention (6th Edition). Lippincott Williams and Wilkins, Wolter Kluwer Company, PA, USA (2000).
- Balter S, Moses J: Managing patient dose in Interventional cardiology. Catheter Cardiovasc. Interv. 70, 244–249 (2007).
- Kuon E: Radiation exposure in invasive cardiology. Heart 94, 667–674 (2008).
- Cousins C, Sharp C: Medical interventional procedures-reducing the radiation risks. Clin. Radiol. 59, 468–473 (2004).
- Limacher MC, Douglas PS, Germano G et al.: American College of Cardiology expert consensus document. Radiation safety in the practice of cardiology. J. Am. Coll. Cardiol. 31, 892–913 (1998).
- Council Directive 97/43/Euratom of 30 June on health protection of individuals against the dangers of ionising radiation in relation to medical exposure. Official Journal of the European Commission NoL 180, (1997).
- Faulkner K: Dose displays and record keeping. Radiat. Prot. Dosim. 94(1–2), 143–145 (2001).
- International Electrotechnical Commission: IEC 2000: Particular Requirements for the Safety of x-ray Equipment for Interventional Procedures (1st Edition). IEC 60601–60602–60643. Geneva, Switzerland (2000).
- Stecker MS, Balter S, Towbin RB et al.: Guidelines for patient radiation dose management. J. Vasc. Interv. Radiol. 20, 263–273 (2009). & Guidelines for patient radiation-dose management developed by the Society of Interventional Radiology Safety and Health Committee.
- Kosunen A, Komppa T, Toivonen M: Evaluation of methods to estimate the patient dose in interventional radiology. Radiat. Prot. Dosim. 117(1–3), 178–184 (2006).
- Publication 60: 1990 recommendations of the International Commission on Radiological Protection, 60. In: Annals of the ICRP. International Commission on Radiological Protection (Eds). Pergammon Press, Oxford, UK 21(1–3) (1991).
- Publication 73: radiological protection and safety in medicine. In: Annals of the ICRP. International Commission on Radiological Protection (Eds). Pergammon Press, Oxford, UK 26(2) (1996).
- Faulkner K, Malone J, Vano E et al.: The SENTINEL (Safety and Efficacy for New Techniques and Imaging using New Equipment to Support European Legislation) project. Radiat. Prot. Dosim. 129(1–3), 3–5 (2008).
- Padovani R, Vano E, Trianni A et al.: Reference levels at European level for cardiac interventional procedures. Radiat. Prot. Dosim. 129(1–3), 104–107 (2008).
- International Atomic Energy Agency: Establishing guidance for x-ray guided medical interventional procedures: a pilot study. In: Safety Reports Series 59 (2009).
- Balter S, Miller D, Vano E et al.: A pilot study exploring the possibility of establishing guidance levels in x-ray directed interventional procedures. Med. Phys. 35(2), 673–680 (2008).
- Onnasch D, Schröder FK, Fischer G, Kramer HH: Diagnostic reference levels and effective dose in paediatric cardiac catheterization. Br. J. Radiol. 80, 177–185 (2007).
- Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ: Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology 254(2), 326–341 (2010).
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M: Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248(1), 254–263 (2008).
- Pantos I, Patatoukas G, Katritsis DG, Efstathopoulos E: Patient radiation doses in interventional cardiology procedures. Curr. Cardiol. Rev. 5, 1–11 (2009).
- Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ: Radiation dose to patients from cardiac diagnostic imaging. Circulation 116, 1290–1305 (2007).
- Bogaert E, Bacher K, Thierens H: A largescale multicentre study in Belgium of dose area product values and effective doses in interventional cardiology using contemporary x-ray equipment. Radiat. Prot. Dosim. 128(3), 312–323 (2008).
- Bogaert E, Bacher K, Thierens H: Interventional cardiovascular procedures in Belgium: effective dose and conversion factors. Radiat. Prot. Dosim. 129(1–3), 77–82 (2008).
- Karambatsakidou A, Sahlgren B, Hansson B, Lidegran M, Fransson A: Effective dose conversion factors in paediatric interventional cardiology. Br. J. Radiol. 82(981), 748–755 (2009).
- National Research Council, Committee on the Biological Effects of Ionizing Radiation: health risks from exposure to low levels of ionizing radiation: BEIR VII. National Academy Press, DC, USA (2006).
- Picano E, Santoro G, Vano E: Sustainability in the cardiac cath lab. Int. J. Cardiovasc. Imaging 23(2), 143–147 (2007).
- Picano E, Vano E, Semelka R, Regulla D: The American College of Radiology white paper on radiation dose in medicine: deep impact on the practice of cardiovascular imaging. Cardiovasc. Ultrasound 5, 37 (2007). & Practical suggestions to minimize radiation risk, including education for all stakeholders in the principles of radiation safety and preferential use of alternative (nonionizing) imaging techniques.
- Amis ES Jr, Butler PF, Applegate KE et al.: American College of Radiology white paper on radiation dose in medicine. J. Am. Coll. Radiol. 4(5), 272–284 (2007).
- Justino H: The ALARA concept in pediatric cardiac catheterization: techniques for managing radiation dose. Pediatr. Radiol. 36(2), 146–153 (2006).
- Bacher K, Bogaert E, Lapere R, De Wolf D, Thierens H: Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation 111(1), 83–99 (2005).
- Koenig TR, Wolff D, Mettler FA, Wagner LK: Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am. J. Roentgenol. 177, 3–11 (2001).
- Wagner LK: Radiation injury is a potentially serious complication to fluoroscopicallyguided complex interventions. Biomed. Imaging Interv. J. e22 (2007). & Reviews the characteristics of radiationinduced skin injuries in interventional cardiology and some actions that can be taken to reduce their likelihood or seriousness.
- Frazier TH, Richardson JB, Fabré VC, Callen JP: Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch. Dermatol. 143(5), 637–640 (2007). & Fluoroscopy-induced chronic radiation dermatitis resulting from prolonged exposure to ionizing radiation during interventional procedures should be considered for any patient who is seen with an acquired vascular lesion, a morphea-like lesion or an unexplained ulcer localized over the scapula, the back or lateral trunk below the axilla.
- Koenig TR, Mettler FA, Wagner LK: Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am. J. Roentgenol. 177, 13–20 (2001).
- Miller DL, Balter S, Noonan PT, Georgia JD: Minimizing radiation-induced skin injury in interventional radiology procedures. Radiology 225(2), 329–336 (2002).
- Li LB, Kai M, Kusama T: Radiation exposure to patients during paediatric cardiac catheterization and angiocardiography. Radiat. Prot. Dosim. 94, 323–327 (2001).
- Wilde P, Pitcher EM, Slack K: Radiation hazards for the patient in cardiological procedures. Heart 85, 127–130 (2001).
- Reiner BI: Quantifying radiation safety and quality in medical imaging, part 1: creating the infrastructure. J. Am. Coll. Radiol. 6(8), 558–561 (2009).
- Rehani M: Training of interventional cardiologists in radiation protection: the IAEA’s initiatives. Inter. J. Cardiol. 114, 256–260 (2007).
- Rehani MM: The IAEA’s activities on radiation protection in interventional cardiology. Biomed. Imaging Interv. J. 3(2), e31 (2007).
Websites
- International Atomic Energy Agency: Safety in Radiological Procedures http://rpop.iaea.org/safrad Asian Network of Cardiologists in Radiation Protection – under RCA/IAEA project.
- Newsletter August 2007, Issue N 1, 1–2 (2007) http://rpop.iaea.org/RPOP/RPoP/Content/ AdditionalResources/Training/2_ TrainingEvents/asian-network.htm


