Research Article - International Journal of Clinical Rheumatology (2016) Volume 11, Issue 5
Polyhydroxylated C60 fullerenes prevent chondrocyte catabolic activity at nanomolar concentrations in osteoarthritis
- Corresponding Author:
- Kazuo Yudoh
Department of Frontier Medicine, Institute of Medical Science
St. Marianna University School of Medicine, Sugao 2-16-1
Miyamae-ku, Kawasaki 216-8512, Japan
E-mail: yudo@marianna-u.ac.jp
Abstract
Aim: Recently, numerous reports have demonstrated that oxidative stress and related chondrocyte aging may participate in the development of osteoarthritis (OA). To further understand the pathogenesis and degenerative process of OA, we have studied water-soluble polyhydroxylated C60 fullerene, a strong free radical scavenger, as an anti-oxidant. Method: We examine the therapeutic effects of five types of water-soluble polyhydroxylated C60 fullerenes [C60(OH)10, C60(OH)24, C60(OH)26, C60(OH)36, C60(OH)44] on OA-related factor-induced catabolic responses in osteoarthritic chondrocytes. In the presence or absence of polyhydroxylated C60 fullerenes [C60(OH)10, C60(OH)24, C60(OH)26, C60(OH)36, C60(OH)44] (0.1, 1.0, 10.0 or 100 nM), human osteoarthritic chondrocytes were treated with IL-1β (10.0 ng/mL). After 24 hours incubation, chondrocyte activities were examined. Results: Water-soluble polyhydroxylated C60 fullerenes inhibited OA-related catabolic responses (IL-1β- upregulation of cartilage degrading enzyme production and downregulation of proteoglycan production) in OA chondrocytes. C60(OH)10 C60(OH)24 and C60(OH)26 showed a stronger chondroprotective effect than C60(OH)36 or C60(OH)44. Conclusion: Our findings indicate that polyhydroxylated C60 fullerenes, especially C60(OH)10 C60(OH)24 and C60(OH)26, may have a part to protect against OA related factor-mediated downregulation of osteoarthritic chondrocyte activities. These data may reveal a novel pathologic mechanism linking oxidative stress-induced development of OA.
Keywords
osteoarthritis, C60 fullerene, oxidative stress, chondrocytes
Abbreviation
OA: Osteoarthritis; ROS: Reactive Oxygen Species; PBS: Phosphate-Buffered Saline; DMEM: Dulbecco’s Modified Eagle’s Medium; FCS: Fetal Calf Serum; HEPES: 2-[4-(2-hydroxyethyl)-1- piperazinyl] ethanesulfonic acid; IL: Interleukin; MMP-13: Matrix Metalloproteinase-13; ELISA: Enzyme- Linked Immunosorbent Assay
Introduction
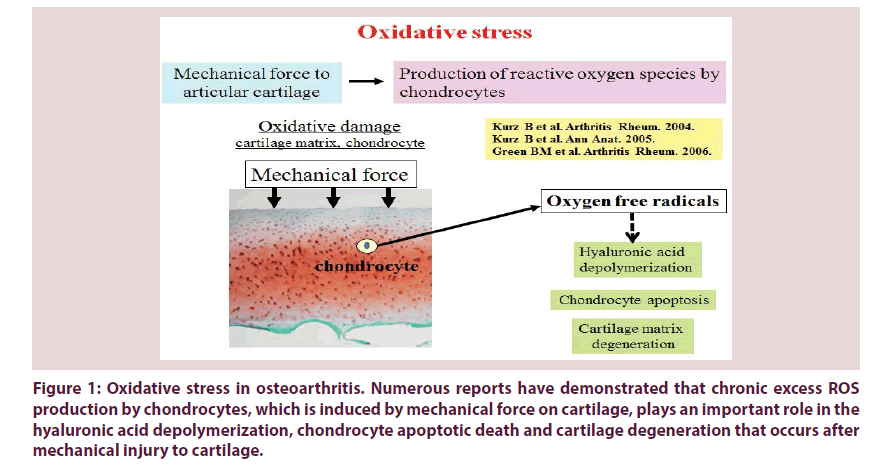
Mechanical stress on articular cartilage down regulates chondrocyte activity and cartilage matrix function [1]. Also, it has been reported that mechanical force to articular cartilage stimulates the secretion of matrix degrading enzymes and proinflammatory cytokines from chondrocytes [1,2]. It has been demonstrated that catabolically-stressed chondrocytes secrete large amounts of reactive oxygen species (ROS) as well as proinflammatory cytokines (Figure 1) [3-5].
Figure 1: Oxidative stress in osteoarthritis. Numerous reports have demonstrated that chronic excess ROS production by chondrocytes, which is induced by mechanical force on cartilage, plays an important role in the hyaluronic acid depolymerization, chondrocyte apoptotic death and cartilage degeneration that occurs after mechanical injury to cartilage.
Imbalance between the production of ROS and the depletion of cellular antioxidants in degenerated articular cartilage tissue has been confirmed by multiple studies. Several reports have already indicated that the cartilage degeneration in osteoarthritis (OA) is mediated by ROS (Figure 1) [1,6,7]. Kurz et al. reported that mechanical force on articular cartilage stimulates excess production of ROS by chondrocytes, facilitating to the degeneration of hyaluronic acid and chondrocyte apoptosis [8]. Green et al. also mentioned that chondrocyte apoptosis is at least partly involved in inducing excess amount of ROS production by chondrocytes in response to mechanical force to cartilage [9]. These studies suggest that mechanical stress-induced ROS produced by chondrocytes may lead to the direct damage to chondrocytes and cartilage matrix in OA.
Recently, attention has been drawn to the theory that aging reduces the maintenance and homeostatic potential of chondrocytes and articular cartilage matrix [3,10]. Martin et al. examined different surrogate biomarkers of aging in human chondrocytes and demonstrated that chondrocyte aging correlates with the age of the donor [10,11]. They concluded that aged chondrocytes cause an inadequate response to anabolic factors, leading to degradation of the cartilage matrix caused by the imbalance of anabolic and catabolic chondrocyte activities. Chondrocyte aging is thought to closely participate in oxidative damage in OA articular cartilage. Recent studies also indicated that ROS directly damage repeat of guanines in the telomeric DNA, resulting in ROSinduced telomere shortening [12]. This finding reveals that oxidative stress may cause cellular senescence due to shortening of telomeric DNA. Chondrocyte senescence caused by oxygen free radicals may implicate the oxidative damage in the progression of OA.
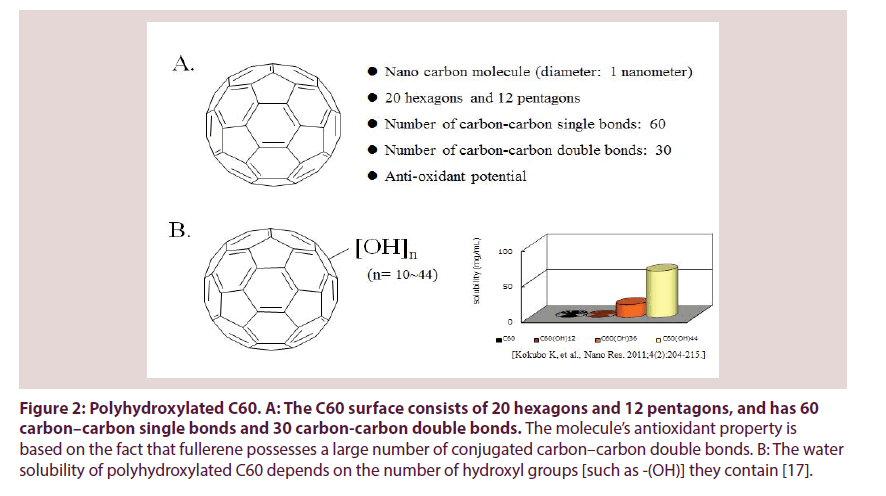
Previously, we have already demonstrated that fullerene (C60), an anti-oxidant, can function as a chondroprotective agent against the progression of OA [13]. C60 is a molecule composed entirely of carbon in a unique spherical structure with 30 conjugated carbon–carbon double bonds (Figure 2A) [14,15]. It is known that one C60 molecule can react with at least in part 15 benzyl radicals or 34 methyl radicals to form stable radical or non-radical adducts and can react with oxygen free radicals without being consumed [16]. C60 is known to be the strongest radical scavenger [14-16]. It has been reported that the anti-oxidative potential of C60 is several hundred-fold higher than that of other antioxidative agents [13,14,16]. Recently, C60 was shown to inhibit cellular apoptosis by scavenging ROS and to inhibit the degeneration of vertebral disc and articular cartilage, suggesting that C60 has a potential as therapeutic agent to protect against oxidative stress-associated pathological features in a variety of diseases. In OA, C60 may be a novel therapeutic agent to prevent both chondrocyte senescence and cartilage matrix degeneration. We have previously confirmed that concentrations of C60 in the μM range level (10- 100 μM) inhibit the OA related factor-mediated production of matrix degrading enzymes and downregulation of cartilage matrix production, as well as chondrocyte aging and apoptosis in vitro. Since C60 is not water soluble factor, we studied cyclodextrin clathrate C60 [13].
Figure 2: Polyhydroxylated C60. A: The C60 surface consists of 20 hexagons and 12 pentagons, and has 60 carbon–carbon single bonds and 30 carbon-carbon double bonds. The molecule’s antioxidant property is based on the fact that fullerene possesses a large number of conjugated carbon–carbon double bonds. B: The water solubility of polyhydroxylated C60 depends on the number of hydroxyl groups [such as -(OH)] they contain [17].
In this study, we examined the therapeutic effects of five types of water-soluble polyhydroxylated C60 fullerenes [C60(OH)10, C60(OH)24, C60(OH)26, C60(OH)36, C60(OH)44] on OA-related factor-induced catabolic responses in osteoarthritic chondrocytes (Figure 2B). We here demonstrate that watersoluble polyhydroxylated C60 fullerenes inhibit the OA-related catabolic reactions (upregulated production of matrix metalloproteinase (MMP)- 13, downregulated production of proteoglycan) in chondrocytes at 1,000-fold lower concentrations (nM order) than those of other C60 fullerenes (μM order) that had previously been reported to have a protective effect [13]. Our study indicates that polyhydroxylated C60 may have a role in protecting against the OA related factor-accelerated development of articular cartilage degradation.
Materials & methods
Chondrocyte isolation and cell culture
Human articular cartilage samples were obtained from bone and cartilage tissues during arthroplastic knee surgery (n=2; 1 female; 81 years of age, 1 male; 84 years of age) after obtaining informed consent from the patients with OA. Chondrocytes were isolated and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma, St. Louis, MO, USA) with 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, 25 mM HEPES (2[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid), and 100 U/mL penicillin and streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2 as previously reported [18].
Measurement of anabolic and catabolic activities of chondrocytes
Cultured chondrocytes were seeded and cultured into 10 cm culture dishes (BD Biosciences, Franklin Lakes, NJ, USA) at approximately 2.0 × 105 cells per dish and incubated in DMEM with 10% FCS in the presence or absence of IL-1β (10.0 ng/mL, R&D Systems Inc., Minneapolis, MN, USA) or C60(OH)10, C60(OH)24, C60(OH)26, C60(OH)36 or C60(OH)44 (0.1, 1.0, 10.0, or 100.0 nM; from VitaminC60 Bio, Tokyo, Japan. We obtained two types of C60(OH)24 in this study.) at 37°C in a humidified atmosphere of 95% air and 5% CO2. After 24-hour incubation period, the culture conditioned medium was collected from each dish and stored at -80°C until analysis.
To study the effect of water-soluble polyhydroxylated C60 on anabolic activity of chondrocytes, the levels of proteoglycan production by chondrocytes were analyzed using an enzyme-linked immunosorbent assay (ELISA) kit in accordance with the manufacturer’s protocol (DIA source Immuno Assays S.A., Nivelles, Belgium). To analyze the catabolic activity of chondrocytes, levels of MMP-13 production from chondrocytes were measured using an ELISA kit (R&D Systems Inc., Minneapolis, MN, USA). Results from each experiment were determined from the mean of triplicate trials.
Statistical analysis
Data are expressed as means ± standard deviation. A two-tailed Student’s t-test was used to examine the statistical significant differences between two groups. Analysis of variance was used for comparisons of more than two groups, and differences between two groups within the set were analysed by a Fisher’s protected least-significant difference test. Probability values of <0.05 were considered statistically significant.
Results
Inhibitory effects of polyhydroxylated C60 on the IL-1β-mediated production of the cartilage matrix degrading enzyme, MMP-13, in chondrocytes
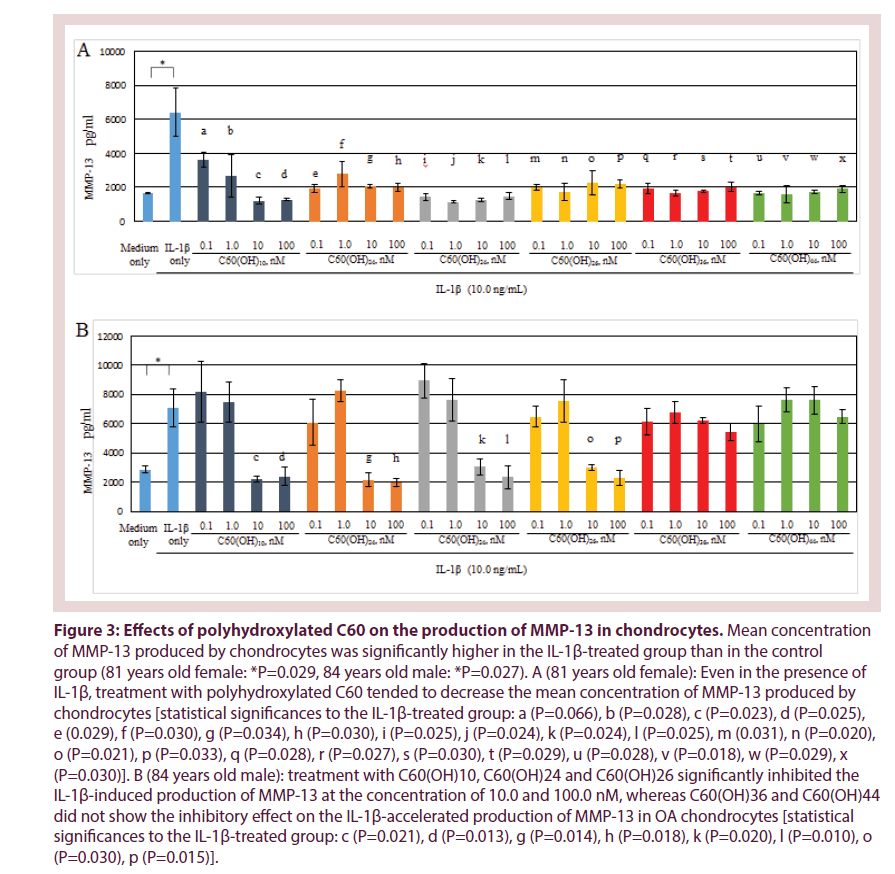
In Figure 3, production of MMP-13 by human osteoarthritic chondrocytes showed higher in the IL-1β-treated group (IL-1β: 10.0 ng/mL) than in the control. IL-1β significantly stimulated MMP-13 production by chondrocytes (81 years old female: P=0.029, 84 years old male: P=0.027). IL-1β is considered as an OA-related catabolic cytokine that closely participate in the progression of OA, for example, in the acceleration of cartilage degrading enzyme production from chondrocytes [1,4]. Our data were consistent with previous reports.
Figure 3: Effects of polyhydroxylated C60 on the production of MMP-13 in chondrocytes. Mean concentration of MMP-13 produced by chondrocytes was significantly higher in the IL-1β-treated group than in the control group (81 years old female: *P=0.029, 84 years old male: *P=0.027). A (81 years old female): Even in the presence of IL-1β, treatment with polyhydroxylated C60 tended to decrease the mean concentration of MMP-13 produced by chondrocytes [statistical significances to the IL-1β-treated group: a (P=0.066), b (P=0.028), c (P=0.023), d (P=0.025), e (0.029), f (P=0.030), g (P=0.034), h (P=0.030), i (P=0.025), j (P=0.024), k (P=0.024), l (P=0.025), m (0.031), n (P=0.020), o (P=0.021), p (P=0.033), q (P=0.028), r (P=0.027), s (P=0.030), t (P=0.029), u (P=0.028), v (P=0.018), w (P=0.029), x (P=0.030)]. B (84 years old male): treatment with C60(OH)10, C60(OH)24 and C60(OH)26 significantly inhibited the IL-1β-induced production of MMP-13 at the concentration of 10.0 and 100.0 nM, whereas C60(OH)36 and C60(OH)44 did not show the inhibitory effect on the IL-1β-accelerated production of MMP-13 in OA chondrocytes [statistical significances to the IL-1β-treated group: c (P=0.021), d (P=0.013), g (P=0.014), h (P=0.018), k (P=0.020), l (P=0.010), o (P=0.030), p (P=0.015)].
Interestingly, there was a tendency to decrease the production of MMP-13 by chondrocytes that were treated with polyhydroxylated C60 even in the presence of IL-1β (10.0 ng/ mL). In OA chondrocytes from 81 years old female, treatment with each polyhydroxylated C60 [C60(OH)10, C60(OH)24, C60(OH)26, C60(OH)36, C60(OH)44] significantly inhibited the IL-1β-accelerated production of MMP- 13 in the entire concentration region (Figure 3A). In OA chondrocytes from 84 years old male, C60(OH)10, C60(OH)24 and C60(OH)26 significantly inhibited the IL-1β-induced production of MMP-13 at the concentration of 10.0 and 100.0 nM, whereas treatment with C60(OH)36 and C60(OH)44 did not show their inhibitory effect on the IL-1β-induced MMP- 13 production by OA chondrocytes (Figure 3B). These findings indicate that C60(OH)10, C60(OH)24 and C60(OH)26, but not C60(OH)36 or C60(OH)44, may inhibit the IL-1β-stimulated production of MMP-13 in OA chondrocytes.
Effect of polyhydroxylated C60 on chondrocyte production of articular proteoglycan
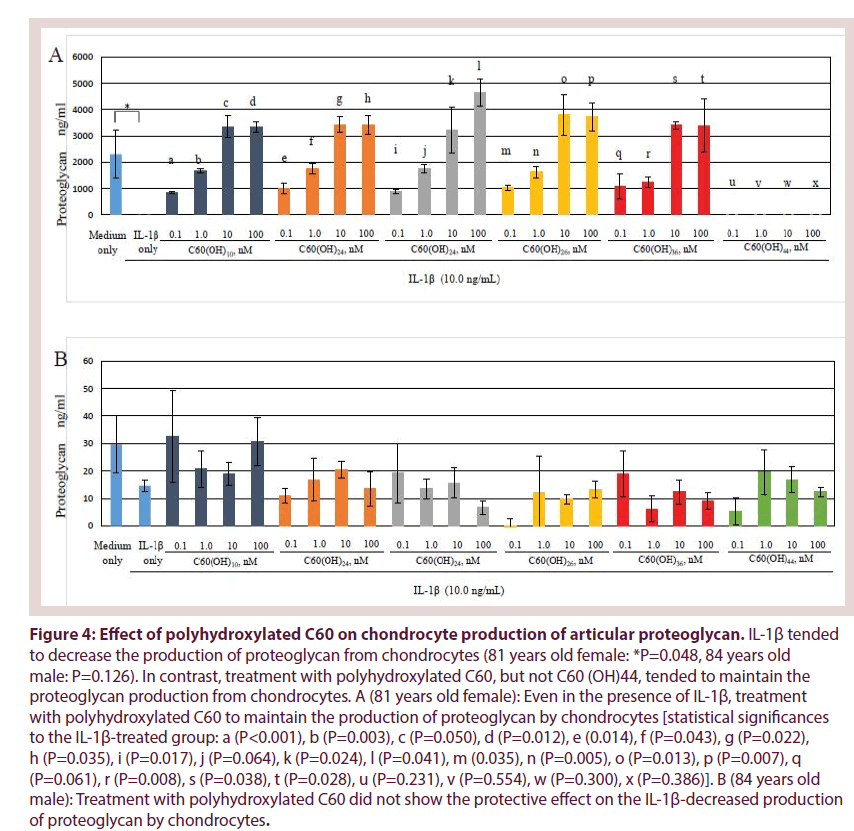
Figure 4 shows that production levels of proteoglycan, which is the articular cartilage matrix component, by chondrocytes tended to decrease in the presence of IL-1β (10.0 ng/ mL) compared to the control (81 years old female: *P= 0.048, 84 years old male: P= 0.062). In contrast, in OA chondrocytes from 81 years old female, treatment with C60(OH)10, C60(OH)24, C60(OH)26 and C60(OH)36, but not C60(OH)44, significantly increased the proteoglycan production from chondrocytes even in the presence of IL-1β (Figure 4A). These findings indicate that polyhydroxylated C60 may have a part to protect against the IL-1β-induced downregulation of proteoglycan production in OA. In OA chondrocytes from 84 years old male, treatment with polyhydroxylated C60 did not show the protective effect on the production of proteoglycan by chondrocytes (Figure 4B).
Figure 4: Effect of polyhydroxylated C60 on chondrocyte production of articular proteoglycan. IL-1β tended to decrease the production of proteoglycan from chondrocytes (81 years old female: *P=0.048, 84 years old male: P=0.126). In contrast, treatment with polyhydroxylated C60, but not C60 (OH)44, tended to maintain the proteoglycan production from chondrocytes. A (81 years old female): Even in the presence of IL-1β, treatment with polyhydroxylated C60 to maintain the production of proteoglycan by chondrocytes [statistical significances to the IL-1β-treated group: a (P<0.001), b (P=0.003), c (P=0.050), d (P=0.012), e (0.014), f (P=0.043), g (P=0.022), h (P=0.035), i (P=0.017), j (P=0.064), k (P=0.024), l (P=0.041), m (0.035), n (P=0.005), o (P=0.013), p (P=0.007), q (P=0.061), r (P=0.008), s (P=0.038), t (P=0.028), u (P=0.231), v (P=0.554), w (P=0.300), x (P=0.386)]. B (84 years old male): Treatment with polyhydroxylated C60 did not show the protective effect on the IL-1β-decreased production of proteoglycan by chondrocytes.
Discussion
In the present study, our findings indicate that C60 hydroxides inhibit OA-related catabolic responses (IL-1β-induced production of MMP-13, IL-1β-induced downregulation of proteoglycan production by OA chondrocytes). C60(OH)10 C60(OH)24 and C60(OH)26 showed a stronger chondroprotective effect than C60(OH)36 or C60(OH)44. These findings suggest that C60 fullerene hydroxides, especially C60(OH)10, C60(OH)24, and C60(OH)26, may have the potential to inhibit the catabolic factorinduced downregulation of chondrocyte activity in OA. These data showed a novel pathologic mechanism explaining the oxidant-mediated degeneration of articular cartilage.
The C60 surface consists of 20 hexagons and 12 pentagons, and has 60 carbon–carbon single bonds and 30 carbon-carbon double bonds (Figure 2A) [16]. All carbon–carbon double bonds are conjugated. The molecule’s antioxidant property is based on the fact that fullerene possesses a large number of conjugated carbon– carbon double bonds which can easily take up an electron, making an attack of radical species highly possible [14-16]. It has been reported that up to 34 methyl radicals have been added onto a single C60 molecule [16]. The antioxidant potential is thought to depend on the number of carbon– carbon double bonds in a C60 fullerene [16]. Reducing the number of carbon–carbon double bonds with a concomitant increase of hydroxyl groups, such as –(OH), may lead to a reduction of the antioxidant potential of polyhydroxylated C60 fullerene. Our data also demonstrate that the chondroprotective potential, which is a reflection of the anti-oxidative potential, of C60(OH)36 and C60(OH)44 were lower than that of C60(OH)10 C60(OH)24 and C60(OH)26.
The water solubility of polyhydroxylated C60 fullerenes is also thought to depend on the number of hydroxyl groups [such as -(OH)] they contain (Figure 2B) [17,19]. A previous report demonstrated that, in contrast to antioxidant potential, an increase in the number of hydroxyl groups of polyhydroxylated C60 fullerenes can improve their water solubility [17]. We also investigated the actual state of dissolved, powdered polyhydroxylated C60. The hydrophilic potential of C60 was lower in C60(OH)10 and C60(OH)24 than in C60(OH)36 or C60(OH)44 (data not shown). In addition, we have also found that C60(OH)24 melted more readily in comparison with C60(OH)10. It has been suggested that fullerenes with fewer than 12 hydroxyl groups have poor water solubility [20-22]. When C60 is derivatized as polyhydroxylated C60, they become water soluble enabling them to cross the cell membrane and localize to the mitochondria, generating oxygen free radicals [23,24].
It is well known that C60 can react with many superoxides without being consumed. It is now thought that C60 may react with ROS as a catalytic substance without metabolic degradation. C60 has been considered as an anti-oxidant and C60 was shown to inhibit cellular apoptosis by scavenging ROS, suggesting that C60 is a useful agent to protect against oxygen free radical-induced pathological features in a variety of diseases [13-17]. These findings suggest that C60, as a strong anti-oxidant, may influence the cellular microenvironment as a catalytic agent. The intracellular amounts of C60 may be quantified by high-performance liquid chromatography (HPLC). Recently, Asada et al. demonstrated that the C60 contents in fibrosarcoma cell line, HT1080 cells, gradually increased and reached the peak after 3 hours of incubation [25]. The C60 fullerene contents in normal fibroblasts reached the peak after 6 hours of incubation. However, in other cell lines and human somatic cells, the level of intracellular uptake of C60 still remains unknown. We are now planning to analyse the level of intracellular uptake of C60 into chondrocytes by HPLC method.
We think that further studies are required to clarify the different mechanisms on chondrocytes originating from osteoarthritis cartilage underlying the biological reaction to various OA-related catabolic factors (such as mechanical stress and proinflammatory cytokines) as compared with chondrocytes from normal cartilage. Thus, we have been studying some experiments using osteoarthritic chondrocytes isolated from degenerated articular cartilages from OA patients who underwent the arthroplastic surgery. In the present study, we have used human osteoarthritic chondrocytes from OA patients and have analyzed the effect of polyhydroxylated C60 fullerenes on chondrocyte activities in vitro. However, during the continuous cell culture to gain efficient chondrocyte population for experiments, chondrocytes easily lose the chondrocytic phenotype and lapse into the dedifferentiation. Thus, in the current study, we made it a role to use cultured chondrocytes within 3 passages. From the point of view of cell source, since it is difficult to obtain efficient chondrocyte population, we think that immortalized chondrocytes are useful for experiments requiring numerous number of chondrocytes. Therefore, we are now planning the study using the immortalized chondrocytes.
Conclusion
Further studies are needed to verify the precise effects of polyhydroxylated C60 on chondrocyte activities in OA. Our results reported here demonstrate that water soluble polyhydroxylated C60 can function as a chondroprotective agent against the OA related catabolic factor-mediated development of OA.
Acknowledgements
We would like to thank M. Suzuki, S. Mogi, M. Tamaki and J. Tamate for excellent technical assistance.
References
- Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis. Cartilage. 11, 747-755(2003).
- DelCarlo M, Loeser RF. Chondrocyte cell death mediated by reactive oxygen species-dependent activation of PKC-beta. Am. J. Physiol. Cell. Physiol. 290, 802-811(2006).
- Loeser RF, Carlson CS, Del Carlo M, et al.Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis. Rheum. 46, 2349-2357(2002).
- Del Carlo M Jr, Loeser RF. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis. Rheum.46, 394-403(2002).
- Carlo MD Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis. Rheum. 48, 3419-3430(2003).
- Yudoh K, Nguyen T, Nakamura H, et al. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis. Res. Ther. 7, 380-391 (2005).
- Vaamonde-García C, Riveiro-Naveira RR, Valcárcel-Ares MN, et al.Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis.Rheum. 64, 2927-2936(2012).
- Kurz B, Lemke A, Kehn M, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis. Rheum. 50, 123-130(2004).
- Green DM, Noble PC, Ahuero JS, et al. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis. Rheum. 54, 1509-1517(2006).
- Martin JA, Buckwalter JA. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa. Orthop. J 21, 1-7(2001).
- Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontol.3, 257-264(2002).
- Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat. Res. 488, 65-76(2001).
- Yudoh K, Shishido K, Murayama H, et al. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via down-regulation of chondrocyte catabolic activity and inhibition of cartilage degeneration during disease development. Arthritis. Rheum. 56, 3307-3318(2007).
- Liu Q, Cui Q, Li XJ, et al. The applications of buckminsterfullerene C60 and derivatives in orthopaedic research. Connect. Tissue. Res. 55, 71-79(2014).
- Bakry R, Vallant RM, Najam-ul-Haq M, et al.Medicinal applications of fullerenes. Int. J.Nanomed. 2, 639-649(2007).
- Kokubo K, Shirakawa S, Kobayashi N, et al.Facile and Scalable Synthesis of a Highly Hydroxylated Water-Soluble Fullerenol as a Single Nanoparticle. Nano Res 4: 204-215(2011).
- Krusic PJ, Wasserman E, Keizer PN, et al.Radical reactions of C60. Science. 254, 1183-1185(1991).
- Liu Q, Jin L, Mahon BH, et al. Novel treatment of neuroinflammation against low back pain by soluble fullerol nanoparticles. Spine. 38, 1443-1451(2013).
- Yui N, Yoshioka H, Fujiya H, et al. The DNA repair enzyme apurinic/apyrimidinic endonuclease (Apex nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis. Int. J. Mol. Sci. 15, 14921-14934(2014).
- Kokubo K, Matsubayashi K, Tategaki H, et al.Facile synthesis of highly water-soluble fullerenes more than half-covered by hydroxyl groups. ACS. Nano. 2, 327-333(2008).
- Chiang LY, Wang LY, Swirczewski JW, et al.Efficient synthesis of polyhydroxylated fullerene derivatives via hydrolysis of polycyclosulfated precursors. J. Org. Chem. 59, 3960-3968(1994).
- Li J, Takeuchi A, Ozawa M, et al. C60 Fullerol formation catalysed by quaternary ammonium hydroxides. J. Chem. Soc. Chem. Commun. 23, 1784-1785(1993).
- Foley S, Crowley C, Smaihi M, et al. Cellular localization of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 294, 116-119(2002).
- Youle RJ, Karbowski M. Opinion: Mitochondrial fission in apoptosis. Nature. Rev. Mol. Cell. Biol. 6, 657-663(2005).
- Asada R, Liao F, Saitoh Y, et al.Photodynamic anti-cancer effects of fullerene [C₆₀]-PEG complex on fibrosarcomas preferentially over normal fibroblasts in terms of fullerene uptake and cytotoxicity. Mol. Cell. Biochem. 390, 175-184(2014).