Review Article - Interventional Cardiology (2014) Volume 6, Issue 2
Neoatherosclerosis: a novel player in late stent failure
- Corresponding Author:
- Rocco A Montone
Institute of Cardiology, Catholic University of the Sacred Heart
Largo Agostino Gemelli, 8, 00168, Rome, Italy
Tel: +39 6 3051166
Fax: +39 6 3055535
E-mail: rocco.montone@gmail.com
Abstract
Neoatherosclerosis is a newly formed atherosclerotic change within the neointima following bare-metal stent (BMS) or drug-eluting stent (DES) implantation. Of importance, recent studies suggested an important role for neoatherosclerosis in late events after both DES and BMS implantation. In particular, the development of neoatherosclerosis has been associated with occurrence of late in-stent restenosis (ISR) and stent thrombosis (ST). In this review, we describe pathogenetic mechanisms responsible for neoatherosclerosis and evidence derived from histopathologic and intravascular imaging studies.Keywords
drug-eluting stent, in-stent restenosis, late events, neoatherosclerosis, stent thrombosis
Neoatherosclerosis is a newly formed atherosclerotic change within the neointima following bare-metal stent (BMS) or drug-eluting stent (DES) implantation. Of importance, recent studies suggested an important role for neoatherosclerosis in late events after both DES and BMS implantation. In particular, the development of neoatherosclerosis has been associated with occurrence of late in-stent restenosis (ISR) and stent thrombosis (ST). In this review, we describe pathogenetic mechanisms responsible for neoatherosclerosis and evidence derived from histopathologic and intravascular imaging studies (Table 1). Finally, we discuss possible approaches for risk stratification and therapeutic strategies.
Mechanisms of neoatherosclerosis
Neoatherosclerosis is characterized by infiltration and accumulation of clusters of foamy macrophages within the neointima, following both BMS and DES implantation, due to the inability to maintain a fully functional luminal surface within the stented segment. Of importance, recent studies demonstrated a key role of neoatherosclerosis for late stent failure [1–3].
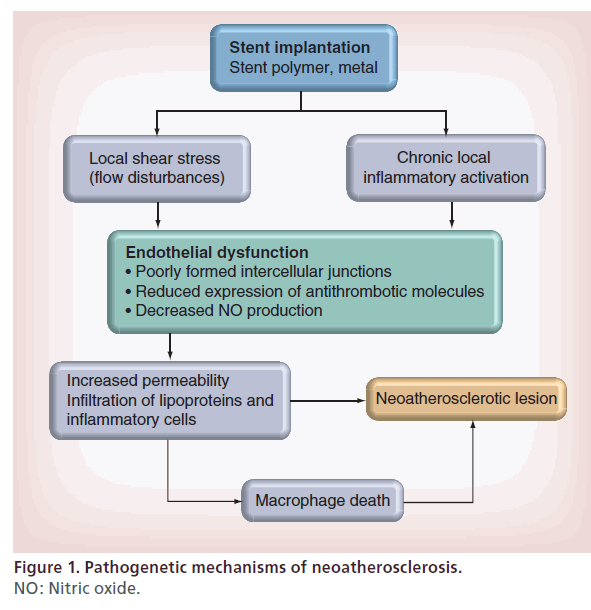
The mechanisms responsible for the development of neoatherosclerotic lesions are multiple (Figure 1) [4,5]. First of all, endothelial dysfunction following stent implantation has been shown to be involved. In normal conditions, the endothelium provides a protection against excessive infiltration of circulating lipids, making the development of the atherosclerotic lesion very slow. Conversely, endothelial cells within the DES stented segment show poorly formed intercellular junctions, reduced expression of antithrombotic molecules and decreased nitric oxide production [6,7].
Endothelial dysfunction may be the consequence of a chronic local inflammatory activation induced by stent polymer or metal [4,5]. Moreover, the local shear stress resulting from flow disturbances may play a role in endothelial dysfunction [8,9]. The activation of endothelial cells results in an increased expression of ICAM-1 and VCAM-1, which attract circulating monocytes into the subendothelial space.
Impaired barrier of endothelium within the stented segment, inflammatory activation and stent-induced shear stress may produce an increased permeability, resulting in an accelerated infiltration and accumulation of lipoproteins and inflammatory cells within the subendothelial space [8–10]. Moreover, lipoproteins in the subendothelial space undergo oxidative modifications, which lead to production of chemoattractant and inflammatory mediators such as MCP-1 and VCAM-1, which are involved in the further recruitment and attachment of monocytes [11].
In addition to endothelial dysfunction, macrophage and vascular smooth muscle cell death appear to play a role in the pathogenesis of neoatherosclerosis. Indeed, release of lipids deriving from macrophage death may contribute to the pool of free cholesterol and cholesterol esters, thereby forming a necrotic core [12]. Finally, vascular smooth muscle cell death and degradation may result in increased levels of free cholesterol and cholesterol esters, which may further attract macrophages [13].
Evidence derived from histopathologic studies
Different histopathological studies have shown an important role for chronic inflammation and/or endothelial dysfunction in the pathogenesis of neoatherosclerosis, following both DES and BMS implantation, suggesting that neoatherosclerosis may be an important player for late ISR and late ST (LST).
Inoue et al. [14] reported histopathologic findings of autopsied samples in 19 patients with noncardiac death after implantation of Palmaz–Schatz coronary stents, suggesting the possibility that peristrut inflammation evoked by a foreign body reaction to the metal corrosion might accelerate new indolent atherosclerotic changes within the stents. Conversely, Hasegawa et al. [15], analyzing 14 BMS restenotic lesions developed beyond 5 years, demonstrated that restenotic tissues retrieved by directional coronary atherectomy were composed of newly developed atherosclerosis facing the underlying intima, regardless of the presence of peristrut inflammation. Furthermore, four samples from the cases presented with acute coronary syndrome showed typical histological morphologies that are similar to vulnerable plaque in native coronary arteries.
However, some studies have pointed out the differences that exist between DES and BMS neoatherosclerosis. Nakazawa et al. [1] reviewed autopsy cases from the CVPath stent registry and compared 66 sirolimus-eluting stent (SES) lesions with 77 BMS lesions. This study showed that neoatherosclerosis occurred in both BMS and DES, but the incidence of this phenomenon is greater in DES (n = 64; 31%) than BMS (n = 31; 16%) lesions (p < 0.001). Moreover, a significant difference in the timing of neoatherosclerosis development was found among BMS and DES. Indeed, atherosclerotic change occurred in shorter implant durations for DES than for BMS (DES: median 420 days; BMS: median 2160 days). In addition, the earliest necrotic core formation began at 9 months, whereas in BMS it occurred at 5 years.
These finding were confirmed more recently in another study from the same group [2]. Regarding DES, data are mostly available for SES and paclitaxel-eluting stent (PES), suggesting a trend for a more rapid neoatherosclerotic changes in SES than in PES. The cumulative incidence up to 6 years are: SES 38% versus PES 24% versus BMS 10%, indicating that differences of drugs or polymers may influence on neointimal tissues. A recent human autopsy analysis by Otsuka et al. evaluated the occurrence and characteristics of neoatherosclerosis after SES, PES or everolimus-eluting stent (EES) implantation, showing that EES had greater strut coverage with less inflammation, less fibrin deposition, and less LST and very late ST (VLST) compared with SES and PES [16]. Nevertheless, the observed frequencies of neoatherosclerosis- related adverse pathological events were comparable in these devices, indicating that careful longterm follow-up remains important even after EES placement.
Taken together, these data suggest that mechanisms of failure after BMS and DES implantation are quite different. Indeed, BMS patients tend to develop ISR early, due to neointima hyperplasia. On the contrary, DES patients tend to develop less neointima in the early period, explaining the benefit in the occurrence of target lesion revascularization (TLR) after 1-year follow-up compared with BMS. However, DES patients tend to develop neoatherosclerosis later, probably explaining the late catch-up phenomenon and the occurrence of VLST.
Evidence deriving from intravascular imaging
Angioscopy
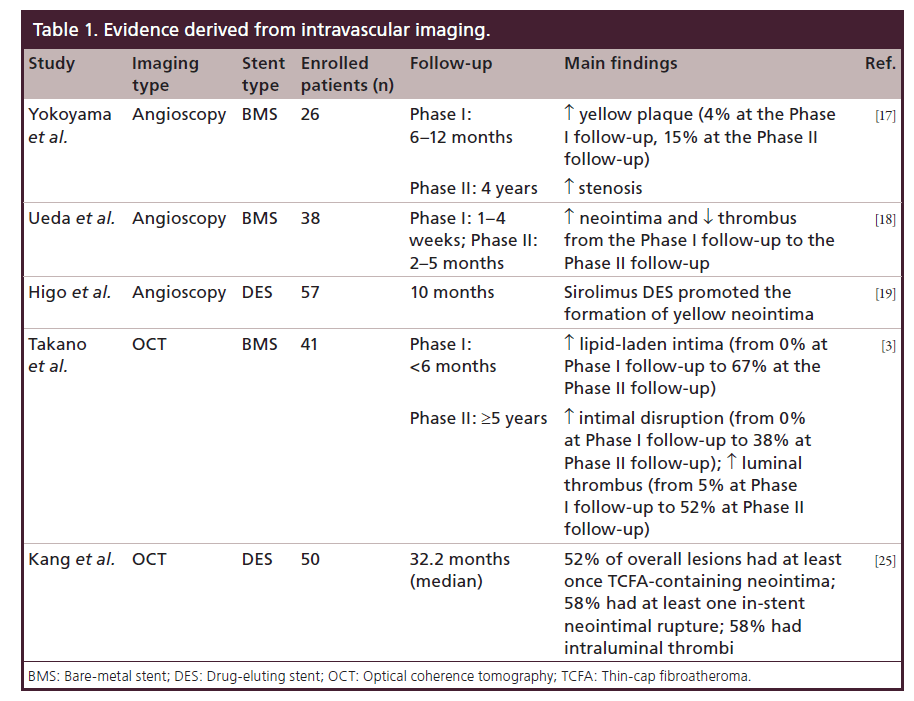
In a serial angioscopic study (at baseline, at 6–12 months and after 4 years) following 26 BMS implantations, Yokoyama et al. investigated the neointimal changes derived from vessel healing response, showing the occurrence of new atherosclerotic lesions, represented as yellow plaque [17]. Although there are no available data regarding the correlation between angioscopic and histologic findings, yellow neointima observed at the angioscopy most likely corresponds to foamy macrophages infiltrating into fibrous cap and/or underlying lipid accumulation. Of note, the intensity of yellow likely signifies thickness of fibrous cap and amount of necrotic core. In this study, an increase in the incidence of yellow plaque, from three cases (4%) at the first follow-up to 15 cases (58%) at the second follow-up, was shown. This increase was accompanied by a rise of the late luminal narrowing, defined as an increase in percentage diameter stenosis between the first and second follow-up, that was significantly greater in segments with yellow plaque than in those without yellow plaque (18.4 vs 3.6%; p = 0.011). This finding indicated that atherosclerotic degeneration inside BMS may contribute to the late luminal narrowing.
Another serial angioscopic examination by Ueda et al. [18] demonstrated that BMSs at 1–4 weeks after implantation were not completely covered by neointima, whereas they were often (45%) accompanied by thrombus. Nevertheless, at 2–5 months, BMS were completely covered by neointima, while thrombus was detected only in 13% of patients. This reduction in the incidence of the thrombus is due to the fact that the neointima over BMS usually covers both stent and yellow plaques under stent completely, and thrombus was no longer detected on the white and smooth neointima even if thrombus was detected on the yellow plaque.
Regarding the DES, Higo et al. [19] demonstrated that sirolimus DESs promoted the formation of atherosclerotic yellow neointima in the stentimplanted lesion at 10-month follow-up. Thrombus was detected more often on the yellow area than on the white area and was never detected where a stent was buried under white neointima. These data suggest that the increased potential risk of LST in DES lesions may be due to the newly formed yellow neointima and cholesterol-laden plaque.
Finally, it is also possible that the angioscopic yellow neointima with advanced atherosclerotic degeneration ruptures and leads to further neointimal progression as well as to late thrombotic events [20].
Virtual histology-intravascular ultrasound
Virtual histology-intravascular ultrasound (VHIVUS) involves spectral analysis for frequency and intensity of back-scattered ultrasound data to construct tissue maps of coronary plaques [21]. Although it is difficult for intravascular ultrasound (IVUS) to determine neointimal tissue because of the signal interference from metal struts, there are several reports attempting discrimination of neointimal tissues by IVUS. Kang et al. [22] reported findings from 70 DESISR and 47 BMS-ISR lesions with intimal hyperplasia in >50% of the stent area by VH-IVUS. The mean follow-up time was 43.5 months for BMS lesions and 11.1 months for DES lesions, and this study showed that both BMS- and DES-treated lesions develop an in-stent necrotic core and dense calcium, suggesting the development of in-stent neoatherosclerosis, especially in lesions with longer implant duration.
Moreover, Sànchez-Recalde et al. performed an IVUS study during primary angioplasty in five patients with very late BMS thrombosis and carried out a histological analysis of the material removed by manual thrombectomy [23]. The mean (standard deviation) time from the index procedure was 7 (±4) years and at IVUS analysis they found calcified atherosclerosis with in-stent plaque rupture, complex plaque in the distal segment of the stent, in-stent neointimal proliferation associated with underexpansion and severe in-stent proliferation.
Optical coherence tomography
Optical coherence tomography (OCT) is a nearinfrared light-based imaging modality with very high resolution. As a consequence of its high resolution (10–20 μm), OCT has been shown to better evaluate the vascular responses after stent implantation [24]. Habara et al. [24], through the use of OCT, have evaluated the progression of in-stent atherosclerotic lesion >5 years after BMS implantation and found a high incidence (90.7%) of possible neoatherosclerotic change, defined as heterogeneous OCT appearance with low-intensity areas, whereas lesions <1 year after BMS implantation showed only 17.9% incidence of neoatherosclerosis. In-stent intimal growth was accompanied by neointimal disruption, which had analogous morphology of ruptured fibroatheroma in a native coronary artery and occurred more frequently in >5-year lesions (18.6%) than in <1-year lesions (0%).
Takano et al. [3] evaluated neointimal OCT characteristics of BMS in early (<6 months) and extended late phases (≥5 years). In the early phase there was no sign of lipid-laden intima, evidenced by the presence of a homogeneous OCT appearance. Conversely, lipid-laden intima, intimal disruption and luminal thrombus formation were more frequently observed in the late phase, when compared with the early phase (67 vs 0%, 38 vs 0%, and 52 vs 5%, respectively; all p < 0.05). These findings suggest that neointima within BMS often undergoes a neoatherosclerotic process during an extended follow-up period and this process, promoting further luminal narrowing, may play a role in the development of in-ST.
By an OCT analysis in 50 patients with DESISR (median follow-up period: 32.2 months), Kang et al. showed that the 52% of overall lesions had at least one TCFA-containing neointima, 58% had instent neointimal rupture and 58% showed intraluminal thrombi [25]. Although Gonzalo et al. previously reported various OCT patterns of restenotic tissue after stenting (84% were various DES), the median follow-up time was only 12 months, too short to manifest the entire spectrum of neoatherosclerosis as suggested in this study [26]. In contrast to patients with stable angina, patients presenting with unstable angina showed a thinner fibrous cap and an increasing number of unstable OCT findings, including TCFAcontaining neointima, neointima rupture and thrombus. Compared with DES <20 months post-implantation (the best cutoff to predict TCFA-containing neointima), DES ≥20 months postimplantation had a higher incidence of TCFA-containing neointima (69 vs 33%; p = 0.012) and red thrombi (27 vs 0%; p = 0.007). These findings suggest that in-stent neoatherosclerosis assessed by OCT may be an important mechanism of DES restenosis, especially late after implantation.
Risk stratification & therapeutic approach
In the past, inflammatory activation has been shown to be associated to clinical recurrence of thrombotic events arising from native coronary artery atherosclerotic lesions [27]. At the same time, inflammatory biomarkers have been shown to predict clinical outcome in patients undergoing stent implantation.
Indeed, in order to stratify the risk of angiographic and clinical outcomes after stent implantation, several inflammatory biomarkers have been investigated. CRP represents the most extensively studied biomarker in patients undergoing percutaneous coronary intervention (PCI). CRP is a sensitive marker of systemic inflammation [28–30] and, in particular, has been shown to be an important marker of poststenting inflammation [31]. Indeed, CRP levels increase after PCI in a time-dependent manner, peaking at 48 h, and the magnitude of CRP change after the procedure has been shown to predict ISR in patients undergoing BMS deployment [32]. Inflammatory biomarkers, however, cannot specifically predict ISR because they may also predict progression and destabilization of atherosclerosis in native coronary arteries. However, some angiographic studies have shown that both baseline and postprocedural CRP levels may allow the identification of patients at higher risk of restenosis after BMS [33–35].
Moreover, different studies evaluated the association between CRP with angiographic and clinical end points after DES deployment showing that baseline CRP levels have been associated with hard clinical end points [36,37]. Indeed, Park et al. demonstrated that higher baseline CRP levels were associated with an increased risk of death, myocardial infarction (MI) and ST, but there was no association with target vessel revascularization [38]. These data suggest that the predictive value of inflammatory activation, as assessed by CRP levels, seems to shift from restenosis as observed in the BMS era to thrombotic events in the DES era. However, a small study by Niccoli et al. has recently investigated the association between baseline CRP levels and restenosis pattern after DES implantation [39]. Interestingly, preprocedural CRP serum levels were an independent predictor of a diffuse pattern of restenosis (odds ratio: 2.5; 95% CI: 1.4–4.3; p = 0.001).
To assess the risk prediction after DES implantation, in addition to CRP, other inflammatory biomarkers have also been evaluated. Several studies evaluated serum levels of matrix metalloproteinases (MMPs), which are involved in extracellular matrix degradation and vascular smooth muscle cells migration, and may play a role in the pathogenesis of ISR. Katsaros et al. showed that baseline MMP-9 and postprocedural (after 24 h) MMP-9 and MMP-2 levels were significantly higher in patients with ISR at 6–8-month angiographic follow-up, as compared with patients without ISR [40]. Also, plasma levels of PAI-1, before and 24 h after PCI, were associated with the occurrence of angiographic ISR [41] and, finally, Speidl et al. found that serum levels of C3a, before and 24 h after PCI, as well as baseline C5a levels, were significantly higher in patients developing ISR at 6–8-month angiographic follow-up [42].
Despite these findings, more studies are needed in order to confirm the association between inflammatory activation assessed by inflammatory biomarkers and the development of neoatherosclerosis. Indeed, as shown in native coronary artery atherosclerotic lesions, the association between inflammatory biomarkers and the risk of ISR and/or ST after stent implantation may reflect the development of neoatherosclerosis. As a consequence, by combining intravascular imaging and inflammatory biomarker assessment, we can better stratify patients undergoing stent implantation to low or high risk of developing neoatherosclerosis. Moreover, a targeted therapy may be considered. Indeed, as PCI-related inflammation is associated with a higher risk of adverse cardiovascular events, periprocedural myocardial necrosis and ISR, several therapies targeting the local or systemic inflammatory response after stent implantation have been investigated. Statins have important anti-inflammatory effects and their efficacy following stent deployment has been documented in many clinical studies [43–49]. In the BMS era, administration of statins has been suggested as a treatment to improve clinical and angiographic outcome, and patients treated with statins, either before or at the time of PCI, had a smaller increase in CRP level after the procedure and a reduced incidence of clinical events and repeat TVR at 6-month follow-up compared with control patients. Moreover, angiographic analysis established a reduction in the occurrence of ISR after BMS implantation, in patients receiving statin therapy [50,51]. Furthermore, the anti-inflammatory effect of statins might contribute to reduce myocardial necrosis owing to microembolization during coronary intervention [52].
Also, a therapy with steroids could reduce the inflammatory burden in the PCI context. In fact, the IMPRESS study in 83 patients undergoing BMS implantation with high CRP levels after the procedure (CRP > 0.5 mg/ dl at 72 h) treated with oral systemic steroid therapy showed a reduction of clinical events at 12 months (28% absolute reduction) and a reduced rate of angiographic restenosis at 6 months (7 vs 33%). This beneficial effect of steroid on ISR occurrence was dose dependent as both clinical and angiographic outcomes worsened when the dose of prednisone was decreased by nearly 50% [53,54].
Pioglitazone, clinically used to treat Type 2 diabetes, acts as a ligand for peroxisome proliferatoractivated receptors (PPARs), in particular the subtype PPAR-g, which is expressed by endothelial cells, monocytes/macrophages and smooth muscle cells in the atherosclerotic plaque, and it is known to regulate anti-inflammatory responses [55–59]. In patients with Type 2 diabetes undergoing BMS implantation, 6-month treatment with pioglitazone at 30 mg/day has been demonstrated to reduce neointimal formation and ISR, as measured by intravascular ultrasound imaging [60], and the TLR rate at 6 months was significantly lower in the pioglitazone group than in the control group (12.5 vs 29.8%; p = 0.04). By contrast, the incidence of death and MI did not differ between the two groups. However, MACEs (major adverse cardiac events: death, MI and TLR) were significantly lower in the pioglitazone group than in the control group (13 vs 31%, respectively; p = 0.02).
Also antithrombotic therapies (e.g., clopidogrel and Gp IIb/IIIa inhibitors) demonstrated anti-inflammatory activity [61] and in the CURE trial, clopidogrel in combination with aspirin reduced the cumulative occurrence of cardiovascular death, MI or the need for urgent TVR in patients presenting with acute coronary syndromes and undergoing PCI, compared with aspirin alone [62–64]. Drugs targeting the Gp IIb/IIIa receptor (e.g., abciximab and eptifibatide) inhibit platelet aggregation and thrombus formation at the injured coronary plaque, reducing the risk of acute ischemic events by up to 50% in patients undergoing PCI [65,66]. In particular, they may also reduce the inflammatory response following PCI. Abciximab demonstrated a potent and direct anti-inflammatory effect, binding to Gp IIb/IIIa or vitronectin (avb3), and reduced interaction between platelets and leukocytes, leukocyte adherence and transmigration across endothelial cells [67–70].
Conclusion & future perspective
Recent studies suggested that neoatherosclerosis represents an important substrate for late ISR and ST after both DES and BMS implantation [4–5,16–19]. Intravascular imaging techniques have been shown to reliably identify and characterize neoatherosclerosis. However, prevention of neoatherosclerosis and its consequences may represent an important target to improve the longterm outcome of patients undergoing stent implantation. Future studies combining biomarkers and intravascular imaging are needed in order to stratify the risk of late stent failure and to target therapy.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• Recent studies suggested an important role for neoatherosclerosis in late events after both drug-eluting stent (DES) and bare-metal stent (BMS) implantation. In particular, the development of neoatherosclerosis has been associated with occurrence of late in-stent restenosis (ISR) and stent thrombosis (ST).
• Neoatherosclerosis is characterized by infiltration and accumulation of clusters of foamy macrophages within the neointima, following both BMS and DES implantation, due to the inability to maintain a fully functional luminal surface within the stented segment.
• Different histopathological studies have shown an important role for chronic inflammation and/or endothelial dysfunction in the pathogenesis of neoatherosclerosis, following both DES and BMS implantation.
• A significant difference in the timing of neoatherosclerosis development was found among BMS and DES. Indeed, atherosclerotic change occurred in shorter implant durations for DES than for BMS.
• Optical coherence tomography and intravascular ultrasound studies confirmed an important role of neoatherosclerosis for late events after DES or BMS implantation.
• Yellow neointima observed at the angioscopy most likely corresponds to foamy macrophages infiltrating into fibrous cap and/or underlying lipid accumulation. Thrombus was detected more often on the yellow area than on the white area and was never detected where a stent was buried under white neointima. These data suggest that the increased potential risk of late ST in DES lesions may be due to the newly formed yellow neointima and cholesterol-laden plaque.
• Inflammatory biomarkers have been shown to predict clinical outcome in patients undergoing both DES and BMS implantation. As in native coronary artery atherosclerotic lesions, the association between inflammatory biomarkers and the risk of ISR and/or ST after stent implantation may reflect the development of neoatherosclerosis. However, further studies are needed in order to confirm this hypothesis.
• As percutaneous coronary intervention-related inflammation is associated with a higher risk of adverse cardiovascular events, periprocedural myocardial necrosis and ISR, several therapies targeting the local or systemic inflammatory response after stent implantation may be useful to reduce the occurrence of neoatherosclerosis. However, further studies are needed in order to confirm this hypothesis.
• Future studies combining biomarkers and intravascular imaging are needed in order to stratify the risk of late stent failure and to target therapy.
References
Papers of special note have been highlighted as:
• of interest; • of considerable interest
- Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-elutingstents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovasc. Imaging 2, 625–628 (2009).
- Nakazawa G, Otsuka F, Nakano M et al. The pathology of neoatherosclerosis in human coronary implants: bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 57, 1314–22 (2011).
- Takano M, Yamamoto M, Inami S et al. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late phase observation by intracoronary optical coherence tomography. J. Am. Coll. Cardiol. 55, 26–32 (2009).
- Otzuka F, Nakano M, Ladich E, Kolodgie FD, Virmani R. Pathologic etiologies of late and very late stent thrombosis following first-generation drug-eluting stent placement. Thrombosis 2012, 608593 (2012).
- Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y. In-stent neoatherosclerosis: a final common pathway of late stent failure. J. Am. Coll. Cardiol. 59, 2051–2057 (2012).
- Joner M, Nakazawa G, Finn AV et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol. 52, 333–342 (2008).
- Nakazawa G, Nakano M, Otsuka F et al. Evaluation of polymer-based comparator drug-eluting stents using a rabbit model of iliac artery atherosclerosis. Circ. Cardiovasc. Interv. 4, 38–46 (2011).
- Jimenez JM, Davies F. Hemodynamically driven stent strut design. Ann. Biomed. Eng. 37, 1483–1494 (2009).
- Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 6, 16–26 (2009).
- Skalen K, Gustafsson M, Rydberg EK et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417, 750–754 (2002).
- Simionescu M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler. Thromb. Vascular Biol. 27, 266–274 (2007).
- Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vascular Biol. 25, 2255–2264 (2005).
- Tulenko TN, Chen M, Mason PE, Mason RP. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J. Lipid Res. 39, 947–956 (1998).
- Inoue K, Abe K, Ando K et al. Pathological analyses of longterm intracoronary Palmaz–Schatz stenting: is its efficacy permanent? Cardiovasc. Pathol. 13, 109–115 (2004).
- Hasegawa K, Tamai H, Kyo E et al. Histopathological findings of new in-stent lesions developed beyond five years. Catheter Cardiovasc. Interv. 68, 554–558 (2006).
- Otsuka F, Vorpahl M, Nakano M et al. Pathology of secondgeneration everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 129(2), 211–223 (2014).
- Yokoyama S, Takano M, Yamamoto M et al. Extended follow-up by serial angioscopic observation for bare-metal stents in native coronary arteries: from healing response to atherosclerotic transformation of neointima. Circ. Cardiovasc. Interv. 2, 205–212 (2009).
- Ueda Y, Nanto S, Komamura K, Kodama K. Neointimal coverage of stents in human coronary arteries observed by angioscopy. J. Am. Coll. Cardiol. 23, 341–346 (1994).
- Higo T, Ueda Y, Oyabu J et al. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovasc. Imaging 2, 616–624 (2009).
- Burke AP, Kolodgie FD, Farb A et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation 103, 934–940 (2001).
- Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 106, 2200–2206 (2002).
- Kang SJ, Mintz GS, Park DW et al. Tissue characterization of in-stent neointima using intravascular ultrasound radiofrequency data analysis. Am. J. Cardiol. 106(11), 1561–1565 (2010).
- Sánchez-Recalde A, González-Obeso E, Reyes Martín et al. Intravascular ultrasound and histology findings in very late bare-metal stent thrombosis. Rev. Esp. Cardiol. 63(12), 1492–1496 (2010).
- Habara M, Terashima M, Suzuki T. Detection of atherosclerotic progression with rupture of degenerated in-stent intima five years after bare-metal stent implantation using optical coherence tomography. J. Invasive Cardiol. 21, 552–553 (2009).
- Kang SJ, Mintz GS, Akasaka T et al. Optical coherent tomographic analysis of in-stent neo-atherosclerosis after drug-eluting stent implantation. Circulation 123, 2913–2915 (2011).
- Gonzalo N1, Serruys PW, Okamura T et al. Optical coherence tomography patterns of stent restenosis. Am. Heart J. 158, 284–293 (2009).
- Liuzzo G, Biasucci LM, Gallimore JR. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N. Engl. J. Med. 331(7), 417–424 (1994).
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 (1999).
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 347, 1557–1565 (2002).
- Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet 349, 462–466 (1997).
- Niccoli G, Montone RA, Ferrante G, Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J. Am. Coll. Cardiol. 56(22), 1783–1793 (2010).
- Gaspardone A, Crea F, Versaci F et al. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am. J. Cardiol. 82, 515–518 (1998).
- Ferrante G, Niccoli G, Biasucci LM et al. Association between C-reactive protein and angiographic restenosis after bare metal stents: an updated and comprehensive meta-analysis of 2747 patients. Cardiovasc. Revasc. Med. 9, 156–165 (2008).
- Segev A, Kassam S, Buller CE et al. Pre-procedural plasma levels of C-reactive protein and interleukin-6 do not predict late coronary angiographic restenosis after elective stenting. Eur. Heart J. 25, 1029–1035 (2004).
- Gomma AH, Hirschfield GM, Gallimore JR Jr, Lowe GD, Pepys MB, Fox KM. Preprocedural inflammatory markers do not predict restenosis after successful coronary stenting. Am. Heart J. 147, 1071–1077 (2004).
- Choi DH, Park KW, Yang HM et al. Renal dysfunction and high levels of hsCRP are additively associated with hard endpoints after percutaneous coronary intervention with drug eluting stents. Int. J. Cardiol. 149, 174–181 (2011).
- Delhaye C, Maluenda G, Wakabayashi K et al. Longterm prognostic value of preprocedural C-reactive protein after drug eluting stent implantation. Am. J. Cardiol. 105, 826–832 (2010).
- Park DW, Yun SC, Lee JY et al. C-reactive protein and the risk of stent thrombosis and cardiovascular events after drugeluting stent implantation. Circulation. 120(20), 1987–1995 (2009).
- Niccoli G, Conte M, Cosentino N et al. Baseline C-reactive protein serum levels and in-stent restenosis pattern after m-TOR inhibitors drug-eluting stent implantation. J. Invasive Cardiol. 23, 16–20 (2011).
- Katsaros KM, Kastl SP, Zorn G et al. Increased restenosis rate after implantation of drug-eluting stents in patients with elevated serum activity of matrix metalloproteinase-2 and -9. JACC Cardiovasc. Interv. 3, 90–97 (2010).
- Katsaros KM, Speidl WS, Kastl SP et al. Plasminogen activator inhibitor-1 predicts coronary in-stent restenosis of drug-eluting stents. J. Thromb. Haemost. 6, 508–513 (2008).
- Speidl WS, Katsaros KM, Kastl SP et al. Coronary late lumen loss of drug eluting stents is associated with increased serum levels of the complement components C3a and C5a. Atherosclerosis 208, 285–289 (2010).
- Serruys PW, de Feyter P, Macaya C et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 287, 3215–3222 (2002).
- Walter DH, Fichtlscherer S, Britten MB et al. Statin therapy, inflammation and recurrent coronary events in patients following coronary stent implantation. J. Am. Coll. Cardiol. 38, 2006–2012 (2001).
- Walter DH, Fichtlscherer S, Britten MB, Auch-Schwelk W, Schachinger V, Zeiher AM. Benefits of immediate initiation of statin therapy following successful coronary stent implantation in patients with stable and unstable angina pectoris and Q-wave acute myocardial infarction. Am. J. Cardiol. 89, 1–6 (2002).
- Schomig A, Mehilli J, Holle H et al. Statin treatment following coronary artery stenting and one-year survival. J. Am. Coll. Cardiol. 40, 854–861 (2002).
- Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) study. Circulation 110, 674–678 (2004).
- Briguori C, Colombo A, Airoldi F et al. Statin administration before percutaneous coronary intervention: impact on periprocedural myocardial infarction. Eur. Heart J. 25, 1822–1828 (2004).
- Walter DH, Schachinger V, Elsner M et al. Statin therapy is associated with reduced restenosis rates after coronary stent implantation in carriers of the Pl(A2)allele of the platelet glycoprotein IIIa gene. Eur. Heart J. 22, 587–595 (2001).
- Walter DH, Schachinger V, Elsner M, Mach S, Auch- Schwelk W, Zeiher AM. Effect of statin therapy on restenosis after coronary stent implantation. Am. J. Cardiol. 85, 962–968 (2000).
- Gaspardone A, Versaci F, Proietti I et al. Effect of atorvastatin (80 mg) initiated at the time of coronary artery stent implantation on C-reactive protein and six-month clinical events. Am. J. Cardiol. 90, 786–789 (2002).
- Jones SP, Lefer DJ. Cardioprotective actions of acute HMG-CoA reductase inhibition in the setting of myocardial infarction. Acta Physiol. Scand. 173, 139–143 (2001).
- Ferrero V, Ribichini F, Rognoni A, Marino P, Brunelleschi S, Vassanelli C. Comparison of efficacy and safety of lowerdose to higher-dose oral prednisone after percutaneous coronary interventions (the IMPRESS-LD study). Am. J. Cardiol. 99, 1082–1086 (2007).
- Versaci F, Gaspardone A, Tomai F et al. Immunosuppressive therapy for the prevention of restenosis after coronary artery stent implantation (IMPRESS study). J. Am. Coll. Cardiol. 40, 1935–1942 (2002).
- Patti G, Pasceri V, Carminati P, D’Ambrosio A, Carcagni A, Di Sciascio G. Effect of dexamethasone-eluting stents on systemic inflammatory response in patients with unstable angina pectoris or recent myocardial infarction undergoing percutaneous coronary intervention. Am. J. Cardiol. 95, 502–505 (2005).
- Ribichini F, Tomai F, Paloscia L et al. Steroid-eluting stents in patients with acute coronary syndrome: the dexamethasone eluting stent Italian registry. Heart 93, 598–600 (2007).
- 57 Law RE, Goetze S, Xi XP et al. Expression and function of PPARg in rat and human vascular smooth muscle cells. Circulation 101, 1311–1318 (2000).
- Ricote M, Huang J, Fajas L et al. Expression of the peroxisome proliferator-activated receptor g (PPARg) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA 95, 7614–7619 (1998).
- Kwak BR, Myit S, Mulhaupt F et al. PPARg but not PPARa ligands are potent repressors of major histocompatibility complex class II induction in atheroma-associated cells. Circ. Res. 90, 356–362 (2002).
- Takagi T, Okura H, Kobayashi Y et al. A prospective, multicenter, randomized trial to assess efficacy of pioglitazone on in-stent neointimal suppression in Type 2 diabetes: POPPS (Prevention of In-Stent Neointimal Proliferation by Pioglitazone Study). JACC Cardiovasc. Interv. 2, 524–531 (2009).
- Kereiakes DJ. Adjunctive pharmacotherapy before percutaneous coronary intervention in non-ST-elevation acute coronary syndromes: the role of modulating inflammation. Circulation 108, 22–27 (2003).
- Chew DP, Bhatt DL, Robbins MA et al. Effect of clopidogrel added to aspirin before percutaneous coronary intervention on the risk associated with C-reactive protein. Am. J. Cardiol. 88, 672–674 (2001).
- Vivekananthan DP, Bhatt DL, Chew DP et al. Effect of clopidogrel pretreatment on periprocedural rise in C-reactive protein after percutaneous coronary intervention. Am. J. Cardiol. 94, 358–360 (2004).
- Quinn MJ, Bhatt DL, Zidar F et al. Effect of clopidogrel pretreatment on inflammatory marker expression in patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 93, 679–684 (2004).
- Lefkovits J, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N. Engl. J. Med. 332, 1553–1559 (1995).
- Lincoff AM, Califf RM, Topol EJ. Platelet glycoprotein IIb/ IIIa receptor blockade in coronary artery disease. J. Am. Coll. Cardiol. 35, 1103–1115 (2000).
- Tam SH, Sassoli PM, Jordan RE, Nakada MT. Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and a(v)b3 integrins. Circulation 98, 1085–1091 (1998).
- Simon DI, Xu H, Ortlepp S, Rogers C, Rao NK. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arterioscler. Thromb. Vasc. Biol. 17, 528–535 (1997).
- Palmerini T, Nedelman MA, Scudder LE et al. Effects of abciximab on the acute pathology of blood vessels after arterial stenting in nonhuman primates. J. Am. Coll. Cardiol. 40, 360–366 (2002).
- Thompson RD, Wakelin MW, Larbi KY et al. Divergent effects of platelet-endothelial cell adhesion molecule-1 and beta 3 integrin blockade on leukocyte transmigration in vivo. J. Immunol. 165, 426–434 (2000).
•• Key article for human pathology of neoatherosclerosis.
• First human pathology study comparing neoatherosclerosis characteristics of first-generation and second-generation drug-eluting stents.
• First angioscopic evidence of neoatherosclerosis.
• Evidence of neoatherosclerosis using optical coherence tomography.