Review Article - Diabetes Management (2017) Volume 7, Issue 6
Management of CV risk in T2DM: The beginning of a new era
- *Corresponding Author:
- Muhammad Abdul-Ghani
Division of Diabetes
University of Texas Health Science Center at San Antonio, USA
E-mail: abdulghani@uthscsa.edu
Abstract
Keywords
type 2 diabetes, antidiabetes drugs, cardiovascular risk, microvascular complications
Introduction
Individuals with type 2 diabetes mellitus (T2DM) experience increased morbidity and mortality secondary to vascular damage involving both the microvasculature [retinopathy, nephropathy, neuropathy] and macrovasculature (myocardial infarction, stroke, amputation) [1]. Diabetes is the leading cause of blindness and end stage renal disease in western countries [2], and T2DM individuals have a 2.5-4 fold increase in the risk of cardiovascular disease [3]. Cross sectional studies have demonstrated that one-third to one-half of all people with diabetes have evidence for organ damage [4]. Although not everyone with diabetes is destined to develop complications, a recent epidemiological study [4] reported that two or more complications are apparent in almost one-fifth of people with diabetes.
Diabetes and Microvascular Complications
Two landmark studies, the Diabetes Control and Complications Trial (DCCT) [5] and United Kingdom Prospective Diabetes Study (UKPDS) [6], as well as others [7-10], unequivocally have documented that improved glycemic control in T2DM subjects reduces the risk of microvascular complications, demonstrating that chronic exposure of tissues to hyperglycemia triggers pathologic processes that lead to eye, kidney and nerve damage. A 1% decrease in HbA1C has been shown to reduce the incidence of microvascular complications by ~35% [5,6,9] and maintaining the HbA1C <6% has been reported to completely prevent the development of retinopathy in T2DM subjects [8]. Further, in the DCCT continuation study (EDIC), subjects who initially were treated aggressively with intensive insulin therapy and achieved a HbA1C <7% maintained a lower risk for microvascular complications many years later, even though their HbA1C increased to 8% compared to subjects who initially were less well controlled [11]. Similar results have been reported in the long term extension of UKPDS [12]. Insulin was utilized to achieve intensive glycemic control in the DCCT and insulin, sulfonylurea and merformin were utilized in the UKPDS to lower the plasma glucose concentration. Conversely, improved glycemic control in T2DM patients with long-standing poor glycemic control has less of an impact to reduce the risk of microvascular complications [13]. More recent studies have demonstrated that newer antidiabetic agents, e.g. GLP-1 receptor agonists (GLP-1 RAs), SGLT2 inhibitors, and pioglitazone, which are very effective in lowering the plasma glucose concentration, also reduce the risk of diabetic microvascular complications. Collectively, these observations emphasize the importance of achieving good glycemic control at the time of diagnosis [14] and maintaining the level of glycemic control, independent of therapeutic strategy utilized, to reduce the risk of microvascular complications. The results of these studies have prompted the professional health care organizations like American Diabetes Association [15], American College of Physicians [16], American Association for Clinical Endocrinology (AACE) [17], the European Association for the Study of Diabetes [15] and the Canadian Diabetes Association [18] to recommend glycemic goals as close to normal as possible while avoiding hypoglycemia in newly diagnosed T2DM patients without clinically manifest vascular disease. The ADA’s target goal for HbA1C is ≤ 7.0%, while the AACE’s and the EASD’s goal is ≤ 6.5%.
Macrovascular Complications in T2DM
Subjects with T2DM have a markedly increased risk of cardiovascular disease [myocardial infarction and stroke] and a worse prognosis following any cardiovascular event [3,19]. Further, T2DM increases the risk of heart failure in the absence of ischemic heart disease, and the presence of T2DM worsens the prognosis in patients with heart failure [20]. Although hyperglycemia is an important risk factor for microvascular complications, improved glycemic control only modestly reduces or has no effect to reduce the increased risk of cardiovascular events [6,13,21,22]. UKPDS demonstrated that hyperglycemia per se has a less prominent role in the development of macrovascular complications compared to microvascular complications [6]. Further, in UKPDS [23] and VADT [24] it took more than 10 years to observe a modest CV benefit following improved glycemic control. Most T2DM individuals manifest moderate - severe insulin resistance which is associated with multiple metabolic abnormalities (obesity, dyslipidemia, hypertension, endothelial dysfunction, procoagulant state), all of which are important risk factors of CVD [25]. This cluster of cardiovascular/metabolic disturbances is known as the Insulin Resistance (Metabolic) Syndrome and likely contributes to the increased CVD risk in T2DM. Hypertension [26,27] and dyslipidemia [28] are major risk factors for coronary artery disease and many studies have documented that reduction of blood pressure and correction of dyslipidemia significantly reduces CVD. Nonetheless, despite reduction of blood pressure [27,28] and plasma LDL cholesterol to target levels [28], cardiovascular risk in T2DM subjects remains greater than in nondiabetic subjects [29,30]. Many recent studies [31-39] have demonstrated that insulin resistance, independent of the associated metabolic abnormalities, is an important risk factor for cardiovascular disease. Although hyperglycemia is only a week risk factor for CVD in T2DM patients, surprisingly, recent clinical outcome trials [37-43] have demonstrated that members of SGLT2 inhibitor class, GLP-1 receptor agonists (GLP-1 RA) and pioglitazone significantly reduce CVD risk in T2DM patients in whom traditional CV risk factors (e.g. blood pressure, dyslipidemia) are optimally controlled. In the following discussion, we will briefly review the results of these studies and discuss the implications of these findings for the care of patients with T2DM.
Insulin Sensitizers and CVD
Because insulin resistance is closely linked with CVD risk in T2DM, it follows that improving insulin sensitivity with an insulin sensitizers would reduce CV risk, independent of their glucose lowering action. Pioglitazone is the only true insulin sensitizer available for treatment of T2DM [44-46]. In addition to lowering plasma glucose concentration, pioglitazone decreases insulin resistance (by 35-40%) in skeletal muscle and liver [47] decreases plasma triglyceride concentration, increases HDL cholesterol converts small dense atherogenic LDL particles to larger more buoyant ones, and reduces blood pressure [48,49], Pioglitazone also reduces plasma FFA, adipocytokines/other inflammatory markers/procoagulant factors, and increases plasma adiponectin [44-48], all of which would be expected to provide cardiovascular benefit. Thus, pioglitazone would be expected to provide additional cardiovascular benefits, independent of the reduction in plasma glucose concentration [50,51]. Consistent with this, pioglitazone has ben shown to slow the progression in carotid intima medial thickness in the Chicago [52] and ACT NOW studies [53], and to reduce coronary atheromna volume [54] in subjects with type 2 diabetes in the Periscope study. In large clinical outcome studies, pioglitazone significantly lowered the incidence of 3-point MACE [non-fatal myocardial infarction, non-fatal stroke, CV death] in T2DM patients. In PROactive [41], 5238 T2DM patients with existing CVD were treated for 2.9 years with pioglitazone or placebo plus standard of care for glycemic control and CV risk factors. 3-point MACE, the main secondary endpoint, was significantly reduced by 16% (HR=0.84, p=0.027). In IRIS [40], 3876 insulin resistant (HOMA-IR >3.0), nondiabetic, insulin resistant individuals with recent [within 6 months] ischemic stroke or TIA were randomized to pioglitazone or placebo for 4.8 years. Pioglitazone caused a 24% reduction in fatal/nonfatal stroke plus myocardial infarction (HR=0.76, P=0.007). Because glycemic control in subjects in the placebo arm in the PROActive study were treated to target and participants in IRIS study did not have T2DM, the results of these studies demonstrate that pioglitazone reduced CVD risk independent of its glucose lowering action.
GLP-1 RAs and CVD risk in T2DM
Multiple mechanisms contribute to the glucose lowering action of GLP-1 RA, e.g. enhanced beta cell function, inhibition of plasma glucagon concentration, delayed gastric emptying, and suppression of hepatic glucose production GLP-1 RAs also suppress appetite, resulting in significant weight loss and, indirectly, improving insulin sensitivity [55]. GLP-1 RAs also decrease plasma triglyceride and increases plasma HDL concentrations and reduce blood pressure [56]. These later actions of GLP-1 RA would be expected to have a favorable effect to reduce cardiovascular risk [56]. Three recent large outcome trials, LEADER [37] SUSTAIN [38] and EXSCEL [43] have examined the effect of once daily liraglutide, once weekly semaglutide and once weekly exenatide, respectively on cardiovascular risk (3-point MACE: non-fatal myocardial infarction, non-fatal stroke and CV death) in T2DM patients. All 3 studies primarily recruited T2DM patients with established CVD. In LEADER [37], 9340 T2DM patients (82% with prior CV event) were randomized to liraglutide, 1.8 mg/day, or placebo for a mean of 3.8 years. Investigators were blinded to the study intervention and instructed to maintain HbA1c <7.0% with any antidiabetic medication except GLP-1 RA or DPP4 inhibitor. Compared to placebo, liraglutide caused a 13% reduction in 3-point MACE. In SUSTAIN [38] 3297 T2DM patients (83% with established CVD) were randomized to semaglutide, 0.5 and 1 mg/day, or placebo and followed for 2 years. Similar to LEADER, investigators were instructed to lower HbA1c to <7.0% according to local guidelines without using incretin-based therapies.
Semaglutide caused a 26% reduction in the primary outcome (3-point MACE) which was driven by a 39% reduction in stroke (p=0.04) and 26% reduction in nonfatal MI (p=0.12). Of note, the decrease in body weight, HbA1c and blood pressure was greater in SUSTAIN compared to LEADER. In EXSCEL [43], 14,752 T2DM patients (73% with established CVD) were randomized to exenatide (2 mg/week) or placebo and followed for 3.2 years. Similar to SUSTAIN and LEADER, investigators were instructed to treat HbA1c to target (<7.0%). At the end of the study, there was a small but significant difference in body weight, HbA1c and blood pressure between exenatide-treated and placebo-treated subjects. Exenatide caused a 9% reduction in the primary outcome (3-point MACE) which fell just short of statistical significant (p=0.06) but discontinuation [~25%] of exenatide was very high in the EXSCEL study. Two aspects of LEADER, SUSTAIN and ESXCEL deserve emphasis: (i) patients at higher CVD risk benefited more from GLP-1 RA treatment. In a combined analysis of the three long acting GLP-1 RAs, the risk of CVD in subjects with T2DM and established CVD (secondary CV prevention) was significantly reduced (by 13%) in subjects receiving GLP-1 RA (liraglutide, semaglutide and exenatide) (HR=0.87, 95% CI=0.81-0.93, p=0.001). Conversely, subjects with T2DM without established CVD (primary CVD prevention) did not benefit from GLP-1 therapy (HR=1.07, 95% CI=0.88-1.3, p=0.49). (ii) the benefit of liraglutide, semaglutide and exenatide was evident on top of optimal control of traditional CV risk factors [37,38,43].
SGLT2 Inhibitors and CVD risk
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have a unique mechanism of action [57]. They lower the plasma glucose concentration by inhibiting renal glucose reuptake and produce glucosuria. This unique mechanism of action, in addition to lowering plasma glucose concentration, results in multiple other metabolic and hemodynamic actions which could favorably benefit CVD risk in T2DM patients [57-61]. SGLT2 inhibitors cause (i) weight loss of 2-3 kg; (ii)reduction of ~5 mmHg in systolic blood pressure; (iii) osmotic diuresis which results is a modest decrease in extracellular volume of ~ 5-10%; (iv) increase in total body fat oxidation and ketone production; (v) a small increase in plasma HDL cholesterol and decrease in plasma triglycerides; (vi) improvement in total body insulin sensitivity (17%). Because these metabolic actions of SGLT2i could improve CVD risk, one might expect that this class of drugs would lower CVD risk in T2DM, independent of their glucose lowering effect. Two large clinical outcome studies have examined the effect of empagliflozin and canagliflozin on 3-point MACE in T2DM patients. In EMPA REG OUTCOME trial [39], empagliflozin caused a 14% reduction (P=0.04) in 3-point MACE in 7020 T2DM patients with established cardiovascular disease over 3.1 years and this was driven by a robust 38% reduction in CV mortality. Further, the reduction in 3-ponit MACE was accompanied by a 39% reduction in the rate of hospitalization for heart failure. One surprising finding in EMPA-REG, unlike LEADER, SUSTAIN, EXSCEL, IRIS and PROactive, separation between the empagliflozin and placebo curves occurred very early, such that reduction in the primary outcome was evident at 3 months after starting treatment. In CANVAS/ CANVAS-R [42], 10,142 T2DM patients (66% with established CVD) were randomized to receive canagliflozin or placebo for 3.6 years. The reduction in 3-point MACE caused by canaglifloizn was identical to that observed with empagliflozin (14%, p<0.001). Similar to EMPA-REG, the separation between the two curves occurred early after starting therapy and was accompanied by a 33% reduction in the rate of hospitalization for heart failure. Collectively, the results of CANVAS and EMPA-REG OUTCOME studies suggest that: (i) it is likely that the CV benefits of canagliflozin and empagliflozin are class effect; (ii) although the mechanisms underlying the CV benefits of both drugs are not completely understood [62], it is likely their mechanism of action to reduce MACE is not due to inhibition of atherosclerosis. (iii) the rapid onset of CV benefit, and the marked reduction in hospitalization for heart failure suggest that the hemodynamic actions of SGLT2 inhibitors play an important role in their CV protective effect; (iv) similar to the LEADER, SUSTAIN, EXSCEL, IRIS and PROactive studies, only subjects with established CVD benefited from the treatment, while T2DM without existing CVD did not benefit from SGLT2 inhibitor therapy; (v) similar to GLP-1 RAs and pioglitazone, the CV benefit of SGLT2 inhibitors was observed in subjects with optimal treatment of traditional CVD risk factors, e.g. blood pressure, LDL and aspirin treatment.
Implication or Care
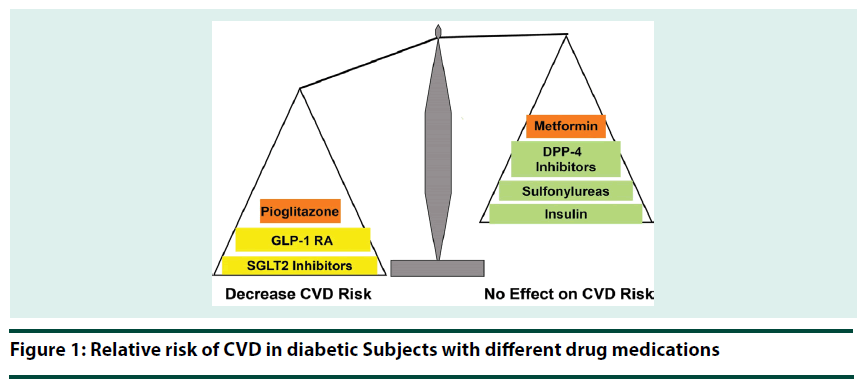
Overwhelming evidence supports that early glycemic control, regardless of the therapeutic strategy utilized, reduces the risk of microvascular complication in T2DM patients. However, the results of recent clinical outcome trials demonstrate that antidiabetic agents vary in their effect on CVD risk in T2DM (Figure 1) and we are entering a new era in T2DM management [63]. Despite improved glycemic control, DPP-4 inhibitors and basal insulin failed to reduce CV risk in T2DM patients [64-67], while GLP-1 RAs, SGLT2 inhibitors, and pioglitazone significantly reduce both plasma glucose concentration and CV risk (3-point MACE). Further, the CV benefit of GLP-1 RAs, SGLT2 inhibitors and pioglitazone is independent of their glucose lowering effect and occurs on top of optimal treatment of traditional CV risk factors. Further, the beneficial CV action of GLP-1 RAs, SGLT2 inhibitors and pioglitazone was evident only in T2DM patients with established CVD. Thus, GLP-1 RA, SGLT2 inhibitors and pioglitazone, not other antihyperglycemic agents, will reduce both microvascular and macrovascular complications in this group of T2DM patients. Therefore, evidence-based medicine dictates that in newly diagnosed T2DM patients with established CVD and in long-standing T2DM patients who experience a CV event, GLP-1 RAs, SGLT2 inhibitors and pioglitazone should be favored over other antidiabetic agents. On the other hand, in T2DM patients without established CVD, emphasize should be placed on lowering the HbA1c to target regardless of the antihyperglycemic strategy utilized.
References
- Schalkwijk C, Stehouwer C. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin. Sci. 109(2), 143–159 (2005).
- He Z, King G. Microvascular complications of diabetes. Endocrinol. Metab. Clin. North. Am. 33(1), 215–238 (2004).
- Haffner S, Lehto S, Ronnemaa T et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339(4), 229–234 (1998).
- Morgan C, Currie C, Stott N et al. The prevalence of multiple diabetes-related complications. Diabet. Med. 17, 146–151 (2000).
- The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
- UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 352(9131), 837–853 (1998).
- Nakagami T, Kawahara R, Hori S et al. Glycemic control and prevention of retinopathy in Japanese NIDDM patients. A 10-year follow-up study. Diabetes. Care. 20(4), 621–622 (1997).
- Klein R, Klein B, Moss S et al. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann. Intern. Med. 124, 90–96 (1996).
- Ohkubo Y, Kishikawa H, Araki E et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diab. Res. Clin. Pract. 28(2), 103–117 (1995).
- Nathan D, Cleary P, Backlund J et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 353(25), 2643–2653 (2005).
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes and Complications Research Group: Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N. Engl. J. Med. 342, 381–389 (2000).
- Holman R, Paul S, Bethel M et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
- Duckworth W, Abraira C, Moritz T et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
- Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia. 52(7), 1219–1226 (2009).
- Nathan D, Buse J, Davidson M et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes. Care. 32(1), 193–203 (2009).
- Qaseem A, Vijan S, Snow V et al. Glycemic Control and Type 2 Diabetes Mellitus: The Optimal Hemoglobin A1c Targets. A Guidance Statement from the American College of Physicians. Ann. Int. Med. 147(6), 417–422 (2007).
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr. Pract. 13(1), 1–68 (2007).
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee, Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes. 32(1), S1-S201 (2008).
- Mazzone T. Reducing cardiovascular disease in patients with diabetes mellitus. Curr Opin Cardiol. 20, 245–249 (2005).
- Dauriz M, Mantovani A, Bonapace S et al. Prognostic Impact of Diabetes on Long-term Survival Outcomes in Patients With HeartFailure: A Meta-analysis. Diabetes. Care. 40(11), 1597–1605 (2017).
- Gerstein H, Miller M, Byington R et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358(24), 2545–2559 (2008).
- Patel A, MacMahon S, Chalmers J et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358(24), 2560–2572 (2008).
- Holman R, Paul S, Bethel M et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008).
- Hayward R, Reaven P, Wiitala W et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 997–978 (2015).
- Reaven G. Role of insulin resistance in human disease. Diabetes. 37(12), 1595–1607 (1988).
- Stratton I, Adler A, Neil H et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 321, 412–419 (2000).
- Turnbull F, Neal B, Algert C et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch. Intern. Med. 165(12), 1410–1419 (2005).
- Costa J, David C, Carneiro A et al. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials BMJ. 332, 1115–1124 (2006).
- Rawshani A, Rawshani A, Franzén S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 376, 1407–1418 (2017).
- Colhoun H, Betteridge D, Durrington P et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 364(9435), 685–696 (2004).
- Haffner S, D Agostino R, Mykkänen L et al. Insulin sensitivity in subjects with type 2 diabetes. Relationship to cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 22, 562–568 (1999).
- Bonora E, Formentini G, Calcaterra F et al. HOMA-estimated insulin resistance is an independent preditor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes. Care. 25(7), 1135–1141 (2002).
- Isomaa B, Almgren P, Tuomi B. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes. Care. 24(4), 683–689 (2001).
- Bonora E, Kiechl S, Willeit J et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in Caucasian subjects from the general population: the Bruneck study. Diabetes. Care. 30, 318–324 (2007).
- Hanley A, Williams K, Stern M et al. Homestatis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes. Care. 25(7), 1177–1184 (2002).
- Rutter M, Meigs J, Sullivan L et al. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes 54(11), 3252–3257 (2005).
- Marso S, Daniels G, Brown-Frandsen K et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 375, 311-322 (2016).
- Marso S, Bain S, Consoli A, Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
- Zinman B, Wanner C, Lachin J et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 373(22), 2117–2128 (2015).
- Kernan W, Viscoli C, Furie K et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N. Engl. J. Med. 374(14), 1321–1331 (2016).
- Dormandy J, Charbonnel B, Eckland D et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitazone Clinical Trial in macrovascular Events): a randomized controlled trial. Lancet. 366(9493), 1279–1289 (2005).
- Neal B, Perkovic V, Mahaffey K. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 377, 644–657 (2017).
- Holman R, Bethel M, Mentz R Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 377, 1228–1239 (2017).
- Yki-Järvinen H. Thiazolidinediones. N. Engl. J. Med. 351(11), 1106-1118 (2004).
- Mudaliar S, Henry R. New oral therapies for type 2 diabetes mellitus: the glitazones or insulin sensitizers. Annu. Rev. Med. 52, 239–257 (2001).
- Saltiel AR, Olefsky JM. Thiazolidinediiones in the treatment of insulin resistance in type 2 diabetes. Diabetes 45(12), 1661–1669 (1996).
- Miyazaki Y, Glass L, Triplitt C et al. Effect of rosiglitazone on glucose and free fatty acid metabolism in type 2 diabetic patients. Diabetologia. 44(12), 2210–2219 (2001).
- Miyazaki Y, Matsuda M, DeFronzo R. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes mellitus. Diabetes. Care. 25(3), 517–523 (2002).
- Goldberg R, Kendall D, Deeg M et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes. Care. 28(7), 1547–1554 (2005).
- Blaschke F, Spanheimer R, Khan M et al. Vascular effects of TZDs: new implications. Vascul Pharmacol. 45, 3–18 (2006).
- Satoh N, Ogawa Y, Usui T et al. Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabetes. Care. 26(9), 2493–2499 (2003).
- Mazzone T, Meyer P, Feinstein S et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 296(21), 2572–2581(2006).
- DeFronzo R, Tripathy D, Schwenke D et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364, 1104–1115 (2011).
- Nissen S, Nicholls S, Wolski K et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 299(13), 1561–1573 (2008).
- Drucker D, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu. Rev. Physiol. 76, 561–583 (2014).
- Nauck M, Meier J, Cavender M et al. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 136(9), 849–870 (2017).
- Abdul-Ghani M, Norton L, DeFronzo RA. Renal sodium-glucose co-transport in the management of type 2 diabetes mellitus. Am. J. Physiol. 309(11), F889–900 (2015).
- Daniele G, Xiong J, Merovci A et al. Dapagliflozin enhances fat oxidation and ketone production in type 2 diabetes patients. Diabetes Care 39(11), 2036–2041 (2016).
- Ferrannini E. Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology. Cell. Metab. 26(1), 27–38 (2017).
- Oliva R, Bakris G. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 8(5), 330–339 (2014).
- Merovci A, Solis-Herrera C, Daniele G et al. Dapagliflozin improves muscle insulin sensitivity but enhances glucose production. J. Clin. Invest. 124(2), 509–514 (2014).
- Abdul-Ghani M, Del Prato S, Chilton R SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME Trial. Diabetes. Care. 39(5), 717–725 (2016).
- Abdul-Ghani M, DeFronzo R, Del Prato S et al. Cardiovascular disease and T2DM: has the dawn of a new era arrived? Diabetes Care. 40(7), 813–820 (2017).
- Gerstein H, Bosch J, Dagenais G et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 367, 319–328 (2012).
- Green J, Bethel M, Armstrong P et al. TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 373, 232–242 (2015).
- Scirica B, Bhatt D, Braunwald E et al. Savor-Timi 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013).
- White W, Cannon C, Heller S et al. EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013).