Research Article - Journal of Experimental Stroke & Translational Medicine (2011) Volume 4, Issue 1
Isoflurane anesthesia alters the brains electroencephalograhical response to ischemia: EEG parameters associated with high-grade CA1 neuronal injury following global cerebral ischemia in the rat
- *Corresponding Author:
- Li-Xian Li MD
Department of Neurosurgery, The First Clinical College of Harbin Medical University
Heilongjiang, China.
Fax: 86 45153670428.
Email address: llxzhdy@sina.com (L. Li).
Abstract
This study reports our re-assessment of the rat 2-vessel occlusion plus hypotension model using isoflurane (in N2O/O2). To identify the animals that developed high-grade CA1 injury (<10% neuronal survival) following 8-minutes carotid occlusion plus hypotension of 37 mmHg, we observed electroencephalogram (EEG) recordings and CA1 neuronal survival rates of 60 ischemic rats. In addition, in a neuroprotective trial, we treated ischemic rats with mild hypothermia (35°C/24h) and magnesium (IV: loading dose 360 μmol/kg; 48h infusion 120 μmol/kg/h) commencing 2 hours post-ischemia. Assessed 7 days after ischemia, the mean percentage of CA1 neuronal survival rate of the 60 rats was 12.6 ± 15.2% (mean ± SD). EEG recovery times of >14 minutes and ≤12 minutes consistently resulted in CA1 neuronal survival rates of <10% and >10%, respectively. Animals that recorded an isoelectric EEG and recovered their EEG activity between >12 minutes to 14 minutes post-ischemia, also developed high-grade CA1 injury; the reliability of these parameters was confirmed in a prospec-tive trial. Finally, hypothermia/magnesium treatment increased CA1 neuronal survival from 7.9 ± 0.9% to 55.3 ± 11.6%. By classifying EEG patterns during and post-ischemia we can identify (by surgery completion) animals that will develop high-grade CA1 injury. We also demonstrated that neuroprotection was achievable in our model.
Keywords
Isoflurane, EEG, Global cerebral ischemia, 2-vessel occlusion, CA1 neurons
Introduction
The 2-vessel occlusion (2VO) model of rat global cerebral ischemia produces near-complete forebrain ischemia, which results in reproducible and readily-quantifiable damage in selectively vulnerable brain regions such as caudoputamen, neocortex and the CA1 pyramidal neurons of the hippocampus (Small and Buchan, 2000). The model, which utilises transi-ent bilateral occlusion of the common carotids com-bined with systemic hypotension, is relatively straight-forward to perform and has long been a valuable tool for conducting physiological, molecular, biochemical and neuroprotective studies.
While anesthetic regimens vary between laboratories, among those that use inhalant anesthesia, halothane has been (and remains) the most common agent used for this model. However, with the introduction of newer and safer halogenated anesthetics such as isoflurane, sevoflurane and desflurane (Ginsberg and Busto, 1989; Murrell et al., 2008), the demand for halothane has decreased to the extent that halothane has not been available in Australia since 2008. In the Australian veterinary market, the natural replacement for halothane has been isoflurane; for this reason, and because this agent is commonly used in rat focal cerebral ischemia models, we have also adopted isoflurane in our laboratory as the anesthetic agent for the 2VO rat model.
Following this change, however, it emerged that our existing surgical protocol, namely using a blood pres-sure level of 45 mmHg and the timing of ischemia from onset of an isoelectric EEG during carotid oc-clusion, did not directly translate to the use of isoflu-rane. In particular, we found that our protocol result-ed in unacceptably high variability in our standard outcome measure, the survival rate of hippocampal CA1 neurons. In this report, we describe an observa-tional study that was conducted to establish the use of a blood pressure level of 37 mmHg and EEG pa-rameters that would dispose to the success of the model. Also, we report a short prospective study to confirm the conclusions drawn from the observational study, and a short treatment study to demonstrate the feasibility of neuroprotective strategies in the adapted model.
Material and Methods
This study was approved by the Animal Ethics Com-mittee of the University of Western Australia and conducted according to guidelines established for the use of animals in experimental research as outlined by the Australian National Health and Medical Re-search Council.
Two-vessel occlusion model
Adult male Sprague-Dawley rats aged 8-10 weeks were fasted overnight but allowed water ad libitum. Anesthesia was induced with 4% isoflurane/27% O2/balance N2O. The animals were intubated, para-lysed with pancuronium (0.02mg IV bolus) and venti-lated on a rodent ventilator (Ugo, Basile, Italy). Dur-ing pre- and post-ischemic surgical manipulations, isoflurane was maintained at 2.0% (Note: 4% isoflu-rane produces an isoelectric EEG, while 2% isoflu-rane results in burst suppression). For continuous monitoring of mean arterial blood pressure and blood sampling during ischemia, the tail artery was cathe-terized with polyethylene tubing (OD 0.8mm x ID 0.4mm; Biocorp, Melbourne, Australia) filled with heparinized saline (15 Units/ml). Via a ventral mid-line neck incision, the common carotid arteries were exposed and silk thread was placed loosely around the isolated arteries, in readiness for subsequent clipping. For induction of hemorrhagic hypotension during ischemia, the right internal jugular vein was exposed and a length of PVC tubing (OD 1mm x ID 0.5mm; Biocorp), primed with heparinized saline, was inserted into the vein. Both cranial and rectal tem-peratures were measured via a thermocouple (Phy-sitemp, New Jersey, USA), and were maintained at 37.5 ± 0.2°C during ischemia and 37.5 ± 0.5°C before and after ischemia with a heating fan and pad.
A bipolar EEG was recorded using two active lateral subdermal needle electrodes (right and left frontopa-rietal) and a reference central electrode, which were interfaced with a bioamplifier (AD Instruments, Mel-bourne, Australia). The cutoff frequencies for EEG recordings were set at 0.3 and 10 Hz for the high-pass and low-pass filters respectively. These fre-quencies were selected to minimise electrical inter-ference while allowing detection of delta and theta waves. As an additional benefit, these cut-offs re-duced the data sampling rate and thus also mini-mised data storage requirements.
EEG and arterial blood pressure were recorded with a PowerLab data acquisition system (LabChart; AD Instruments). Plasma glucose levels were measured with a blood glucose meter (Miles Laboratories, Indi-ana, USA). Ten minutes before the ischemic insult, PaCO2, PaO2, and pH were measured with a pH/blood gas analyzer (ABL5 Radiometer, Copenha-gen, Denmark). PaCO2 was maintained between 35 - 45 mmHg, PaO2 between 90 - 120 mmHg and pH between 7.35 and 7.45 by adjusting ventilation.
Before ischemia induction, isoflurane was reduced to 1% to allow EEG recovery from burst suppression (usually 4 - 5 min). Ischemia was subsequently in-duced by withdrawing venous blood into a syringe containing 0.5 ml heparinized saline and then occlud-ing the common carotids using aneurysm clips. Mean arterial blood pressure was reduced to 37 ± 2 mmHg (hereafter also referred to as 37 mmHg) and maintained for the ischemic period. The 8-minute ischemic period was deemed to have begun from the time that target blood pressure was obtained. After the 8-minute period, the aneurysm clips were re-moved and the blood, which had been maintained at 37°C, was reinfused. EEG recordings were contin-ued post-ischemia and isoflurane was increased to 2% after the re-appearance of burst suppression spikes. Blood gases and glucose measurements were then repeated. Arterial and venous lines were removed and the wounds were sutured. On return of spontaneous respiration, isoflurane was discontinued, animals were extubated, allowed to recover and re-turned to their cages with free access to food and water in a 25°C temperature controlled holding room. Sham-operated animals received identical anesthesia and surgery as experimental animals but were not rendered ischemic.
Sixty rats were used in the study to collect the EEG data that were used to set up the EEG parameters to predict outcomes, 18 rats were used in the prospec-tive trial to verify the EEG parameters and 6 rats were used as sham-operated animals.
Neuroprotective treatment trial
Once we had established a satisfactory predictive model (see Results and Discussion), we sought to confirm that neuroprotection could be achieved using the altered protocol. We conducted a trial using a mild hypothermia/magnesium treatment regimen we have previously shown to be effective in our laborato-ry. For full details of the treatment protocol, see Zhu et al. (2005). Briefly, rats were implanted with an in-traperitoneal probe which sent temperature data to a control system that maintained them mildly hypo-thermic (at 35°C) for 24 hours. Hypothermic rats also received a 48 hour intravenous infusion of magnesi-um sulphate (starting 1h:40 min post-ischemia - load-ing dose: 360 μmol/kg; infusion: 120 μmol/kg/h). A 20-minute cooling period commenced 100 minutes after ischemia, so that the rats were at the target temperature by 2 hours after ischemia. Normother-mic control animals were held in the 25°C holding room. Six rats were used in this trial. Physiological variables were recorded and remained within normal ranges for the different experimental groups.
Histological and statistical analysis
All rats were euthanised 7 days after surgery with pentobarbital (100 mg/kg IP) and transcardially per-fused with 200 ml of normal saline followed by 200 ml of 10% neutral buffered formalin. The brains were removed and post-fixed for 1 week before being par-affin embedded. Brains were sectioned in 5 μm cor-onal slices at bregma section -3.8 mm according to a standard rat brain atlas (Paxinos and Watson, 1998) and stained with cresyl violet. The number of normal-appearing pyramidal neurons per high-power field (400X) in 1000 μm segments in the medial, interme-diate and lateral sections of the left and right hippo-campal CA1 regions were counted and a neuronal count for the CA1 region for each animal was ob-tained (Zhu et al., 2004). Neuronal survival rate in the hippocampal CA1 region was expressed as a percentage of the mean neuronal cell count obtained in sham animals, which was taken as 100%. Neu-ronal cell counts were performed by an operator blinded to EEG observations and whenever possible to blood pressure experimental groups. Histological data were analysed by ANOVA, followed by post hoc Bonferroni/Dunn.
Results
Physiological variables
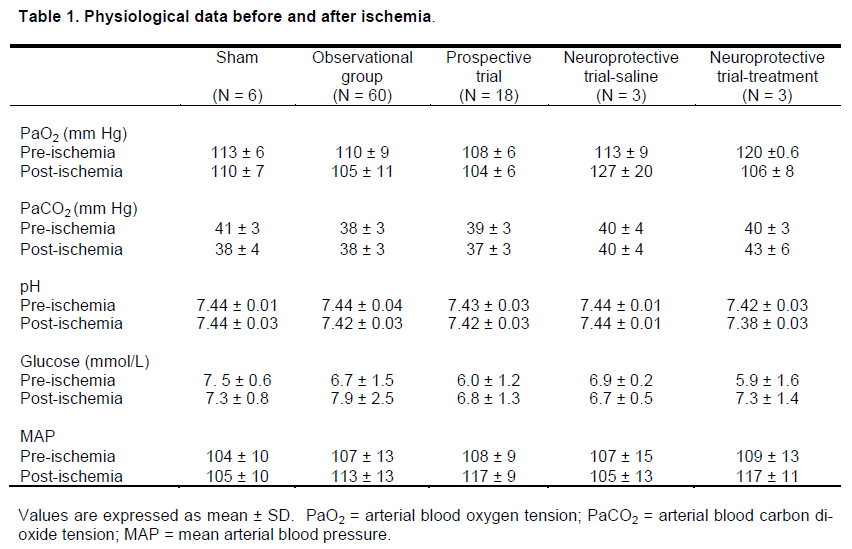
Excluding mean arterial blood pressure, which was controlled at 37 ± 2 mmHg during the 8-minutes of ischemia, all other physiological variables remained within normal ranges for the different experimental groups (Table 1).
EEG findings
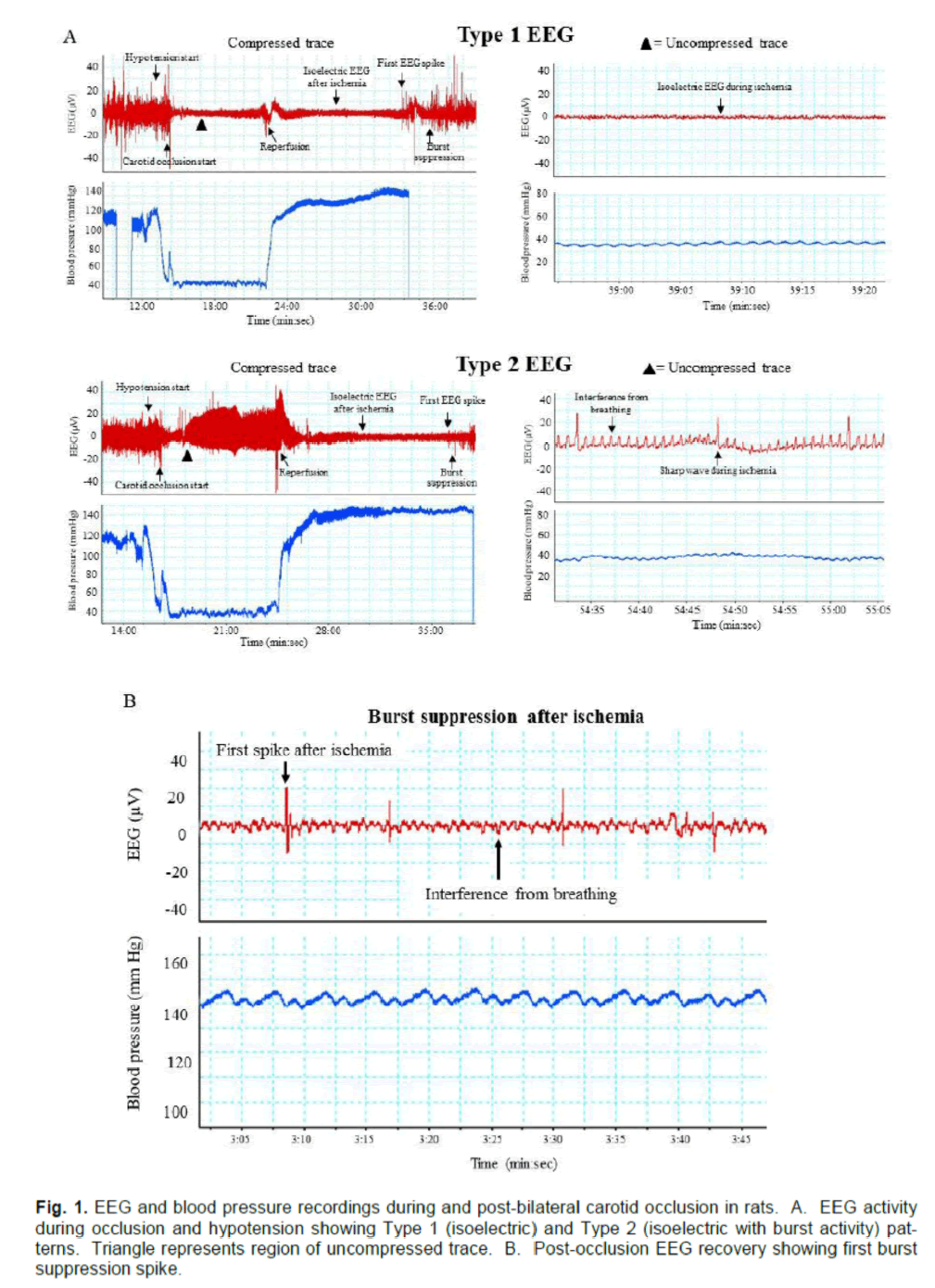
As expected, all animals displayed changes in EEG activity during ischemia compared with pre-ischemia recordings. However, not all animals displayed the clean isoelectric EEG that is ordinarily associated with ischemic brain quiescence. Instead, we found that ischemic EEGs could be categorised as one of two types. The first, which we refer to as Type 1,consisted of a typical isoelectric EEG (with only inter-ference activity of <20 μV), while the second type (Type 2) displayed an isoelectric EEG, but with regu-lar spikes of electrical activity >20 μV (Fig. 1A). Note that these EEG types were seen only during the 8-minute period of hypotension and carotid occlusion; after reperfusion, all animals went on to display an isoelectric EEG.
The time elapsed from start of reperfusion to the re-covery of EEG activity was also recorded. Note here that recovery of EEG activity is defined as occurring at the appearance of the first burst suppression spike (Figure. 1B), and does not imply recovery of normal pre-ischemic EEG.
Figure 1. EEG and blood pressure recordings during and post-bilateral carotid occlusion in rats. A. EEG activity during occlusion and hypotension showing Type 1 (isoelectric) and Type 2 (isoelectric with burst activity) pat-terns. Triangle represents region of uncompressed trace. B. Post-occlusion EEG recovery showing first burst suppression spike.
EEG recovery and CA1 neuronal injury
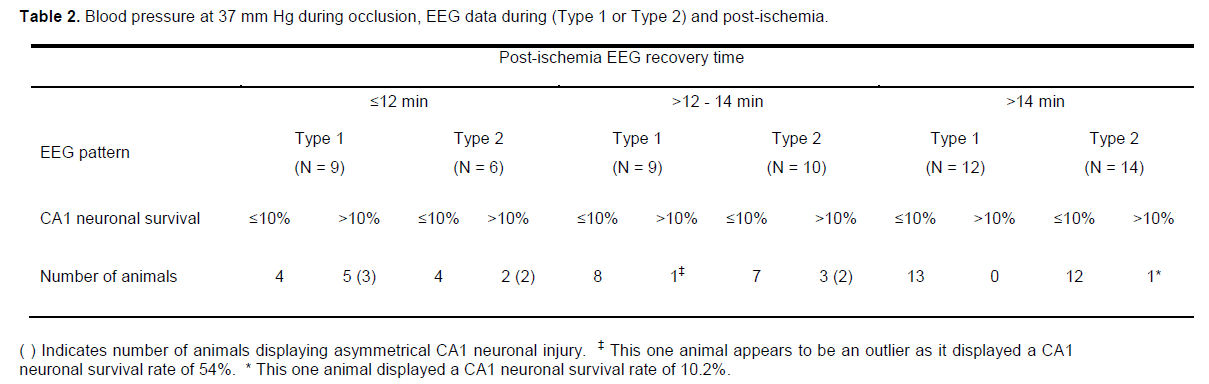
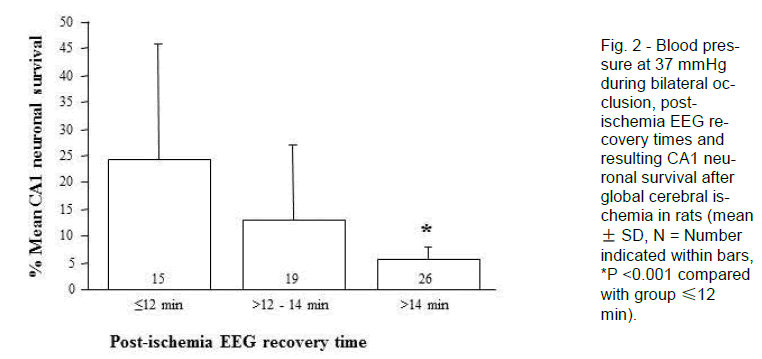
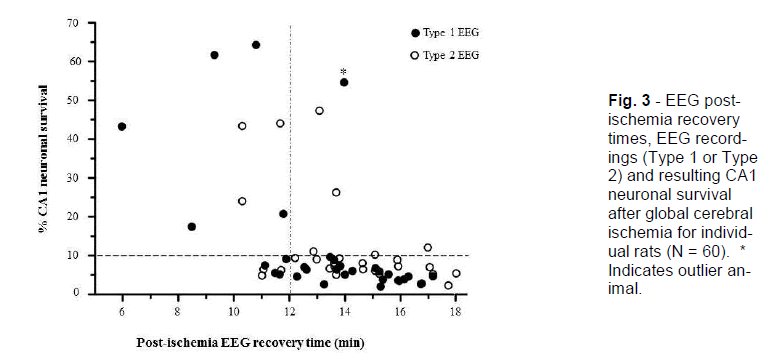
To identify patterns in the data, we stratified the results by post-hoc analysis of recovery of EEG activity and by CA1 neuronal survival dichotomised to <10% or >10%, this level being chosen as representing a reasonable cut-off for a high-grade ischemic injury (Table 2; Figure. 2 and 3). The CA1 neuronal survival rate for the 60 rats was 12.6 ± 15.2%. The majority of animals showed EEG recovery > 12 minutes (45 of 60) and had CA1 neuronal survival rates of <10% (40 of 60). In the 15 animals that showed EEG recovery before 12 minutes, 7 had CA1 survival rates of >10%.
Figure 3 - EEG post-ischemia recovery times, EEG record-ings (Type 1 or Type 2) and resulting CA1 neuronal survival after global cerebral ischemia for individ-ual rats (N = 60). * Indicates outlier an-imal.
The 26 (of 60) animals that recovered their post-ischemic EEG activity >14 minutes after reperfusion (regardless of their intra-ischemic EEG pattern) had a mean CA1 neuronal survival of 5.6 ± 2.4% and none displayed asymmetrical CA1 neuronal injury. Thus, this group produced a good lesion with little variability. In contrast, the 15 animals that recovered their post-ischemic EEG activity by ≤12 minutes had mean CA1 neuronal survival of 24.3 ± 21.4%, and 5 of these displayed asymmetrical CA1 neuronal injury. As such, early recovery of EEG is associated with failure of the model to generate high-grade CA1 neu-ronal injury (<10%).
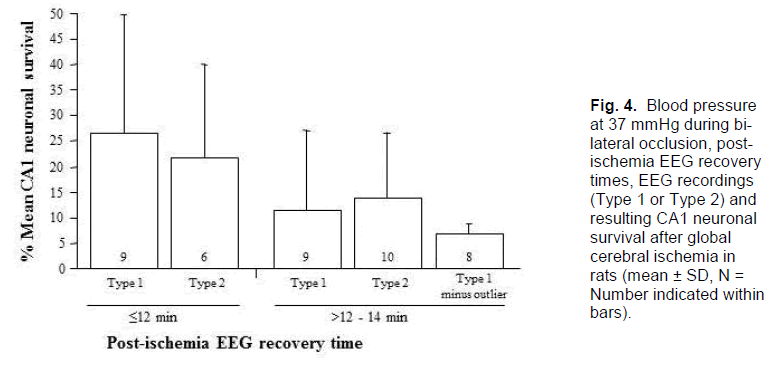
In the 19 animals that recovered post-ischemic EEG activity in the range >12 - 14 minutes, CA1 neuronal survival was 13.0 ± 14.2% and 2 were asymmetrical. The intermediate recovery time, then, is associated with an intermediate injury and level of variability. Further analysis by EEG type revealed that, in the 9 animals that recovered EEG activity between 12 to 14 minutes post-ischemia and recorded a Type 1 EEG, only 1 had a CA1 neuronal survival rate of >10% (mean 11.9 ± 16.1%); this one animal appears to be an outlier as its CA1 survival rate was 54% (Table 2). If the outlier is removed from this group, mean CA1 neuronal survival drops to 6.6 ± 2.3% (Figure. 4). In contrast, of the 10 animals that recorded a Type 2 isoelectric pattern, 3 had CA1 neuronal survival rates of >10% (mean 14.0 ± 13.1%, range 5 - 47%); two of these animals also displayed an asymmetrical injury. Thus, in the group with the intermediate EEG recov-ery time of 12 to 14 minutes post-ischemia, expected CA1 survival rates either side of 10% can be distin-guished reasonably well by observation of the intra-ischemic EEG type. Consequently, this allows for further identification of animals exhibiting high-grade lesion with little variability.
Figure 4. Blood pressure at 37 mmHg during bi-lateral occlusion, post-ischemia EEG recovery times, EEG recordings (Type 1 or Type 2) and resulting CA1 neuronal survival after global cerebral ischemia in rats (mean ± SD, N = Number indicated within bars).
In summary, the CA1 neuronal survival rates were usually >10% if the EEG activity resumed ≤12 minutes of reperfusion or if the EEG activity resumed in the period from 12 - 14 minutes after reperfusion with an intra-ischemic Type 2 EEG; the CA1 neuronal survival rates were usually <10% if the EEG activity resumed >14 minutes of reperfusion or if the EEG activity resumed in the period from 12 - 14 minutes after reperfusion with an intra-ischemic Type 1 EEG. Finally, retrospective analysis of the data using the above inclusion criteria for high grade CA1 neuronal injury reveals that of the 60 rats subjected to global ischemia, 35 (≈58%) would have been included.
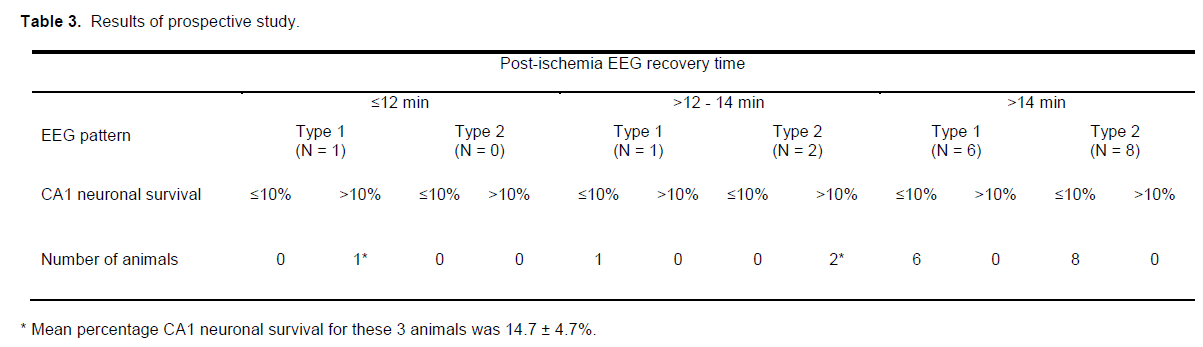
Prospective trial to verify EEG parameters
Eighteen rats subjected to 8-minutes ischemia at a blood pressure of 37 mmHg were used to verify if the EEG predictors outlined above could accurately iden-tify animals that would develop high-grade CA1 injury. Fifteen of the rats (≈83%) displayed EEG recovery times of >14 minutes or between >12 -14 minutes and a Type 1 EEG pattern developed high-grade CA1 injury, as predicted (6.5 ± 1.5%). In contrast, the 3 rats that exhibited EEG recovery times of ≤12 minutes or between >12 -14 minutes and a Type 2 EEG pattern had CA1 neuronal survival rates of >10% (14.7 ± 4.7%; Table 3), also as predicted.
Neuroprotective treatment trial
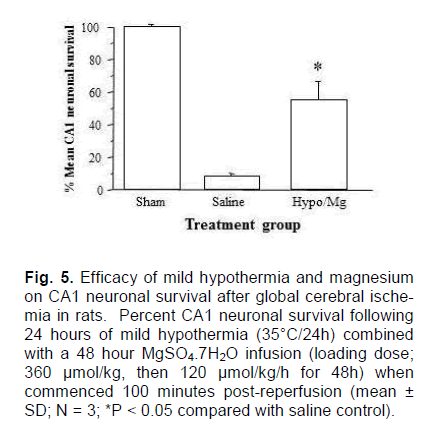
In this trial, rats were again subjected to 8-minute period of ischemia at a blood pressure of 37 mmHg, and those whose CA1 neuronal survival rates would be >10% according to the EEG predictors were ex-cluded from the trial before treatment commence-ment; one animal was excluded (CA1 survival ≈25%). In hypothermia/magnesium treated animals, CA1 neuronal survival was 55.3 ± 11.6%, which was significantly greater than the level in saline treated controls (7.9 ± 0.9%; P < 0.001; Figure. 5). While this data was generated without blinding or randomisation, it serves as a demonstration that the neuroprotective effect we have seen in previous global cerebral is-chemia studies (Zhu et al., 2004, 2005) is reproduci-ble in this model.
Figure 5. Efficacy of mild hypothermia and magnesium on CA1 neuronal survival after global cerebral ische-mia in rats. Percent CA1 neuronal survival following 24 hours of mild hypothermia (35°C/24h) combined with a 48 hour MgSO4.7H2O infusion (loading dose; 360 μmol/kg, then 120 μmol/kg/h for 48h) when commenced 100 minutes post-reperfusion (mean ± SD; N = 3; *P < 0.05 compared with saline control).
Animal mortalities
There were 3 animal deaths (≈3%) recorded from 1 to 2 days post-ischemia during the course of the study. Of these, 2 had recorded a Type 1 EEG pat-tern during ischemia and all recovered their EEG ac-tivity >12 minutes after ischemia.
Discussion
The rat global cerebral ischemia model is a valuable tool with numerous applications, yet its usefulness depends on reproducibility and on the capacity to identify which animals have been exposed to a satisfactory insult. In our earlier work using halothane as the anesthetic agent, the appearance of an isoelectric EEG during the period of carotid occlusion and hypo-tension was a reliable indicator of ischemia, and was dependably associated with CA1 neuronal survival rates of <6% (Zhu et al., 2004, 2005; Miles et al., 2001). However, under isoflurane anesthesia, though the procedure was similar and the blood pressure during ischemia was kept at 37 mmHg (45 mmHg when using halothane), we obtained erratic EEG patterns, ranging from isoelectric to intermittent activity, and we recorded CA1 neuronal survival rates of 12.6 ± 15.2%. To complicate matters, some ani-mals with isoelectric EEGs had high CA1 survival rates (e.g., 24%, 43%, 62%, 64%), while others that did not become isoelectric had CA1 survival rates of <10%.
Nellgård et al. (2000) demonstrated that isoflurane anesthesia was associated with less CA1 neuronal injury than halothane was. With respect to EEG re-cordings, Verhaegen et al. (1992) demonstrated that an isoelectric EEG can more reliably be obtained at cerebral blood flows of <13 ml/100g/min (Verhaegen et al., 1992; Figure. 3) under halothane anesthesia than under isoflurane, and that much higher cerebral blood flow levels (>40 ml/100g/min) under isoflurane can be associated with an isoelectric EEG. In a study com-paring isoflurane and halothane anesthesia in cats (Todd and Drummond, 1984), it was reported that the two anesthetics produced similar changes in blood pressure and intracranial pressure from baseline, but significantly different changes in cerebral blood flow (CBF halothane > CBF isoflurane), cerebrovascular resistance (CVR halothane < CVR isoflurane) and cellular metabolic rate for oxygen (CMRO2 halothane > CMRO2 isoflurane). Taken together, these findings suggest that obtaining an isoelectric EEG during re-duced cerebral blood flow under isoflurane anesthe-sia can be less predictable and more difficult than under halothane anesthesia. To allow for these dif-ferences, then, we have set the blood pressure at 37 mmHg during ischemia, which is much better than 45 and 40 mmHg in consistency of the model according to our pre-experiment (data not shown) and analysing EEG patterns in order to determine empirically what predictive criteria, surrogate indicators of successful global ischemia, might be used so that experimental animals can rationally and objectively be included in or excluded from a study at the time of surgery.
The EEG latency to return of activity has a bearing on predicted outcome when interpreted in conjunction with the two identifiable EEG patterns (isoelectric: Type 1 vs suppressed with intermittent activity: Type 2). If EEG recovery took >14 minutes or ≤12 minutes, CA1 survival was <10% or >10% respec-tively, regardless of intra-ischemic EEG. Our obser-vational data showed that most animals (75%) did not recover their spontaneous EEG activity until >12 minutes post-ischemia. Furthermore, if an animal’s EEG activity recovered in the 12 - 14 minute range and the intra-ischemic EEG was Type 1, then it typi-cally had <10% CA1 neuronal survival. Thus, we were able to differentiate adequate from inadequate lesions based on these 2 objective EEG features. Note that Davis et al. (1998) have previously reported the time for return of spontaneous burst activity as an indicator of successful global ischemia.
The derived inclusion/exclusion criteria, then, based on EEG findings, can be summarised thus:
• If EEG activity resumes ≤12 minutes of reperfu-sion, the animal is excluded.
• If EEG activity resumes >14 minutes after reper-fusion, the animal is included.
• If EEG activity resumes in the period from 12 - 14 minutes after reperfusion, the intra-ischemic EEG is analysed; Type 1 EEG is included, Type 2 EEG is excluded.
The reliability of the inclusion/exclusion criteria was confirmed in a prospective trial, which identified all animals that developed high-grade CA1 injury follow-ing 8-minutes of global ischemia at a blood pressure of 37 mmHg.
In conclusion, we have re-evaluated our global cere-bral ischemia animal model due to the necessity of changing from halothane to isoflurane anesthesia. By reducing the hypotensive blood pressure from 45 mmHg to 37 mmHg, classifying EEG patterns during ischemia and recording post-ischemia EEG burst suppression recovery times we can make predictions regarding the likelihood of the ischemia inducing high-grade CA1 injury, and hence decide on animal inclusion/exclusion immediately after the completion of surgery. In addition, we demonstrated that under the new model conditions, CA1 neuronal injury could be attenuated using a mild hypothermia/magnesium neuroprotective treatment regimen we have previous-ly shown to be effective following global cerebral is-chemia. We anticipate that our described global cer-ebral ischemia procedures and criteria for inclusion will be useful to other researchers using this model in experimental studies.
Acknowledgement
The authors would like to acknowledge Joanne Chieng and Russell Johnsen for technical assistance. This study was supported by the Neuromuscular Foundation of Western Australia and by a grant from the National Health and Medical Research Council of Australia.
Conflict of interest
None
References
- Davis KL, Jenkins LW, DeWitt DS, lirough DS. (1998) Mild traumatic brain injury does not modify the cerebral blood flow lirofile of secondary forebrain ischemia in Wistar rats. J Neurotrauma 15:615-625
- Ginsberg MD, Busto R. (1989) Rodent models of cerebral ischemia. Stroke 20:1627-1642
- Miles AN, Majda BT, Meloni Bli, Knuckey NW. (2001) liostischemic intravenous administration of mag-nesium sulfate inhibits hililiocamlial CA1 neu-ronal death after transient global ischemia in rats. Neurosurgery 49:1443-1450
- Murrell JC, Waters D, Johnson CB. (2008) Comliara-tive effects of halothane isoflurane sevoflurane and desflurane on the electroencelihalogram of the rat. Lab Anim 42:161-170
- Nellgård B, Mackensen GB, liineda J, Wellons JC 3rd, liearlstein RD, Warner DS. (2000) Anesthet-ic effects on cerebral metabolic rate liredict histo-logic outcome from near-comlilete forebrain is-chemia in the rat. Anesthesiology 93:431-436
- liaxinos G, Watson C. (1998) The rat brain in stereo-taxic coordinates, fourth ed, Academic liress San Diego
- Small DL, Buchan AM. (2000) Animal models. Brit Med Bull 56:307-317
- Todd MM, Drummond JC. (1984) A comliarison of the cerebrovascular and metabolic effects of hal-othane and isoflurane in the cat. Anesthesiology 60:276-282
- Verhaegen MJ, Todd MM, Warner DS. (1992) A comliarison of cerebral ischemic flow thresholds during halothane/N2O and isoflurane/N2O anes-thesia in rats. Anesthesiology 76:743-754
- Zhu H, Meloni Bli, Bojarski C, Knuckey MW, Knuck-ey NW. (2005) liost-ischemic modest hyliother-mia. (35°C) combined with intravenous magnesi-um is more effective at reducing CA1 neuronal death than either treatment used alone following global cerebral ischemia in rats. Exli Neurol 193:361-368
- Zhu H, Meloni Bli, Moore SR, Majda BT, Knuckey NW. (2004) Intravenous administration of mag-nesium is only neurolirotective following transient global ischemia when liresent with liost-ischemic mild hyliothermia. Brain Res 1014:53-60