Review Article - Imaging in Medicine (2010) Volume 2, Issue 4
Imaging to optimize liver tumor ablation
Bradley B Pua1 and Constantinos T Sofocleous†1
1Memorial Sloan-Kettering Cancer Center, NY, USA
- *Corresponding Author:
- Constantinos T Sofocleous
Department of Radiology, Memorial Sloan-Kettering Cancer Center
1275 York Avenue, H-118, New York, NY 10021, USA
Tel: +1 212 639 3379
Fax:+1 212 717 3325
E-mail: sofoclec@mskcc.org
Abstract
Surgical resection remains the ideal treatment for hepatocellular carcinoma and metastasis to the liver. Many alternatives are available for treatment of nonsurgical candidates. Regardless of treatment, optimizing imaging in the pretreatment, treatment and post-treatment settings is critical in order to lower the rates of local tumor progression and maximize the effectiveness of treatment that may result in prolonged survival. This article summarizes some basic imaging techniques of primary and metastatic liver tumors with a focus on how to optimize their treatment with ablation.
Keywords
ablation; colon cancer liver metastases; hepatocellular cancer; image-guided ablation; liver imaging; liver metastases; liver tumors; radiofrequency
Hepatocellular carcinoma (HCC) and colorectal metastases to the liver are the most common primary and secondary cancers of the liver. While the mainstay of curative treatment is surgery, only 10–20% of patients with HCC and 20–25% of patients with colorectal liver metastasis are resectable at the time of detection [1,2]. Resectability is often limited by location size or number of lesions, inadequate liver remnant, extrahepatic metastasis, as well as medical comorbidities that preclude open surgery. Various locoregional and systemic therapies have been investigated to target this large group of unresectable patients, including systemic chemotherapy, regional chemotherapy (hepatic artery infusion pump), transarterial therapies such as transarterial chemoembolization (TACE), bland embolization and Yttrium 90 radioembolization, as well as ablative therapies (radiofrequency [RF] ablation, cryoablation, microwave ablation). Regardless of treatment modality, imaging characteristics of the tumor before, during and after treatment is important. Determination of tumor characteristics before treatment will allow the choice of the safest and most efficacious treatment. An understanding of tumor characteristics and its relationship to adjacent structures will allow optimization of treatment, decreasing the likelihood of local tumor progression (LTP; recurrence) and minimizing risks. Knowledge of the expected posttreatment changes and the imaging evolution of the treated tumor will allow early detection of LTP to facilitate early retreatment.
This article will focus on the utilization of imaging in the preprocedural and procedural phase of treatment of liver tumors with RF ablation and combination therapies. The postablation appearance of liver tumors has been discussed extensively elsewhere [3]. These imaging techniques are applicable to most ablative technologies; however, due to the widespread usage of RF ablation, this will be discussed primarily.
RF ablation
Radiofrequency ablation utilizes heat generated by frictional energy secondary to oscillating tissue ions, which are created by passing an alternating current through them. This current is created across an electrode placed within the target lesion and dispersive pads placed across the patient’s skin. When tissues are heated, protein denaturation and cellular apoptosis occur leading to irreversible cell death in the form of coagulation necrosis in a zone around the electrode. These changes are time and temperature dependent with coagulation necrosis occurring when tissues are heated to 50°C for approximately 5 min, instantaneous cell death occurring at temperatures above 60°C and charring occurring at temperatures in the range of 100 to 110°C [4].
Radiofrequency ablation can be accomplished percutaneously, during an open surgical procedure or laparoscopically, and each has its advantages and disadvantages. Early surgical literature suggested that performing RF ablation open and laparoscopically increases the opportunity to detect previously unknown intrahepatic or extrahepatic disease [5]. Ablation has been used to treat lesions that are detected during surgery (utilizing intraoperative sonography) that are not resectable. This approach has improved the clinical outcomes after resection of liver tumors [6].
Imaging HCCs
Most patients will undergo a tri-phasic CT (nonenhanced, arterial and portal-equilibrium phases) evaluation prior to any therapy. Since the most efficacious treatment of HCC (percutaneous or open) as well as treatment of postsurgical recurrences have yet to be defined, imaging is directed initially to assess for operative candidacy [7,8]. Tumors limited to a single lobe with adequate residual hepatic reserve after resection and without findings of cirrhosis or portal hypertension, are considered ideal operative candidates. In addition to tumor burden, it is important to determine involvement and proximity to vasculature. Tumors that are close to or abut the porta hepatis may preclude complete surgical resection. For the most part, curative surgical management depends on whether a complete surgical resection with a clear margin (R0 resection) is achievable. Tumors that are deemed unresectable are then further evaluated for other potential treatments. Alternatives to surgical resection include systemic and regional chemotherapy, transarterial therapy, ablative techniques and combination therapy. The utilization of RF ablation alone in the treatment of HCC remains a much debated topic [9]. Size and number of HCC lesions is linked with efficacy, and should be the first characteristic evaluated on preprocedural CT. Survival rates at 5 years of up to 64% have been reported in treating solitary lesions of less than 3.5 cm or in treatment of less than three lesions that are each smaller than 3.0 cm in size [10]. In fact, two randomized controlled studies have demonstrated that percutaneous RF ablation may yield similar overall and disease-free survival to partial hepatectomy [11–13].
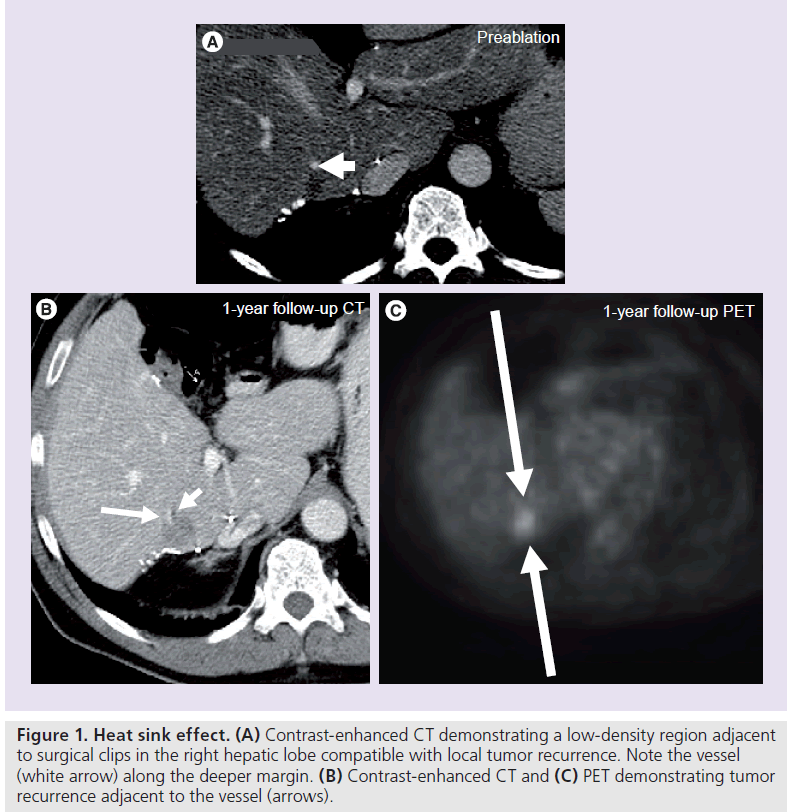
While size and number of lesions are important characteristics, additional characteristics such as proximity to large vascular structures, vessel patency, vascular supply to the tumor and associated bile duct obstruction should be assessed. Ablation of tumors abutting major vascular structures has been shown to be feasible, however, this location allows the flowing blood within the nearby vessel to carry the ablative energy away from the tumor, thus acting like a ‘heat sink’, diminishing the efficacy of the ablation and resulting in a higher local tumor recurrence rate (Figure 1). Mulier et al., in a metaanalysis, demonstrated that tumors that are at least 5 mm away from a large vessel had a significantly lower LTP rate (6.3%) compared with those that were less than 5 mm from a large vessel (37%) [14]. Lu et al. demonstrated that vessel size is also a factor, suggesting that peritumoral vessels larger than 3 mm were associated with incomplete tumor destruction [15]. Owing to the inherent nature of this technology, it is not simply a matter of increasing the power (wattseconds) of the ablation to increase the zone of coagulation necrosis to compensate; with many investigators looking at ways to fine-tune the ablation protocol to increase ablative effectiveness [16,17]. As a matter of fact, thermal damage can rarely lead to damage and thrombosis in even large vessels, such as the main portal vein, if blood flow within that vessel is decreased [18]. Recently microwave technology and irreversible electroporation have been used and at least in vitro and in animal models, these modalities appear to be less or not at all influenced by the heat sink phenomenon.
Figure 1. Heat sink effect. (A) Contrast-enhanced CT demonstrating a low-density region adjacent to surgical clips in the right hepatic lobe compatible with local tumor recurrence. Note the vessel (white arrow) along the deeper margin. (B) Contrast-enhanced CT and (C) PET demonstrating tumor recurrence adjacent to the vessel (arrows).
Assessment of the relationship between the biliary tree and target tumor is important since RF ablation may damage a bile duct leading to biliary stenosis with secondary dilatation of the peripheral biliary tree [19]. Stenosis and dilatation of these peripherally located ducts may progress to biloma formation, which are generally subclinical. Periprocedural antibiotic prophylaxis is prudent to prevent secondary infection. While it has been shown that the central biliary tree may be protected by the heat sink effect of adjacent large vascular structures such as the portal vein, care should be taken [20].
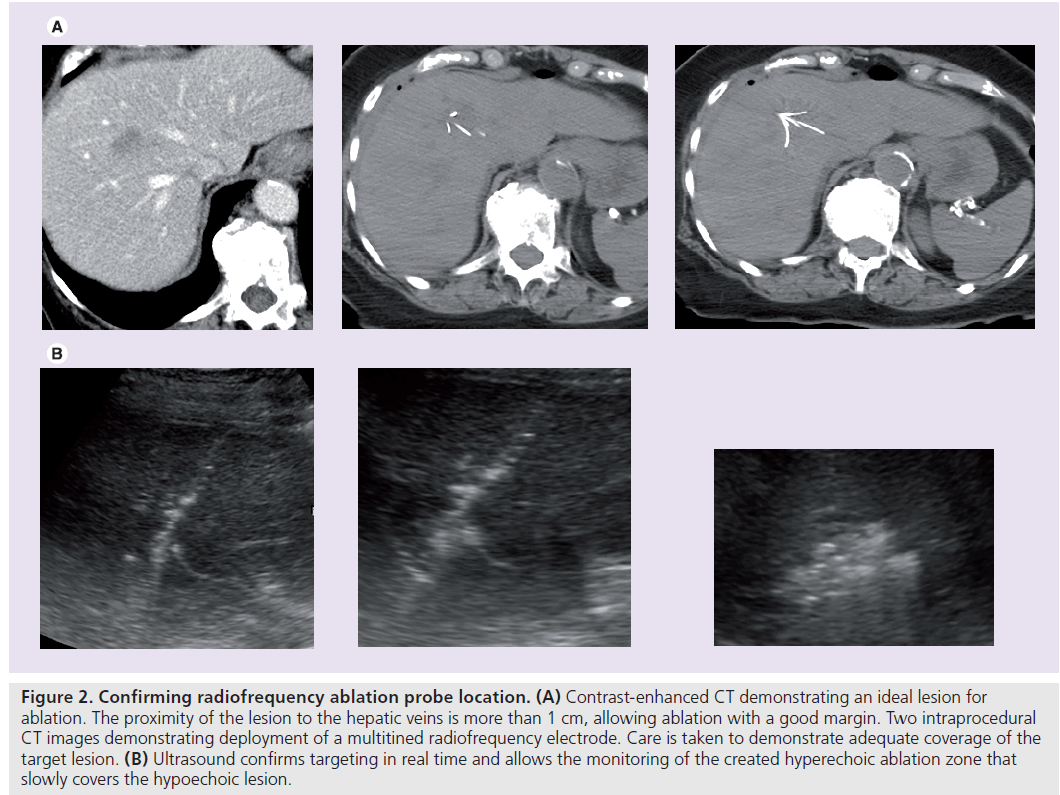
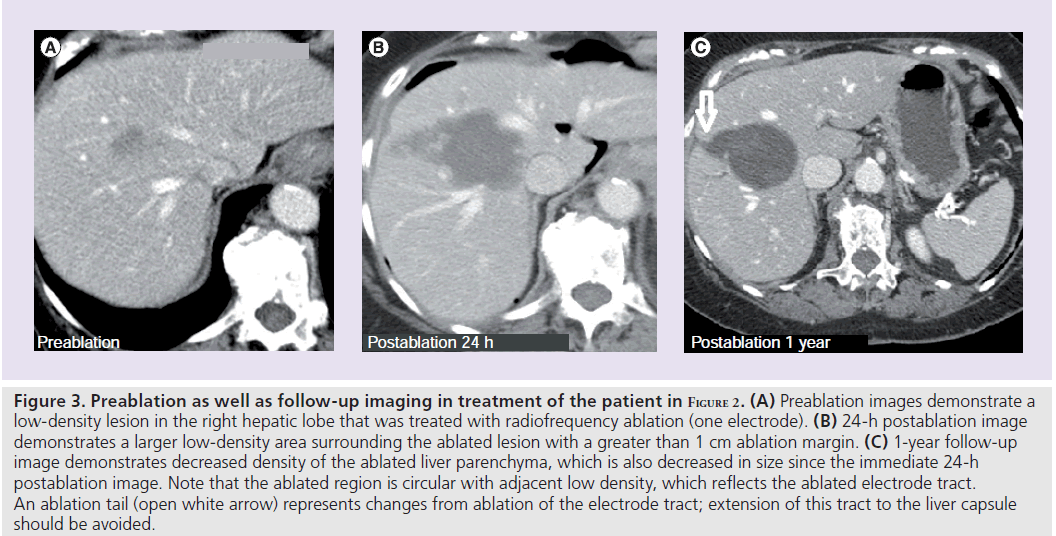
The dramatic differences in outcomes and LTP in patients who have received ‘optimal’ RF ablation highlight the importance of proper imaging to determine both electrode positioning and ablation margins (Figure 2). Indeed, some studies have demonstrated that operator experience is associated with fewer local recurrences, and we surmise, that this may be related at least partly to comfort with imaging appearances during ablation (Figure 3) [21,22].
Figure 2. Confirming radiofrequency ablation probe location. (A) Contrast-enhanced CT demonstrating an ideal lesion for ablation. The proximity of the lesion to the hepatic veins is more than 1 cm, allowing ablation with a good margin. Two intraprocedural CT images demonstrating deployment of a multitined radiofrequency electrode. Care is taken to demonstrate adequate coverage of the target lesion. (B) Ultrasound confirms targeting in real time and allows the monitoring of the created hyperechoic ablation zone that slowly covers the hypoechoic lesion.
Proper RF ablation requires intimate knowledge of the equipment being utilized. It is important to understand the different RF electrodes being used and their respective ablation size as well as shape. Earlier electrodes consisted of a single insulated needle design with a noninsulated tip. These electrodes produced either a spherical or oblong ablation zone. Newer probes, some of which are multi-tined, allow for a more reproducible shaped spherical ablation zone, with some internally cooled probes found in animal studies to produce a larger ablation zone [23]. Utilization of this newer technology has also been shown to translate to lower local tumor recurrence rates [24].
Percutaneous placement of the RF ablation electrode, unlike that of open placement, is heavily dependent upon imaging. One of the advantages of ultrasound is its capability for real-time imaging. Ultrasound-guided placement of the RF electrode allows the operator to avoid large vessels as well as compensate for respiratory variation of the target lesion. Disadvantages of ultrasound include its limited utility in targeting superficial lesions and its dependence on air-less interfaces. Adjacent air-filled structures (such as bowel and lungs) result in artifact and limits visualization of structures deep to this air interface. This is often where CT-guided placement of the RF electrode is necessary. Most interventional radiology departments (including ours) have both CT and ultrasound guidance ability. The use of both modalities can increase accurate targeting and monitoring of the ablation zone in order to provide the best possible outcome (Figure 2).
Figure 3. Preablation as well as follow-up imaging in treatment of the patient in Figure 2. (A) Preablation images demonstrate a low-density lesion in the right hepatic lobe that was treated with radiofrequency ablation (one electrode). (B) 24-h postablation image demonstrates a larger low-density area surrounding the ablated lesion with a greater than 1 cm ablation margin. (C) 1-year follow-up image demonstrates decreased density of the ablated liver parenchyma, which is also decreased in size since the immediate 24-h postablation image. Note that the ablated region is circular with adjacent low density, which reflects the ablated electrode tract. An ablation tail (open white arrow) represents changes from ablation of the electrode tract; extension of this tract to the liver capsule should be avoided.
Although CT is not a real-time modality (if CT fluoroscopy is not employed), it offers a wider field of view and is not operator dependent. The current ability to create multiplanar reconstruction on CT also allows for improved placement of the RF electrode, which, in turn will likely result in a more complete ablation. Multiplanar imaging has been found to be helpful for assessing the ablation zone in the postprocedural setting. A recent study demonstrated that 2D imaging of the ablation zone in porcine livers may underestimate its size, and that 3D imaging with volumetric measurements may be of benefit [25]. This ability improves accuracy and allows the operator to ablate, in the same session, areas that may not have the desired ‘ ablation margin’.
As alluded to earlier, each RF electrode is limited by the size of the effective ablation zone it can create. Charring within the ablation area decreases the ability for the current to be conducted, thus limiting effectiveness at the periphery. It has been determined that LTP occurs at the periphery of the ablation zone [26]. In fact, inadequate ablation margin, rather than subcapsular location and proximity to vascular structures, has been shown to be the strongest predictor of LTP [27]. Tumors that are larger than the known ‘effective’ ablation diameter or are irregular in shape often require multiple ablations to cover the entire area. This technique is often named ‘overlapping ablations/electrodes’. Secondary to technological limitations, multiple RF electrodes cannot be ‘on’ or ablating simultaneously. Therefore, careful preprocedural planning in the placement of one electrode performing multiple burns or of a cluster of electrodes prior to ablation is important. Anatomy and imaging appearance of the lesion changes secondary to bleeding or the normal postablation changes, making successive ablations and electrode positioning challenging during the same treatment session.
Similar to adequate surgical margins, ‘adequate’ ablation margins have been studied. An ablative margin of at least 5 mm has been associated with decreased LTP rates [28]. At our institution, we generally strive to achieve an ablation margin of 1 cm, which can be considered an ‘A0’ ablation, likened to a R0 resection for hepatectomy where no tumor is noted at surgical margins.
Large & multiple HCC lesions
Secondary to studies demonstrating increased LTP rates as high as 20% in treating tumors greater than 3.5 cm with RF ablation alone and poor tumor necrosis in treatment of lesions larger than 5.0 cm, alternative and combined therapies have been suggested [29,30]. Large and multiple lesions, in addition to being assessed for basic imaging characteristics, need to be assessed for tumor arterial supply. A characteristic of HCC is its highly vascular nature with preferential blood supply from the hepatic artery, making them suitable to transarterial therapies such as TACE, bland transarterial embolization (TAE) and combined transarterial therapy and ablation. Standard hepatic anatomy may not be found in many patients, underscoring the importance of an appropriate preprocedural imaging workup. One study demonstrated that in 350 HCCs, up to 11.9% of patients derived hepatic arterial supply from the superior mesenteric artery [31]. Collateral arterial feeders to HCC lesions may also be found and treated accordingly to improve treatment effectiveness [32].
Transarterial therapies such as TACE and TAE have been studied extensively in treatment of large HCC, certain HCC subtypes as well as in treatment of recurrent HCC after resection [33–35]. While the superiority of TACE over TAE remains controversial [36,37], several studies have demonstrated that combination of either TACE or TAE with RF ablation is superior to either therapy alone. Sugimori et al. demonstrated in an animal model that TAE in combination with RF ablation resulted in a larger region of coagulative necrosis [38]. In fact, a small study has shown some potential survival benefit in combination therapy in HCCs larger than 5.0 cm [39]. For the treatment of lesions that are refractory to TACE/TAE or with arterial anatomy not amendable to transarterial therapy, percutaneous ethanol injection or ablation can be utilized. Prior studies demonstrated that RF ablation is associated with better outcomes when compared with ethanol injection for lesions up to 4 cm in diameter [40,41]. Similar to other combination therapies, the combination of RF ablation-percutaneous ethanol injection has been shown to be more effective than either alone [42].
Imaging in colorectal metastasis
Preprocedural imaging of colorectal metastasis usually commences with a contrast-enhanced CT. However, we advocate the use of triphasic CT (similar to HCC). Although the arterial phase may not be as important as it is in the treatment planning for HCC, the noncontrast phase of the scan is important to identify the tumor in a similar manner to which it is going to be visualized by the CT used for guidance in the ablation procedure. Metastasis in different phases of growth, as well as partially treated metastasis, may demonstrate different phases of vascular enhancement. Reminiscent of HCC imaging, assessment of the size and number of colorectal metastasis, proximity to vasculature and arterial supply are important. Several studies have demonstrated that MRI, paricularly with liverspecific contrast, is more sensitive in detecting colorectal metastasis [43].
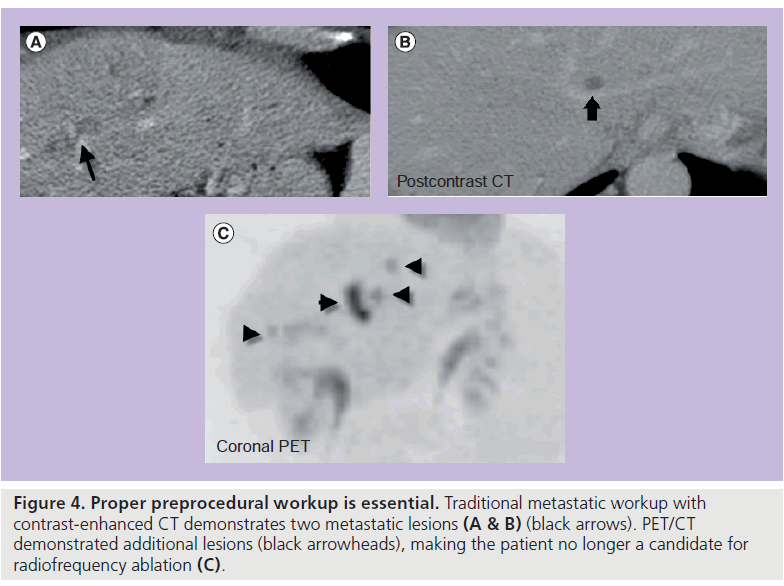
A preprocedural PET/CT should also be performed to determine if extrahepatic disease is present, which could alter treatment strategy (Figure 4). PET/CT has been shown to be more sensitive in detecting extrahepatic metastasis than traditional contrast-enhanced CT [44]. This same study also demonstrated that a manganese dipyridoxyl diphosphate liver MRI can be more effective in detecting intrahepatic disease than PET/CT. Studies comparing treatment of colorectal metastasis to the liver are varied [45]. In evaluation of the role of RF ablation in the treatment of colorectal cancers, a recent retrospective study asserts that hepatic resection should remain the mainstay of therapy for patients with solitary liver metastasis and RF ablation can be used in nonsurgical candidates with tumors less than 3 cm [46]. Additional studies would appear to confirm this, and as techniques for RF ablation advance, survival rates in patients treated with RF ablation are expected to improve [47]. In the meta-analysis by Mulier et al. it appears that overall survival after RF ablation is similar to that of resection, despite the fact that the ablation series included the patients that were not candidates for surgery. LTP rates remain higher after RF ablation when compared with surgery and this justifies the current recommendation of surgery as the gold standard [14].
Figure 4. Proper preprocedural workup is essential. Traditional metastatic workup with contrast-enhanced CT demonstrates two metastatic lesions (A & B) (black arrows). PET/CT demonstrated additional lesions (black arrowheads), making the patient no longer a candidate for radiofrequency ablation (C).
Recently, a ‘test of time’ paradigm has been used in treatment of patients with metastatic disease to the liver [48]. In this model, patients are first treated with RF ablation and followed for a short interval. For those that demonstrate LTP, these patients can be treated with either followup RF ablation or resection. For those that are cured with RF ablation, these patients have been spared surgery. For those with multiple new sites of metastasis, they have been spared the potential morbidity associated with noncurative surgery. This series determined that 59% of patients were spared unnecessary surgery, either because they were free of disease after RF ablation (44%) or they developed multiple new sites of disease within a short period of time, which would have made them unsuitable for resection (56%).
A recent meta-analysis of 763 colorectal tumors metastatic to the liver demonstrated that, similar to HCC, tumor size, tumor distance from large vessels and adequate treatment margins were associated with decreased local recurrence rates [14]. Tumors treated with percutaneous RF ablation that were less than 3 cm in size had a 16.0% recurrence rate, while treated tumors that were 3–5 cm in size or larger than 5 cm had reported recurrence rates of 25.9 and 60.0%, respectively.
Similar to the treatment of HCC, large or multiple colorectal metastases may be treated with TACE, hepatic chemoperfusion (utilizing a hepatic arterial infusion pump) or radioembolization therapy with Yttrium 90 microspheres. The superiority of chemoperfusion over TACE is under debate [49].
Preparation of a patient for Yttrium 90 radioembolization requires a meticulous assessment of tumor vasculature. Since these particles are impregnated with a pure b‑emitter and lodged in their target organ, a very intense local radiotherapeutic effect is created. Assessment of arteries feeding the tumor and potential branches is important to reduce nontarget embolization. Evidence of uncorrectable flow to the GI tract at angiography or on technetium-88m macroaggregated albumin scintigraphy is a contraindication to this procedure [50].
Other metastases
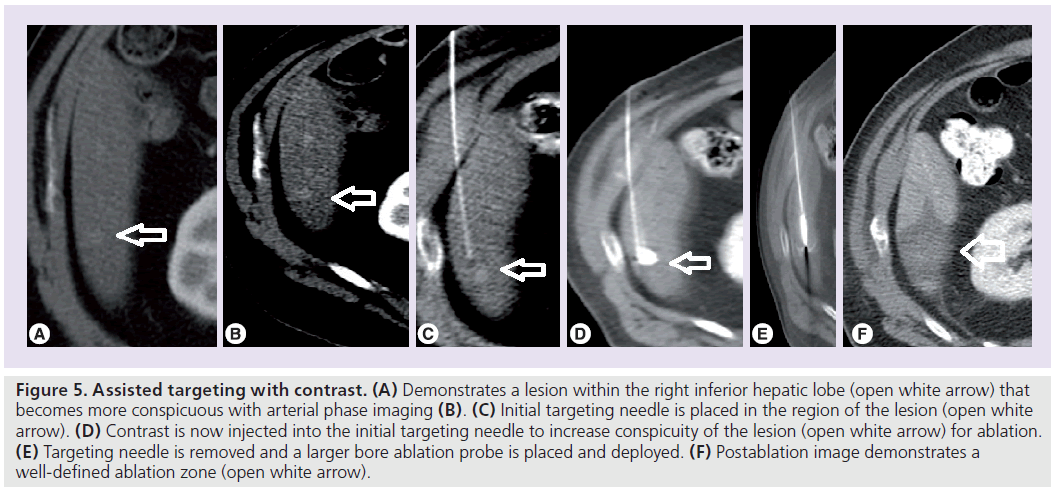
Evaluation of other, less common, metastases to the liver is directed at determining stage (often with PET/CT) as well as vascularity. Knowledge of the primary tumor is also important as certain metastases are better detected using certain imaging modalities. Highly vascular tumors such as neuroendocrine, renal cell, thyroid and gastrointenstinal stromal tumor metastases will be better visualized on a triphasic CT, making these tumors more amenable to transarterial therapies. It must be noted that advanced localization techniques can also be used if RF ablation is the treatment of choice for a highly vascular lesion. As demonstrated in Figure 5, an initial arterial phase CT can be used to localize the lesion using local landmarks, allowing for placement of an initial localizing needle. Through this needle, contrast can be injected to make the target lesion more conspicuous for ablation.
Figure 5. Assisted targeting with contrast. (A) Demonstrates a lesion within the right inferior hepatic lobe (open white arrow) that becomes more conspicuous with arterial phase imaging (B). (C) Initial targeting needle is placed in the region of the lesion (open white arrow). (D) Contrast is now injected into the initial targeting needle to increase conspicuity of the lesion (open white arrow) for ablation. (E) Targeting needle is removed and a larger bore ablation probe is placed and deployed. (F) Postablation image demonstrates a well-defined ablation zone (open white arrow).
Less vascular tumors such as breast cancer metastasis can simply be treated with ablative therapies such as RF ablation. In fact, our group and others have demonstrated that RF ablation is both safe and efficacious in controlling breast metastasis to the liver in selected patients with limited liver metastases and stable or no extrahepatic disease [51–53]. For these patients, PET/CT may be the most sensitive imaging modality to detect hepatic and extrahepatic lesions and should be used for staging and procedure planning prior to ablation.
Adjunctive imaging & techniques
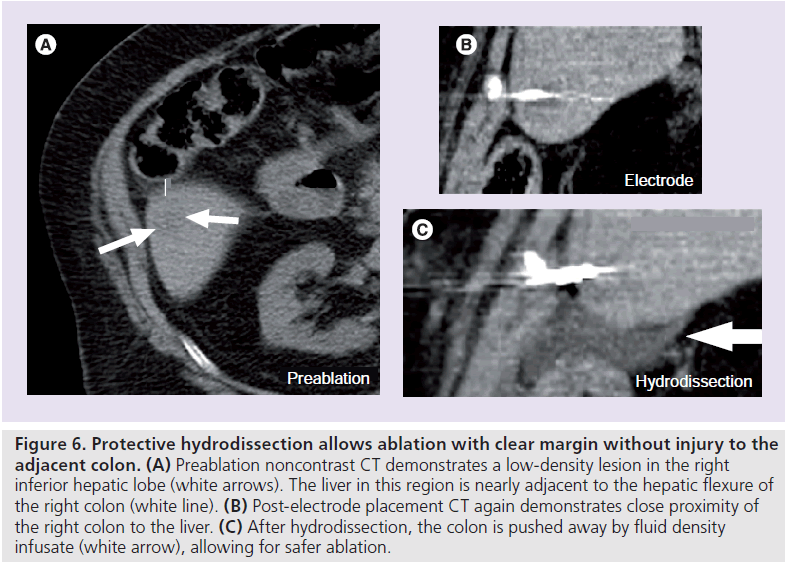
Recent advances in sonography have given operators the ability to merge CT images with that of sonography. This ability will allow real-time placement of an RF electrode in lesions that may not be well visualized by ultrasound alone. Patients may undergo a contrast- enhanced or triphasic CT that shows the target lesions and these images may be seen in conjuction with ultrasound images, which will aid in targeting. In a similar fashion, newer fusion PET techniques may be utilized to target F-18-fluorodeoxyglucose avid lesions that do not demonstrate an obvious CT abnormality. Knowledge of the location of the tumor in relation to the liver capsule as well as adjacent organs also aids in planning of ablation. As discussed previously, an air-gap created by the lungs or bowel may hinder visualization during ultrasound-guided placement of the RF ablation electrode. Additionally, tumors adjacent to the diaphragm and bowel may risk injury to these organs if a complete ablation is sought after. Various investigators have proposed and shown feasibility of different isolation techniques that solve both problems with ultrasound visualization and nontarget organ insulation. Some of these measures include placement of fluid into the pleural space to improve ultrasound visualization, as well as hydrodissection, where fluid is introduced into the peritoneal space to separate a peripheral hepatic lesion from either the diaphragm, an adjacent organ or the GI tract [54,55]. While most early studies utilized saline as an infusate, recent studies suggest that 5% dextrose in water may act as a better buffer and in a small series, has been shown to decrease procedural pain and length of hospital stay (Figure 6) [56,57].
Figure 6. Protective hydrodissection allows ablation with clear margin without injury to the adjacent colon. (A) Preablation noncontrast CT demonstrates a low-density lesion in the right inferior hepatic lobe (white arrows). The liver in this region is nearly adjacent to the hepatic flexure of the right colon (white line). (B) Post-electrode placement CT again demonstrates close proximity of the right colon to the liver. (C) After hydrodissection, the colon is pushed away by fluid density infusate (white arrow), allowing for safer ablation.
Future perspective
As the role of thermal ablation increases in the treatment of liver tumors, it is imperative for all those treating these patients to understand the importance of imaging in the diagnosis/planning phase as well as during the actual treatment and follow-up. Traditional approaches, such as hepatectomy, affords the operator direct visualization of the treatment field in 3D space. This allows the surgeon to continue resection until the entire tumor is removed and until he receives pathologic confirmation of a clear margin (whenever feasible). Additionally, open techniques allow for manipulation of the target organ to more advantageous positions, akin to what hydrodissection is striving to achieve. Surgery also has the advantage of pathologically evaluating the resected tumor in order to confirm that no residual tumor remains and that the resection has achieved a clear margin. Although the entire tumor cannot be examined in the case of locoregional treatments, tissue may be found on the electrodes and may be used to identify patients at risk for LTP. In a recent study we demonstrated that identification of prolific marker ki-67-positive cells on the RF electrode after ablation of liver malignancies up to 5 cm in diameter, is associated with overall six-times higher risk for LTP when compared with those tumors where only coagulation necrosis was found. This risk is ten-times higher for tumors under 3 cm [58].
Percutaneous approaches rely solely on imaging appearances. Advanced imaging techniques, such as multiplanar imaging, must be utilized to close the gap between open surgery and percutaneous techniques. It is important to evaluate the ablation margin at the end of each procedure in order to confirm that it encompasses the tumor with a clear margin. To that effect we strongly advocate the performance of immediate post-ablation contrast-enhanced CT that may identify part of the tumor being inadequately treated. In such a case additional ablation should be performed. This immediate CT should, however, not be used as the imaging study to evaluate the success of the ablation. Technical success should be evaluated on a contrast-enhanced CT (we again advocate a triphasic examination), as detailed in the reporting standards in image guided tumor ablation described by Goldberg et al., within 4–12 weeks after the ablation [59].
Similar to that for surgery, risk of liver failure and postprocedural morbidity and mortality for RF ablation is linked to the degree of preprocedural hepatic cirrhosis, as measured by the Child- Pugh classification. While further research will be required to better correlate imaging with clinical classification of liver disease, and thus better prediction of post-treatment liver function, several preliminary studies appear promising. Koda et al. determined that liver parenchymal function is only transiently decreased in patients with a low Child-Pugh score (<8) by RF ablation or combined TACE-ablation, whereas patients with high Child-Pugh scores (≥8) had an increased risk of serious postprocedural complications, with refractory ascites only seen in this group of patients [60]. This underscores the importance of evaluating not only the target lesion on either MRI or CT, but the residual liver parenchyma and signs of cirrhosis or portal hypertension.

Financial & competing interests disclosure
CT Sofocleous receives research support from the R21 NIH mechanism for a research project evaluating tissue characteristics from liver tumors as predictors of outcome after radiofrequency ablation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Lau WY: Primary hepatocellular carcinoma. In: Surgery of the Liver and Biliary Tract (2nd Edition, Vol II). Blumgart LH, Fong Y (Eds). WB Saunders, London, UK, 1423–1450 (2000).
- Steele G Jr, Ravikumar TS: Resection of hepatic metastasis from colorectal cancer. Biologic perspective. Ann. Surg. 210, 127–138 (1989).
- Kim YS, Rhim H, Lim HK: Imaging after radiofrequency ablation of hepatic tumors. Semin. Ultrasound CT MRI 30, 49–66 (2009).
- Goldberg SN, Dupuy DE: Image-guided radiofrequency tumor ablation: challenges and opportunities – Part 1. J. Vasc. Interv. Radiol. 12, 1021–1032 (2001).
- Tsioulias GJ, Wood TF, Morton DL, Bilchik AJ: Diagnostic laparoscopy and laparoscopic ultrasonography optimize staging and resectability of intraabdominal tumors. Surg. Endosc. 15, 1016–1019 (2001).
- Pearson AS, Francesco I, Fleming RY et al.: Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am. J. Surg. 178, 592–599 (1999).
- Lencioni R, Cioni D, Crocetti L et al.: Early stage hepatocellular carcinoma in patients with cirrhosis: long term results of percutaneous image guided ablation. Radiology 234, 961–967 (2005).
- Yang W, Chen MH, Yin SS et al.: Radiofrequency ablation of recurrent hepatocellcular carcinoma after hepatectomy: therapeutic efficacy on early and late phase recurrence. AJR Am. J. Roentgenol. 186, S275–S283 (2006).
- Lau WY, Lai EC: The current role of radiofrequency ablation in the management of hepatocellular carcinoma. A systematic review. Ann. Surg. 249, 20–25 (2009).
- Salmi A, Turrini R, Lanzani G, Savio A, Anglani L: Efficacy of radiofrequency ablation of hepatocellular carcinoma associated with chronic liver disease without cirrhosis. Int. J. Med. Sci. 5, 327–332 (2008).
- Chen MS, Li JQ, Zheng Y et al.: A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann. Surg. 243, 321–328 (2006).
- Lu MD, Kuang M, Liang J et al.: Surgical resection versus percutaneous thermal ablation for early stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi 86, 801–805 (2006).
- Guglielmi A, Ruzzenente A, Valdegamberi A et al.: Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J. Gastrointest. Surg. 12, 192–198 (2008).
- Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L: Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann. Surg. 242, 158–171 (2005).
- Lu DS, Raman SS, Limanond P et al.: Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J. Vasc. Interv. Radiol. 14, 1267–1274 (2003).
- Frieser M, Schaber S, Peters A et al.: Radiofrequency ablation using perfused needle electrodes – study of intermittent and continuous triple needle application ex vivo. Eur. Surg. Res. 37, 312–316 (2005).
- Lee JM, Han JK, Chang JM et al.: Radiofrequency ablation of the porcine liver in vivo: increased coagulation with an internally cooled perfusion electrode. Acad. Radiol. 13, 343–352 (2006).
- Ng KK, Lam CM, Poon RT, Shek TW, Fan ST, Wong J: Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br. J. Surg. 91, 632–639 (2004).
- Kim SH, Lim HK, Choi D et al.: Changes in bile ducts after radiofrequency ablation of hepatocellcular carcinoma: and clinical significance. AJR Am. J. Roentgenol. 183, 1611–1617 (2004).
- Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK: Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann. Surg. 227, 559–565 (1998).
- Poon RT, Ng KK, Lam CM et al.: Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients at a tertiary institution. Ann. Surg. 239, 441–449 (2004).
- Hildebrand P, Leinecke T, Kleemann M et al.: Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur. J. Surg. Oncol. 32, 430–434 (2006).
- Pereira PL, Trubenbach J, Schenk M et al.: Radiofrequency ablation: in vivo comparison of four commercially available devices in pig livers. Radiology 232, 482–490 (2004).
- Ahman A, Chen SL, Kavanagh MA, Allegra DP, Bilchik AJ: Radiofrequency ablation of hepatic metastases from colorectal cancer: are newer generation probes better? Am. Surg. 72, 875–879 (2006).
- Bangard C, Rosgen S, Wahba R et al.: Large-volume multi-tined expandable RF ablation in pig livers: comparison of 2D and volumetric measurements of the ablation zone. Eur. Radiol. 20(5), 1073–1078 (2010).
- Curley SA: Radiofrequency ablation of malignant liver tumors. Oncologist 6, 14–23 (2001).
- Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS: Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am. J. Roentgenol. 190, 1544–1551 (2008).
- Nakazawa T, Kokubu S, Shibuya A et al.: Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am. J. Roentgenol. 188, 480–488 (2007).
- Lencioni R, Cioni D, Donati F, Bartolozzi C: Combination of interventional therapies in hepatocellular carcinoma. Hepatogastroenterology 48, 8–14 (2001).
- Livraghi T, Goldberg SN, Lazzaroni S et al.: Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology 214, 761–768 (2000).
- Wei M, Qiang L, Jian Y, Jie C: Hepatocellular carcinoma fed by the hepatic artery arising from the superior mesenteric artery: angiographic analysis and interventional treatment. Chinese-German J. Clin. Oncol. 2, 176–178 (2003).
- Miyayama S, Matsui O, Taki K et al.: Extrahepatic blood supply to hepatocellular carcinoma: angiographic demonstration and transcatheter arterial chemoembolization. Cardiovasc. Intervent. Radiol. 29, 39–48 (2006).
- Wang Y, Zhang J, Gao Y, Yu M, Gong Y, Yu G: Therapeutic efficacy of transcatheter arterial embolization of primary hepatocellular carcinoma: discrepancy in different histopathologic subtypes. Chin. Med. J. 112, 264–268 (1999).
- Covey AM, Maluccio MA, Schubert J et al.: Particle embolization of recurrent hepatocellular carcinoma after hepatectomy. Cancer 106, 2181–2189 (2006).
- Malagari K, Pomoni M, Kelekis A et al.: Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc. Intervent. Radiol. 33(3), 541–551 (2010).
- Kawai S, Okamura J, Ogawa M et al.: Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma – a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother. Pharmacol. 31, S1–S6 (1992).
- Pleguezuelo M, Marelli L, Misseri M et al.: TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev. Anticancer Ther. 8, 1623–1641 (2008).
- Sugimori K, Nozawa A, Morimoto M: Extension of radiofrequency ablation of the liver by transcatheter arterial embolization with iodized oil and gelatin sponge: results in a pig model. J. Vasc. Interv. Radiol. 16, 849–856 (2005).
- Takaki H, Yamakado K, Uraki J et al.: Radiofrequency ablation combined with chemoembolization for treatment of hepatocellular carcinomas larger than 5cm. J. Vasc. Interv. Radiol. 35, 217–224 (2009).
- Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC: Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma =4cm. Gastroenterology 127, 1714–1723 (2004).
- Lencioni RA, Allgaier HP, Cioni D et al.: Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228, 235–240 (2003).
- Zhang YJ, Liang HH, Chen MS et al.: Hepatocellcular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randoimized trial. Radiology 244, 599–607 (2007).
- Oliva MR, Saini S: Liver cancer imaging: role of CT, MRI, US and PET. Cancer Imaging 4, S42–S46 (2004).
- Kong G, Jackson C, Koh DM et al.: The use of 18F-FDG PET/CT in colorectal liver metastases – comparison with CT and liver MRI. Eur. J. Nucl. Med. Mol. Imaging 35, 1323–1329 (2008).
- Tsai S, Pawlik TM: Outcomes of ablation versus resection for colorectal liver metastasis: are we comparing apples with oranges? Ann. Surg. Oncol. 16, 2422–2428 (2009).
- Hur H, Ko YT, Min BS et al.: Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal metastases. Am. J. Surg. 197, 728–736 (2009).
- Machi J, Osihi AJ, Sumida K et al.: Long term outcome of radiofrequency ablation for unresectable liver metastases from colorectal cancer: evaluation of prognostic factors and effectiveness in first and second line management. Cancer 12, 318–326 (2006).
- Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS: Percutaneous ablation of liver metastases in potential candidates for resection. The ‘test of time’ approach. Cancer 97, 3027–3035 (2003).
- Vogl TJ, Zangos S, Eichler K, Yakoub D, Nabil M: Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur. Radiol. 17, 1025–1034 (2007).
- Atassi B, Bangash AK, Bahrani A et al.: Multimodality imaging following 90Y radioembolization: a comprehensive review and pictorial essay. Radiographics 28, 81–99 (2008).
- Sofocleous CT, Nascimento RG, Gonen M et al.: Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am. J. Roentgenol. 189, 883–889 (2007).
- Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT Jr: Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation – intermediate and long-term survival rates. Radiology 253, 861–869 (2009).
- Jakobs TF, Hoffmann RT, Schrader A et al.: CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc. Intervent. Radiol. 32, 38–46 (2009).
- Ohmoto J, Tsuzuki M, Yamamato S: Percutaneous microwave coagulation therapy with intraperitoneal saline infusion for hepatocellular carcinoma in the hepatic dome. AJR Am. J. Roentgenol. 172, 65–66 (1999).
- Shibata T, Iimuro Y, Ikai I, Hatano E, Yamaoka Y, Konishi J: Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J. Vasc. Interv. Radiol. 13, 313–315 (2002).
- Rhim H, Lim HK, Kim YS, Choi D: Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am. J. Roentgenol. 190, 91–98 (2008).
- Hinshaw JL, Laeseke PF, Winter TC III, Kliewer MA, Fine JP, Lee FT Jr: Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am. J. Roentgenol. 186, S306–S310 (2006).
- Sofocleous CT, Nascimento RG, Petrovic LM et al.: Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology 249(1), 364–374 (2008).
- Goldberg S, Grassi C, Cardella J et al.: Image-guided tumor ablation: Standardization of terminology and reporting criteria. J. Vasc. Interv. Radiol. 20, S377–S390 (2009).
- Koda M, Ueki M, Maeda Y et al.: The influence on liver parenchymal function and complications of radiofrequency ablation or the combination with transcatheter arterial embolization for hepatocellular carcinoma. Hepatol. Res. 29, 18–23 (2004).