Perspective - Imaging in Medicine (2012) Volume 4, Issue 5
Future prospects for dental cone beam CT imaging
Ruben Pauwels*1, Reinhilde Jacobs1, Hilde Bosmans2 & Ralf Schulze31Oral Imaging Center, Department of Oral Health Sciences, University of Leuven, Leuven, Belgium
2Department of Radiology, University Hospitals Leuven, Leuven, Belgium
3Deptartment of Oral Surgery (and Oral Radiology), University Medical Center of the Johannes Gutenberg University, Mainz, Germany
- Corresponding Author:
- Ruben Pauwels
Oral Imaging Center
Department of Oral Health Sciences
University of Leuven, Leuven, Belgium
Tel: +32 16 3 32410 or +32 16 3 32951
Fax: +32 16 3 32410
E-mail: ruben.pauwels@med.kuleuven.be
Abstract
Cone beam CT (CBCT) has been widely accepted as an imaging tool for a variety of dental and nondental applications. Although dental CBCT has evolved considerably in the last decade, there is still a lot of room for optimization of this modality at different levels. In this perspective, an overview is given of various hardware and software innovations that could be implemented in CBCT imaging in the foreseeable future. Some have already been applied to clinical practice but require further validation; others are being commonly applied by other imaging modalities and could be adapted for use in dental CBCT. The overview covers the x-ray tube, adaptive exposure techniques, beam and rotation geometry, detector technology and reconstruction algorithms. Furthermore, the combination of CBCT with optical imaging is discussed, the possibility of using these devices for nondental applications is evaluated, and the potential use of phase-contrast tomography is discussed.
Keywords
cone beam CT ▪ image quality ▪ image reconstruction ▪ optimization ▪ perspectives ▪ radiation dose ▪ x-ray imaging technology
Dental radiography has undergone a continual evolution since the acquisition of the first dental radiograph in 1896 by Friedrich Otto Walkhof, only 14 days after the report on the discovery by Roentgen [1]. For this inaugural radiograph, an exposure time of 25 min was used, resulting in exposures that were large enough to cause mild deterministic effects, such as local hair loss. Evidently, there have been a series of developments in x-ray imaging since that point and radiography has become an indispensable part of all diagnostic imaging fields, including dental practice. Currently, a variety of intra- and extraoral dental radiographic techniques are applied for diagnosis, treatment planning and follow-up of dental patients [2]. Although 2D radiographs are conventionally applied in dental practice, they often fail to answer the clinical question because of the superposition of tissues on the lesion or anatomical structure of interest.
Similar to planar radiography, 3D imaging has undergone a tremendous evolution throughout the years. Different methods of combining 1D or 2D projections into a 3D representation of the scanned objects have been applied over time, originating from the use of two images obtained at right angles. Film-based methods and basic tomographic techniques have evolved into CT, which is still undergoing continual innovation itself. The introduction of 3D imaging techniques in dental practice faces the practitioner with the selection of the most appropriate imaging modality for any given clinical indication. The currently existing range of available imaging techniques necessitates the definition of proper guidelines and clear selection criteria. This need became accentuated when cone beam CT (CBCT) was introduced into dental practice. CBCT uses a cone- or pyramid- shaped x-ray beam in conjunction with a 2D detector array to obtain a large series of projections from the scanned objects that are reconstructed into a 3D image stack [3,4].
Cone beam CT
CBCT has been applied in medicine as early as the 1980s, with its first application being primarily in the field of angiography [5]. Currently, the CBCT technique is commonly used in different fields of diagnostic medicine, such as radiotherapy planning and cardiology [6–8]. The first commercial dental CBCT device was introduced in 1998 [9,10]. Over a decade later, a large number of manufacturers are distributing one or several types of CBCT scanners. They are used for a wide array of clinical indications, mainly in the areas of implant surgery, endodontics, orthodontics and maxillofacial surgery [11–13]. From a radiological perspective, the main advantage of CBCT is its ability to acquire 3D reconstructions of the dentomaxillofacial region with high levels of detail. As a result, reconstructed data sets can be manipulated in a versatile way, and visualized and reformatted for an optimal evaluation. Improvements in patient treatment when using CBCT instead of 2D radiography or medical CT have been demonstrated for different clinical indications [12,13]. When compared with multislice CT (MSCT), a few aspects have supported the acceptance process of CBCT in dental practice: the size of the scanner, the cost and its ease of use make it accessible for both private dental practices and radiology clinics, as well as dental and general hospitals [11–13].
In recent years, a few advancements in hardware and software technology have resulted in a range of current and future opportunities for the optimization of CBCT imaging in dentistry. This perspective article provides an overview of the state-of-the-art and future outlooks on optimization of varying parts of the CBCT imaging chain (Box 1).

■ X-ray tube
A first parameter associated with the x-ray tube, which may be improved in the near future, is the focal spot, that is one of the key elements determining the resolution of an x-ray image [14–17]. Currently, the typical nominal focal spot size in dental CBCT is 0.5 mm. This relatively small focal spot enables high resolution imaging but may result in overheating and damaging of the tube anode. Overheating is currently avoided because of the relatively low exposure used in CBCT. Still, improvements in x-ray tube technology can result in even smaller focal spots. The use of a rotating anode has enabled one CBCT manufacturer to use a focal spot of 0.3 mm for its latest device. Further evolutions, such as the use of a floating focal spot and improved anode cooling, could result in more common use of smaller focal spots, with a potential improvement in resolution as a result. However, this improvement can only be achieved if other parts of the imaging chain are optimized as well. As seen below, the final spatial resolution can be limited by the detector, reconstruction or patient movement.
The main difference between current-generation CBCT devices regarding the x-ray tube is found in the peak voltage (kVp), which varies between 60 and 120 kVp for CBCT devices currently available. In combination with the filtration, the kVp value determines the spectrum of x-ray energies, which is a key determinant for both image quality and radiation dose [18]. Contrast between tissues differs for varying kVp values, as x-ray attenuation is highly dependent on x-ray energy. It can be expected that for each type of tissue (e.g., cortical bone, muscle, fat and lung tissue) there is an optimal beam energy for which the ratio between image quality and radiation dose is the highest. For dental CBCT imaging, as it is focused on the hard tissues, bony canals and air cavities in the oral region, this optimal beam energy is still to be determined. It is not possible to investigate the effect of the kVp on image quality experimentally, as existing dental CBCT scanners typically fix the kVp value. A few devices allow for the selection of a kVp between 60 and 90 kV. However, this does not represent the kVp range used by different CBCT devices. Therefore, it is not possible to single out the effect of the kVp while keeping all other exposure factors constant. Monte Carlo simulations may overcome this experimental limitation and aid in the determination of the optimal beam energy for dental CBCT imaging, and could lead to a standardization of kVp and filtration values between manufacturers. It is quite possible that this optimal beam energy differs for varying patient sizes, anatomical regions (e.g., maxilla and mandible) and diagnostic applications (e.g., sinus pathology vs implant planning).
A recent evolution and hot topic in MSCT imaging is the use of real-time dual energy imaging, in which two sets of projections are obtained at different energies, either by using two x-ray tubes operated at a different tube voltage, rapid tube voltage switching or successive acquisitions of low- and high-voltage scans [19–21]. There are various applications for dual energy imaging, as it allows for a broadening of the contrast range. Most notably, it can be applied in angiography for different purposes and can be a valuable replacement for the noncontrast image in a contrast-enhanced study [22,23]. In dental imaging, at first sight, the clinical application of dual energy imaging seems questionable, as it mainly involves the visualization of tissues with a high inherent contrast. A potential application is sialography, for which CBCT is currently applied using an injected contrast agent [24,25]. For CBCT imaging of the teeth, facial skeleton and air cavities, a single, optimal kVp may suffice, and the added value of dual-energy imaging may be limited to a few rare applications where low-contrast resolution is needed, although alternative nonionizing imaging modalities may be preferred in those cases [24–29]. Still, the added diagnostic value of dual energy dental imaging and its balance with radiation dose remains to be investigated.
■ Adapted exposure
Although the CBCT user is always able to select certain exposure parameters according to the clinical indication and patient size, this is a suboptimal and subjective approach towards proper x-ray exposure. Both tube voltage and tube load (mAs) should be adjusted to provide a set detector signal.
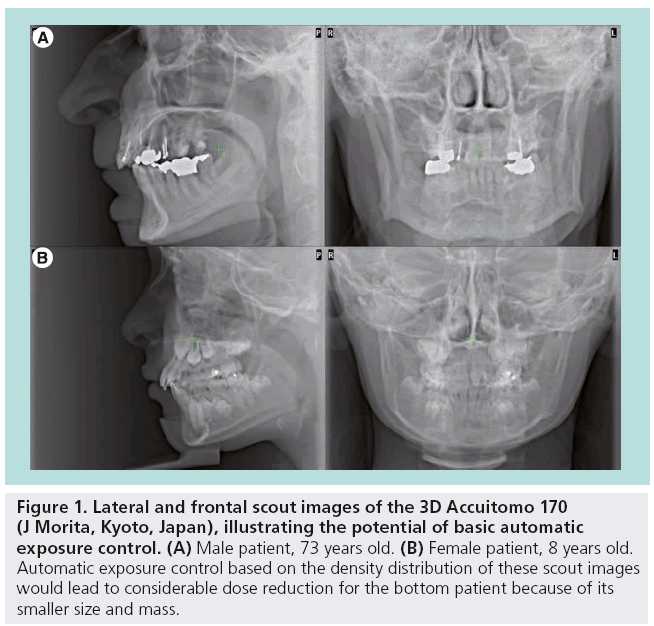
A first possibility for objective adapted exposure is the introduction of automatic exposure control (AEC) [30,31]. There are two levels of AEC. In its basic form, it implies that the mAs (possibly in combination with the kVp) is automatically determined based on the gray level (‘density’) histogram of the scout image, leading to an automatic reduction of exposure levels for smaller (i.e., size and mass) patients (Figure 1). This technique has been implemented by one CBCT manufacturer for many years, but according to our knowledge, it has not been adapted by others. Most CBCT devices are simply provided with either preset protocols for different patient sizes, or allow for manual selection of the exposure within certain ranges.
Figure 1: Lateral and frontal scout images of the 3D Accuitomo 170 (J Morita, Kyoto, Japan), illustrating the potential of basic automatic exposure control. (A) Male patient, 73 years old. (B) Female patient, 8 years old. Automatic exposure control based on the density distribution of these scout images would lead to considerable dose reduction for the bottom patient because of its smaller size and mass.
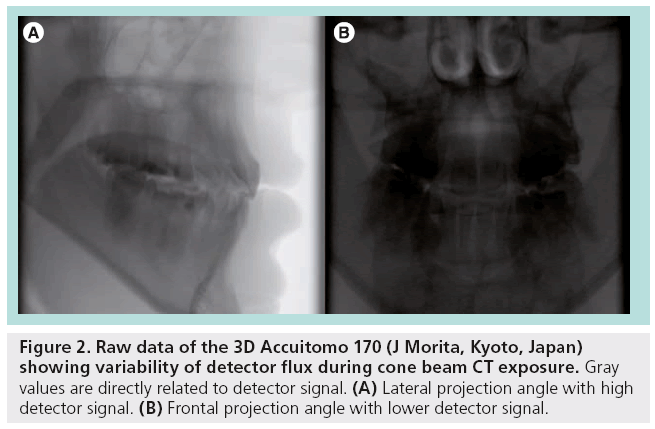
In addition, dynamic AECs could be used that adapt the exposure during the acquisition. As the primary beam encounters varying depths and densities of attenuating tissues during the rotation (Figure 2), a dynamic adapted exposure would ensure an adequate detector signal during the entire acquisition, avoiding over- or underexposure of the detector for all projection angles. For any type of AEC, the range of image quality criteria for different dental indications needs to be taken into account, seeing that a single reference level for AEC would not be suitable for the range of diagnostic requirements found within the various dental imaging applications. Similar to the use of AEC in MSCT, the user should still be able to select a task-specific reference dose level to ensure adequate image quality is attained.
Figure 2: Raw data of the 3D Accuitomo 170 (J Morita, Kyoto, Japan) showing variability of detector flux during cone beam CT exposure. Gray values are directly related to detector signal. (A) Lateral projection angle with high detector signal. (B) Frontal projection angle with lower detector signal.
Another area of development in CBCT imaging can be found in the beam collimation. As of now, all CBCT devices allow for one or more discrete options in terms of field of view (FOV) size. Although certain devices allow for a variety of volume sizes, the user is always limited by the available options. Even for those devices with an array of FOV options, an additional dose reduction could still be achieved if the FOV size could be any value within an available range (with an upper limit determined by the detector size), allowing it to be varied depending on the patients anatomy and the diagnostic indication. Seeing that the operator can exactly determine the region of interest (ROI) size based on a frontal and lateral scout image, the use of a dynamic FOV size that is drawn by the operator on the scout images warrants further investigation. It should be noted that the radiation dose provided by the scout image cannot be ignored and that it should also be collimated as much as possible based on prior information (i.e., the ROI, as stated in the referral). Still, the ability of a scout image to reduce overshooting of the actual scan to a minimum and to avoid mispositioning of the FOV greatly outweighs its radiation dose.
Unlike physical collimation of the beam, reconstruction-based collimation of the FOV should be avoided as much as possible. It is seen that, prior to or after reconstruction, the edges of the FOV are cut off by certain devices. Although these regions can be susceptible to artifacts, cropping the FOV implies that a small part of the exposure was not used for actual imaging, which does not fully comply with the principle of dose optimization. The importance of FOV reduction is further discussed in the next section.
■ Beam & rotation geometry
Although the geometric shape of the beams used in CBCT is always a symmetrical or nonsymmetrical cone or pyramid, there are a few specific adaptations of the beam shape that have important repercussions on imaging performance. A first technique that was introduced in CBCT imaging is off-axis scanning, where a ‘half cone’ is used with a small overlap in the isocenter. It is used to increase the size of the FOV beyond the limitation set by the detector size, achieving FOVs with almost twice the diameter compared with full cone scanning for a given detector panel width. Although it also leads to dose reduction compared with a full-cone scan, the added value of this technique compared with a straightforward mAs reduction or a half rotation can be questioned.
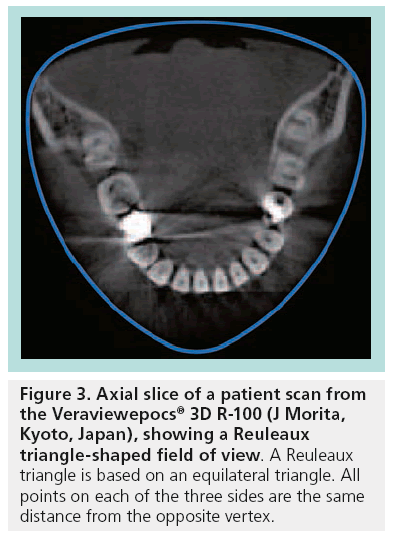
A special technique that was recently introduced is the use of a Reuleaux triangle-shaped FOV, which corresponds more to the dental arch compared with a cylindrical or spherical FOV (Figure 3). Although this leads to dose reduction for scans of the dental region, it somewhat focuses (but not limits) the application range of the device on the dental arches. The topic of specializing CBCT scanners for dentistry, as opposed to broadening the range of clinical applications, is further discussed below.
A further evolution that coud lead to considerable dose reduction is the inclusion of a smallsized FOV option for each device. For different reasons, it makes sense that every CBCT device should be able to scan using a small FOV, but not every device needs a medium or large FOV size, depending on the application range. In current practice, certain devices have a fixed large FOV, whereas other are limited to one or a few small- or medium-sized FOVs. Others allow for a full FOV range between small-sized (e.g., 4 cm diameter) and large (e.g., 15 cm or more) FOVs. The issue with fixed large FOVs is that they may lead to a large amount of unjustified exposure, as they are often applied in cases where only a small part of this FOV is required for diagnostic purposes [32–34]. Although this overshooting may result in incidental findings outside the ROI, these rare findings may still not be justified by the large amount of unnecessary radiation given to the majority of patients where these findings are absent [101]. More evidence is needed on the frequency and the severity of incidental findings in order to make a risk–benefit analysis at the population level, balancing the added exposure of consistently using large FOVs with the potential incidental detection of various pathologies. However, this kind of analysis will always be hampered by the large uncertainty associated with the relation between radiation dose and cancer risk at low exposure levels. When adhering to the generally accepted linear nonthreshold model for radiation risk at low doses, it is highly questionable that the consistent use of large FOVs, in line with a screening approach, will be acceptable in terms of radioprotection [34]. In addition, overscanning of a patient implies that the dentist or oral radiologist should take the time to read the entire scan, without purely focusing on the referred indication. Also, FOVs extending beyond the oral region may require the input of a general or a head and neck radiologist. Therefore, it is not purely from a radioprotection point of view that it should be recommended to collimate the FOV to the ROI. As it has been pointed out that, for the majority of patients, a small FOV would suffice, the reduction in population dose is expected to outweigh the small added benefit owing to incidental findings.
Regarding the rotation geometry, the use of a half (i.e., 180°) rotation can be considered as a useful technique for certain patient groups. At the moment, some devices are using a rotation arc between 180° and 220°. Others allow for the choice between a 360° and 180° rotation. In the latter case, a 180° rotation will have a similar mA compared with a 360° rotation, with a 50% reduction in mA or acquired projections leading to similar effects on the image, primarily expressed as an increase in noise. However, there are two added benefits to a 180° rotation. First of all, it cuts the scanning time in half, which is an important issue for patient groups with high risk of motion during scanning, such as young, old or handicapped patients. Furthermore, a 180° rotation may lead to dose reductions of more than 50% compared with 360° rotations, depending on the size and position of the FOV and the rotational direction of the tube. According to general intuition, for dental CBCT acquisitions, the tube moving at the anterior side would lead to higher doses to radiosensitive organs compared with the posterior side, as the radiosensitive tissues in the head and neck are primarily located on the anterior side (e.g., salivary glands and thyroid gland). However, dosimetric results have shown that this is not the case, mainly due to the anatomical location of the salivary glands, lymph nodes and extrathoracic airways, which are mostly posterior to the isocenter of a FOV positioned in the dental region. An effective dose evaluation performed in the SEDENTEXCT project on a CBCT scanner using various FOV sizes in combination with full and half rotations, and keeping all other exposure factors constant, has pointed out that if the tube moves on the anterior side during a 180° rotation, the reduction in dose is less than 40% [35]. This implies that tube movement on the posterior side would result in a dose reduction of 60% or more compared with a full rotation. If these results are confirmed, for example, by Monte Carlo simulations, it could be useful for manufacturers using half rotation to adapt their devices by making the tube move anteriorly, assuming that this does not significantly affect image quality compared with posterior movement.
■ Detector
Recent innovations in digital x-ray detection have resulted in increasingly higher detector efficiencies. Current CBCT devices use flat panel detectors (FPD); detectors containing image intensifiers that are still in use, although these are generally considered to result in inferior image quality [36–38]. FPDs have a layered structure consisting of a scintillator that converts x-rays into light and a photodiode array that transfers the light signal into an electrical signal, which is in turn transferred for image formation and processing. Recently, FPDs have evolved from amorphous silicon panels into large-area complementary metal oxide semiconductor detectors. At each detection level of the FPD, future developments may lead to marketable detectors getting closer and closer to the ‘perfect detector’. Increased efficiency in x-ray detection and transmission could lead to the use of increasingly smaller detector pixel sizes. In addition, an increased temporal resolution may lead to shorter scan times, and the ability to discriminate incident x-ray photon energies could lead to significant improvements in x-ray reconstruction.
■ Reconstruction
Optimization of image reconstruction has always been a hot topic in CT imaging. Along with the ever-increasing computational power of modern central and graphics processing units (CPU and GPU), new and adapted reconstruction algorithms are being introduced and evaluated on a preclinical and clinical level.
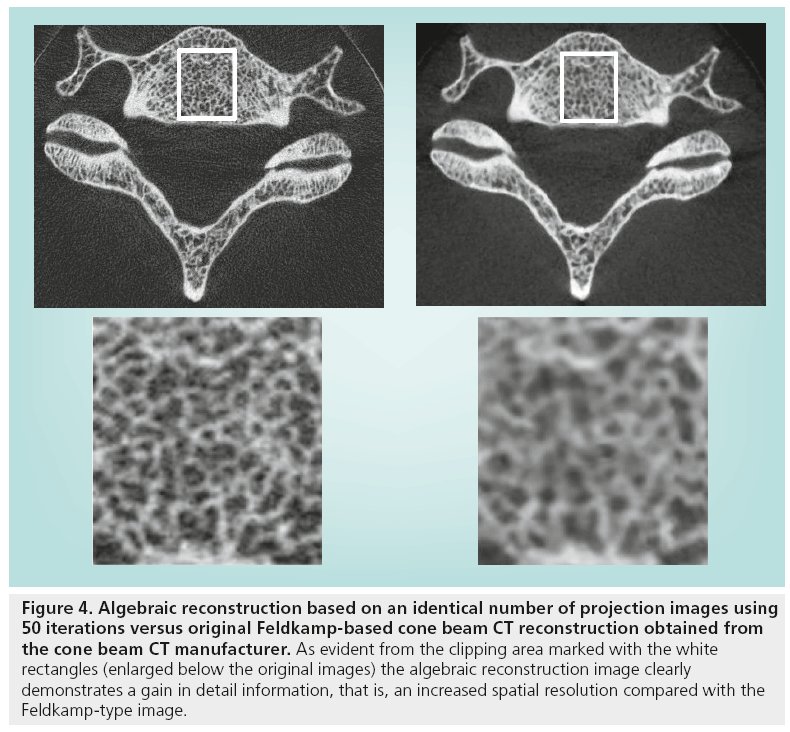
Most CBCT reconstruction algorithms are based on the Feldkamp algorithm, which is a modified back projection technique applied to 2D projections [39]. Recently, there has been a buzz about the algebraic reconstruction technique (ART); a class of iterative reconstructions algorithms. ART algorithms approach the reconstruction problem by searching for the optimal solution for a large number of algebraic equations determined by the projection data. Iterative reconstruction has been introduced in clinical MSCT imaging, resulting in dose reduction for various applications [40–42]. The use of ART is now considered for CBCT as well, seeing as the versatility of this technique may solve many issues that the Feldkamp algorithm cannot. One fundamental theoretical advantage of ART over direct inversion back projection techniques, such as the Feldkamp algorithm, lies in the fact that ART requires approximately only half of the number of projection images for reconstruction [43]. Apart from the raw data provided by the projections, it is possible to incorporate additional information into an ART algorithm to improve image quality by removing or minimizing known issues in x-ray tomographic reconstruction. As an example, the polyenergetic nature of an x-ray beam could be taken into account, and physical effects such as beam hardening and scatter could be modeled. If the detector was able to determine the energy of incident photons, the algebraic equations could be elaborated even further. As a result, significant improvements in image quality could be obtained. The potency of iterative algebraic reconstruction is illustrated in Figure 4, where the fine detail of the information clearly exceeds that of the Feldkamp-type reconstruction. Although a basic ART algorithm has already been applied in CBCT, more complicated algorithms have still not been brought into clinical practice. An optimization of the computational efficiency of these algorithms in combination with an increasing processing power of reconstruction hardware should result in the clinical implementation of iterative algorithms in the near future.
Figure 4: Algebraic reconstruction based on an identical number of projection images using 50 iterations versus original Feldkamp-based cone beam CT reconstruction obtained from the cone beam CT manufacturer. As evident from the clipping area marked with the white rectangles (enlarged below the original images) the algebraic reconstruction image clearly demonstrates a gain in detail information, that is, an increased spatial resolution compared with the Feldkamp-type image.
Two other potential improvements at the level of reconstruction, which are gradually being introduced into practice, are the use of metal artifact reduction (MAR) algorithms and motion correction. The presence of high-density metals inside the scanned area leads to a loss or alteration of projection data compared with a normal anatomic situation, which current reconstruction algorithms cannot resolve. This leads to parts of the reconstructed image, mostly in the vicinity of the metal, being prone to excessive artifacts. MAR is being applied by certain manufacturers but when it is used in combination with backprojection, the clinical improvement in image quality is limited, as it typically involves an adjustment of the projection data and an interpolation or averaging to reduce the artifacts [44–49]. Therefore, improvements in the appearance of the metal artifacts are often artificial (i.e., there is no actual gain of information in the image) or balanced with a general loss in image quality. Particularly for cases in which there is a loss of projection data due to complete absorption of the x-rays by a metal object, the replacement of this information could lead to an image that appears suitable but is in fact faulty, which could mislead the clinician into an incorrect diagnosis. With or without the use of MAR, it is always best to evaluate the vicinity of metal objects with great care and to avoid any quantitative use of gray values [50]. However, MAR used in combination with iterative reconstruction could largely overcome these issues. To a large extent, MAR can be easily incorporated into iterative reconstruction, as it allows for the modeling of the polychromatic beam spectrum, which would take the large amount of scatter, absorption and beam hardening by metal objects into account. However, as mentioned above, the computational power of current-generation reconstruction units can be considered as a bottleneck for the clinical use of advanced iterative reconstruction in dental CBCT. Next to MAR, the effect of patient motion on image quality is also being explored at the moment for various applications of CT [51–53]. In dental CBCT, patient motion could gravely affect image quality, as scan times are relatively long (typically ~20 s). In addition, reconstructed voxel sizes are small ( 0.08–0.4 mm) and even the slightest motion will have some effect on image sharpness. Ideally, motion detection and correction should be done at the level of the raw data, comparing consecutive projection images or comparing the first and last projection, providing that they are taken from the same angle. Although excessive movement can only be compensated for by removing the projections in which the movement has occurred, small movements or tremor could be resolved through matching of the projections, leading to increased image sharpness.
A final potential improvement in reconstruction that needs to be carefully considered by manufacturers is the calibration of gray values for density estimations. The main clinical use of quantitative gray values is the assessment of trabecular bone (i.e., bone quality, although this is not a consistent term) at implant sites [54]. Although different studies have shown that different factors (e.g., varying beam energy and local tomography effect) affect the variability of absolute gray values in CBCT [55–59], future improvement in reconstruction techniques (e.g., artifact reduction) may lead to acceptable gray value accuracy, at least for bony tissues. Although certain CBCT manufacturers claim to be using Hounsfield units, there is still room for improvement in terms of gray value calibration, ensuring the stability of gray values within and between images. The use of a reference object with known density in the scanned volume during clinical scanning could be explored, as it may provide a partial solution to this problem (Figure 5). The use of antiscatter grids could be of value as well, although this does not seem to be under consideration in current CBCT practice, as its use would require greater radiation exposure to the patient due to the partial absorption of primary x-rays. As it can be expected that the quantitative use of gray values in CBCT will remain susceptible to variability and error in the foreseeable future, the main focus in research should be the investigation and validation of alternative methods of assessing bone structure. When adapted for CBCT imaging, morphometric and structural analysis of trabecular bone may provide a more suitable prediction and evaluation of treatment outcome [60–62].
Figure 5: Quantitative CT–Bone Mineral Phantom (Image Analysis Inc., Columbia, KY, USA) containing three materials of known density. Similar, smaller density phantoms could be applied in dental cone beam CT for gray value calibration, although uniformity of gray values throughout the field of view remains an issue.
■ Optical imaging
Apart from the optimization of the x-ray imaging process itself, there are a few external imaging techniques which, in combination with a CBCT image, could lead to various possibilities in different dental areas. Recently, optical scanning has been introduced in dental imaging for intra- and extra-oral purposes. If these techniques are combined with CBCT scanning, they could result in new or improved patient treatment options. Digital impressions made with an intra-oral scanner could be merged with a CBCT image that is acquired for treatment planning. Using the complimentary information from both scans, optimal patient treatment and comfort could be achieved in prosthetic and implant dentistry [63].
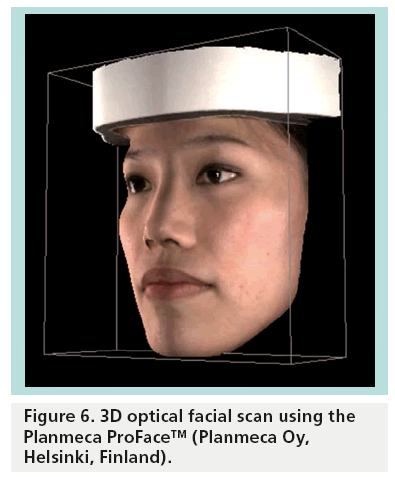
An additional optical technique that is now starting to be applied in combination with CBCT imaging is facial scanning (Figure 6) [64]. Currently, two CBCT devices allow for the acquisition of a facial scan, simultaneous with or apart from the CBCT exposure. The optical scan of the facial surface, when merged with the CBCT image of the hard and soft tissues, can be used to visualize the effect of orthodontic treatment or maxillofacial surgery on the facial features. It should be noted that the CBCT scan itself is able to distinguish between the soft tissue and the surrounding air. A volume rendering from a CBCT scan, preferably with seated or standing patient positioning, can provide a monochromatic representation of the facial topography, and the added value of a 3D photograph matched to this surface is still to be validated.
■ Nondental applications of CBCT
As mentioned above, the CBCT technique is currently applied in various fields of diagnostic medicine. Although dental CBCT devices are mainly used for implant planning, impacted teeth, endodontic evaluation and other dental applications, there are various potential uses for these devices, providing that they are designed in a way which allows for this broader application spectrum.
First of all, the use of CBCT for nondental head and neck indications (e.g., sinus and ear imaging) is being strongly considered. For sinus imaging, CBCT is already commonly applied [65–67]. Although CBCT is capable of providing high quality images of the sinuses at a reasonable radiation dose, it should be noted that modern MSCTs are capable of achieving very low exposure levels for sinus imaging. For imaging of the middle ear and surrounding structures, CBCT has a potential for significant patient dose reduction compared with MSCT, which typically uses high exposure protocols for this application [68–70]. Although the spatial resolution of CBCT is considered to be superior to that of MSCT, it should be noted that most devices are not able to scan at high enough exposure levels for imaging of the temporal bone, leading to a high degree of noise. Other nondental applications that are still located at the head and neck, and could therefore be implemented for most devices with a sufficiently large FOV size, are cervical spine scanning for orthopedic purposes and upper airway evaluations for various purposes [71–73]. It should be noted that these types of scans may not always be possible due to physical obstruction of the tube-detector gantry by the patient’s shoulders. Still, there is a promising future for CBCT being applied as a multipurpose scanner for dental, and head and neck application in a hospital environment or private radiological practices.
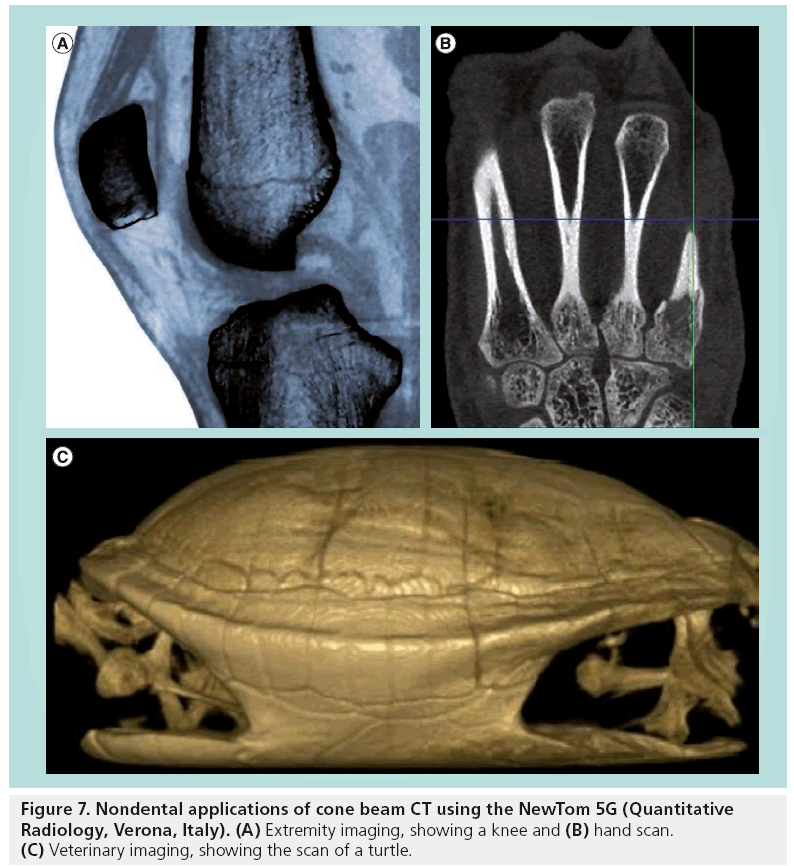
More recently, the use of dental CBCT for musculoskeletal scanning of extremities has been considered (Figure 7) [74,75]. Although most CBCT scanners should be able to provide high-quality images of the upper and lower extremities, it may be impossible to properly install patients for these types of scans using devices with standing or seated patient positioning.
Finally, 3D imaging is starting to find its way into veterinary practice for dental and nondental applications IN a range of animals (Figure 7) [76,77]. Again, veterinary applications of CBCT are limited to those devices with supine patient positioning.
It can be expected that certain CBCT manufacturers will keep their focus on the dental market, while others will choose to design their device for a broader spectrum of dental and nondental applications. The triangular FOV shape of the Veraviewepocs® 3D R-100 shown in Figure 3 is an example of a development tailored for dental imaging, whereas the use of supine patient positioning for the NewTom 5G and other devices allows for different applications that are not possible for devices with seated or standing positioning.
■ Phase-contrast tomography
Until today, medical radiographic imaging has only utilized x-ray absorption in the object for image formation. The phase nature of x-rays had been neglected simply because no technical method was available for using that information. On the other hand, research applying synchrotron x-ray sources has been carried out over many years to investigate x-ray phase-contrast. The interest in this novel technique is driven by the fact that in tissue with equal x-ray attenuation, such as soft tissues, the phase-shift information by far exceeds the attenuation (absorption) information [78]. Recently, sophisticated techniques have been developed that enable phase-contrast detection using conventional x-ray tubes at typical energies for medical radiography [79]. It has been demonstrated that this exciting technique can also be applied for cone beam tomography of nonliving objects [80]. The transfer to clinical practice is still challenging. However, given the current development speed it seems likely that in 10 years time, such machines should be readily available. 2D or 3D phase-contrast radiography. with its outstanding soft-tissue contrast, will mark another revolutionary step in the long history of x-ray imaging.
Conclusion & future perspective
Although CBCT has become an indispensable tool for various dental applications, there is a lot of room for dose reduction of this modality by following the optimization and justification principles. Optimization refers to the fact that each image should be obtained at the lowest possible exposure level. In this perspective, current and future possibilities for optimization are presented and discussed. It can be seen that a few recent innovations are already implemented in clinical practice by one or a few manufacturers and may or may not become the standard for the near future. Further optimization can be possible by adapting recent developments from other imaging modalities, particularly MSCT. A few other potential optimizations are more speculative, and their added value and marketability remains to be investigated. Finally, the combination of CBCT with optical imaging and its use in nondental applications is gradually being explored, and the use of phasecontrast tomography has been demonstrated experimentally and might be introduced into clinical practice.
Apart from the optimization of the CBCT technique itself, it is equally important to continue investigating the correct implementation of the justification principle to dental CBCT. The justification of CBCT exposure implies that practitioners should be able to make educated, individual decisions on the need for CBCT scanning for a given patient. Although this is a basic principle, stating that the expected benefit for any exposure to radiation should outweigh the estimated risk, it can be very complicated to apply and is typically prone to the subjective interpretation of the practitioner. Evidence-based referral criteria, as found in the SEDENTEXCT report published by the European Commission, can provide a guide to the CBCT user and lead to a degree of standardization concerning the selection of patients eligible for CBCT scanning [101]. It can be expected that the increasing evidence on CBCT radiation dose levels, dose–risk relationships at low radiation levels and patient treatment outcome after CBCT scanning will lead to a further refinement of referral criteria.
Acknowledgements
The authors acknowledge J Morita (Kyoto, Japan) and Quantitative Radiology (Verona, Italy) for providing the images for Figures 3 and 7, respectively. The authors also thank P Pittayapat (Oral Imaging Center, KU Leuven) for providing Figure 6.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Sansare K, Khanna V, Karjodkar F. Early victims of X-rays: a tribute and current perception. Dentomaxillofac. Radiol. 40(2), 123–125 (2011).
- Vandenberghe B, Jacobs R, Bosmans H. Modern dental imaging: a review of the current technology and clinical applications in dental practice. Eur. Radiol. 20(11), 2637–2655 (2010).
- Scarfe WC, Li Z, Aboelmaaty W, Scott SA, Farman AG. Maxillofacial cone beam computed tomography: essence, elements and steps to interpretation. Aust. Dent. J. 57, 46–60 (2012).
- Miracle AC, Mukherji SK. Conebeam CT of the head and neck, part 1: physical principles. Am. J. Neuroradiol. 30(6), 1088–1095 (2009).
- Altschuler MD, Censor Y, Eggermont PP et al. Demonstration of a software package for the reconstruction of the dynamically changing structure of the human heart from cone beam x-ray projections. J. Med. Syst. 4(2), 289–304 (1980).
- Boda-Heggemann J, Lohr F, Wenz F, Flentje M, Guckenberger M. kV cone-beam CTbased IGRT: a clinical review. Strahlenther. Onkol. 187(5), 284–291 (2011).
- Maier A, Wigstrom L, Hofmann HG et al. Three-dimensional anisotropic adaptive filtering of projection data for noise reduction in cone beam CT. Med. Phys. 38(11), 5896–5909 (2011).
- Lauzier PT, Tang J, Chen GH. Timeresolved cardiac interventional cone-beam CT reconstruction from fully truncated projections using the prior image constrained compressed sensing (PICCS) algorithm. Phys. Med. Biol. 57(9), 2461–2476 (2012).
- Arai Y, Tammisalo E, Iwai K, Hashimoto K, Shinoda K. Development of a compact computed tomographic apparatus for dental use. Dentomaxillofac. Radiol. 28(4), 245–248 (1999).
- Mozzo P, Procacci C, Tacconi A, Martini PT, Andreis IA. A new volumetric CT machine for dental imaging based on the cone-beam technique: preliminary results. Eur. Radiol. 8(9), 1558–1564 (1998).
- Dawood A, Patel S, Brown J. Cone beam CT in dental practice. Br. Dent. J. 207(1), 23–28 (2009).
- White SC, Pharoah MJ. The evolution and application of dental maxillofacial imaging modalities. Dent. Clin. North Am. 52(4), 689–705 (2008).
- Scarfe WC, Farman AG, Sukovic P. Clinical applications of cone-beam computed tomography in dental practice. J. Can. Dent. Assoc. 72(1), 75–80 (2006).
- Tang X, Narayanan S, Hsieh J et al. Enhancement of in-plane spatial resolution in volumetric computed tomography with focal spot wobbling - overcoming the constraint on number of projection views per gantry rotation. J. Xray Sci. Technol. 18(3), 251–265 (2010).
- Kyriakou Y, Kachelriess M, Knaup M, Krause JU, Kalender WA. Impact of the z-flying focal spot on resolution and artifact behavior for a 64-slice spiral CT scanner. Eur. Radiol. 16(6), 1206–1215 (2006).
- Flohr TG, Stierstorfer K, Ulzheimer S, Bruder H, Primak AN, McCollough CH. Image reconstruction and image quality evaluation for a 64-slice CT scanner with z-flying focal spot. Med. Phys. 32(8), 2536–2547 (2005).
- Prasad SC. Effects of focal spot intensity distribution and collimator width in reconstructive x-ray tomography. Med. Phys. 6(3), 229–232 (1979).
- Jarry G, Graham SA, Moseley DJ, Jaffray DJ, Siewerdsen JH, Verhaegen F. Characterization of scattered radiation in kV CBCT images using Monte Carlo simulations. Med. Phys. 33(11), 4320–4329 (2006).
- Sidky EY, Zou Y, Pan X. Impact of polychromatic x-ray sources on helical, cone-beam computed tomography and dual-energy methods. Phys. Med. Biol. 49(11), 2293–2303 (2004).
- Ko JP, Brandman S, Stember J, Naidich DP. Dual-energy computed tomography: concepts, performance, and thoracic applications. J. Thorac. Imaging 27(1), 7–22 (2012).
- Karçaaltıncaba M, Aktas A. Dual-energy CT revisited with multidetector CT: review of principles and clinical applications. Diagn. Interv. Radiol. 17(3), 181–194 (2011).
- Toepker M, Moritz T, Krauss B et al. Virtual non-contrast in second-generation, dualenergy computed tomography: reliability of attenuation values. Eur. J. Radiol. 81(3), e398–e405 (2012).
- Sommer CM, Schwarzwaelder CB, Stiller W et al. Iodine removal in intravenous dual-energy CT-cholangiography: is virtual non-enhanced imaging effective to replace true non-enhanced imaging? Eur. J. Radiol. 81(4), 692–699 (2012).
- Li B, Long X, Cheng Y, Wang S. Cone beam CT sialography of Stafne bone cavity. Dentomaxillofac. Radiol. 40(8), 519–523 (2011).
- Jadu FM, Hill ML, Yaffe MJ, Lam EW. Optimization of exposure parameters for cone beam computed tomography sialography. Dentomaxillofac. Radiol. 40(6), 362–368 (2011).
- Stoianovici C, Wilder-Smith P, Choi B. Assessment of pulpal vitality using laser speckle imaging. Lasers Surg. Med. 43(8), 833–837 (2011).
- Karayilmaz H, Kirzioglu Z. Comparison of the reliability of laser Doppler flowmetry, pulse oximetry and electric pulp tester in assessing the pulp vitality of human teeth. J. Oral Rehabil. 38(5), 340–347 (2011).
- Lopes S, Costa A, Cruz A, Li L, de Almeida S. Clinical and MRI investigation of temporomandibular joint in major depressed patients. Dentomaxillofac. Radiol. 41(4), 316–322 (2012).
- Li C, Su N, Yang X, Yang X, Shi Z, Li L. Ultrasonography for detection of disc displacement of temporomandibular joint: a systematic review and meta-analysis. J. Oral Maxillofac. Surg. 70(6), 1300–1309 (2012).
- Alibek S, Brand M, Suess C, Wuest W, Uder M, Greess H. Dose reduction in pediatric computed tomography with automated exposure control. Acad. Radiol. 18(6), 690–693 (2011).
- Papadakis AE, Perisinakis K, Oikonomou I, Damilakis J. Automatic exposure control in pediatric and adult computed tomography examinations: can we estimate organ and effective dose from mean MAS reduction? Invest. Radiol. 46(10), 654–662 (2011).
- Pauwels R, Beinsberger J, Collaert B et al. Effective dose range for dental cone beam computed tomography scanners. Eur. J. Radiol. 81(2), 267–271 (2012).
- Okano T, Harata Y, Sugihara Y et al. Absorbed and effective doses from cone beam volumetric imaging for implant planning. Dentomaxillofac. Radiol. 38(2), 79–85 (2009).
- Librizzi ZT, Tadinada AS, Valiyaparambil JV, Lurie AG, Mallya SM. Cone-beam computed tomography to detect erosions of the temporomandibular joint: effect of field of view and voxel size on diagnostic efficacy and effective dose. Am. J. Orthod. Dentofacial Orthop. 140(1), e25–e30 (2011).
- Pauwels R. Optimisation of cone beam computed tomography for dentomaxillofacial applications. Doctoral Thesis, University of Leuven, Belgium (2012).
- Jaffray DA, Siewerdsen JH. Cone-beam computed tomography with a flat-panel imager: initial performance characterization. Med. Phys. 27(6), 1311–1323 (2000).
- Baba R, Konno Y, Ueda K, Ikeda S. Comparison of flat-panel detector and image-intensifier detector for cone-beam CT. Comput. Med. Imaging Graph. 26(3), 153–158 (2002).
- Baba R, Ueda K, Okabe M. Using a flat-panel detector in high resolution cone beam CT for dental imaging. Dentomaxillofac. Radiol. 33(5), 285–290 (2004).
- Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J. Opt. Soc. Am. 1(1), 612–619 (1984).
- Korn A, Fenchel M, Bender B et al. Iterative reconstruction in head CT: image quality of routine and low-dose protocols in comparison with standard filtered back-projection. AJNR Am. J. Neuroradiol. 33(2), 218–224 (2012).
- Nelson RC, Feuerlein S, Boll DT. New iterative reconstruction techniques for cardiovascular computed tomography: how do they work, and what are the advantages and disadvantages? J. Cardiovasc. Comput. Tomogr. 5(5), 286–292 (2011).
- Renker M, Ramachandra A, Schoepf UJ et al. Iterative image reconstruction techniques: applications for cardiac CT. J. Cardiovasc. Comput. Tomogr. 5(4), 225–230 (2011).
- Mueller K. Fast and accurate threedimensional reconstruction from cone-beam projection data using algebraic methods. Dissertation Thesis, The Ohio State University, OH, USA (1998).
- Nakae Y, Sakamoto K, Minamoto T et al. Clinical evaluation of a newly developed method for avoiding artifacts caused by dental fillings on X-ray CT. Radiol. Phys. Technol. 1(1), 115–122 (2008).
- Bal M, Spies L. Metal artifact reduction in CT using tissue-class modeling and adaptive prefiltering. Med. Phys. 33(8), 2852–2859 (2006).
- Prell D, Kyriakou Y, Beister M, Kalender WA. A novel forward projection-based metal artifact reduction method for flat-detector computed tomography. Phys. Med. Biol. 54(21), 6575–6591 (2009).
- Wang G, Vannier MW, Cheng PC. Iterative X-ray cone-beam tomography for metal artifact reduction and local region reconstruction. Microsc. Microanal. 5(1), 58–65 (1999).
- Watzke O, Kalender WA. A pragmatic approach to metal artifact reduction in CT: merging of metal artifact reduced images. Eur. Radiol. 14(5), 849–856 (2004).
- Zhang Y, Zhang L, Zhu XR, Lee AK, Chambers M, Dong L. Reducing metal artifacts in cone-beam CT images by preprocessing projection data. Int. J. Radiat. Oncol. Biol. Phys. 67(3), 924–932 (2007).
- Pauwels R, Stamatakis H, Bosmans H et al. Quantification of metal artifacts on cone beam computed tomography images. Clin. Oral Implants Res. doi:10.1111/ j.1600-0501.2011.02382.x (2011) (Epub ahead of print).
- Schulz B, Jacobi V, Beeres M et al. Quantitative analysis of motion artifacts in high-pitch dual-source computed tomography of the thorax. J. Thorac Imaging doi:10.1097/ RTI.0b013e3182575729 (2012) (Epub ahead of print).
- Udrescu C, Jalade P, de Bari B, Michel- Amadry G, Chapet O. Evaluation of the respiratory prostate motion with fourdimensional computed tomography scan acquisitions using three implanted markers. Radiother. Oncol. 103(2), 266–269 (2012).
- Pauchard Y, Liphardt AM, Macdonald HM, Hanley DA, Boyd SK. Quality control for bone quality parameters affected by subject motion in high-resolution peripheral quantitative computed tomography. Bone 50(6), 1304–1310 (2012).
- Turkyilmaz I, Tumer C, Ozbek EN, Tözüm TF. Relations between the bone density values from computerized tomography, and implant stability parameters: a clinical study of 230 regular platform implants. J. Clin. Periodontol. 34(8), 716–722 (2007).
- Bryant JA, Drage NA, Richmond S. Study of the scan uniformity from an i-CAT cone beam computed tomography dental imaging system. Dentomaxillofac. Radiol. 37(7), 365–374 (2008).
- Katsumata A, Hirukawa A, Okumura S et al. Relationship between density variability and imaging volume size in cone-beam computerized tomographic scanning of the maxillofacial region: an in vitro study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 107(3), 420–425 (2009).
- Katsumata A, Hirukawa A, Okumura S et al. Effects of image artefacts on gray-value density in limited-volume cone-beam computerized tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 104(6), 829–836 (2007).
- Nackaerts O, Maes F, Yan H, Couto Souza P, Pauwels R, Jacobs R. Analysis of intensity variability in multislice and cone beam computed tomography. Clin. Oral Implants Res. 22(8), 873–879 (2011).
- Pauwels R, Stamatakis H, Manousaridis G et al. Development and applicability of a quality control phantom for dental cone-beam CT. J. Appl. Clin. Med. Phys. 12(4), 245–260 (2011).
- Hua Y, Nackaerts O, Duyck J, Maes F, Jacobs R. Bone quality assessment based on cone beam computed tomography imaging. Clin. Oral Implants Res. 20(8), 767–771 (2009).
- Fanuscu MI, Chang TL. Three-dimensional morphometric analysis of human cadaver bone: microstructural data from maxilla and mandible. Clin. Oral Implants Res. 15(2), 213–218 (2004).
- Torres SR, Chen CS, Leroux BG, Lee PP, Hollender LG, Schubert MM. Fractal dimension evaluation of cone beam computed tomography in patients with bisphosphonateassociated osteonecrosis. Dentomaxillofac. Radiol. 40(8), 501–505 (2011).
- Lee SJ, Gallucci GO. Digital vs. conventional implant impressions: efficiency outcomes. Clin. Oral Implants Res. doi:10.1111/ j.1600-0501.2012.02430.x (2012) (Epub ahead of print).
- Ma L, Xu T, Lin J. Validation of a threedimensional facial scanning system based on structured light techniques. Comput. Methods Programs Biomed. 94(3), 290–298 (2009).
- Bachar G, Barker E, Nithiananthan S et al. Three-dimensional tomosynthesis and cone-beam computed tomography: an experimental study for fast, low-dose intraoperative imaging technology for guidance of sinus and skull base surgery. Laryngoscope 119(3), 434–441 (2009).
- Güldner C, Pistorius SM, Diogo I, Bien S, Sesterhenn A, Werner JA. Analysis of pneumatization and neurovascular structures of the sphenoid sinus using cone-beam tomography (CBT). Acta Radiol. 53(2), 214–219 (2012).
- Prisman E, Daly MJ, Chan H, Siewerdsen JH, Vescan A, Irish JC. Real-time tracking and virtual endoscopy in cone-beam CT-guided surgery of the sinuses and skull base in a cadaver model. Int. Forum Allergy Rhinol. 1(1), 70–77 (2011).
- Peltonen LI, Aarnisalo AA, Kortesniemi MK, Suomalainen A, Jero J, Robinson S. Limited cone-beam computed tomography imaging of the middle ear: a comparison with multislice helical computed tomography. Acta Radiol. 48(2), 207–212 (2007).
- Dahmani-Causse M, Marx M, Deguine O, Fraysse B, Lepage B, Escudé B. Morphologic examination of the temporal bone by cone beam computed tomography: comparison with multislice helical computed tomography. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 128(5), 230–235 (2011).
- Peltonen LI, Aarnisalo AA, Käser Y et al. Cone-beam computed tomography: a new method for imaging of the temporal bone. Acta Radiol. 50(5), 543–548 (2009).
- Karandikar M, Mirza SK, Song K et al. Complex pediatric cervical spine surgery using smaller nonspinal screws and plates and intraoperative computed tomography. J. Neurosurg. Pediatr. 9(6), 594–601 (2012).
- Munera F, Rivas LA, Nunez DB Jr, Quencer RM . Imaging evaluation of adult spinal injuries: emphasis on multidetector CT in cervical spine trauma. Radiology 263(3), 645–660 (2012).
- Chiang CC, Jeffres MN, Miller A, Hatcher DC. Three-dimensional airway evaluation in 387 subjects from one university orthodontic clinic using cone-beam computed tomography. Angle Orthod. doi:10.2319/122811-801.1 (2012) (Epub ahead of print).
- Prakash P, Zbijewski W, Gang GJ et al. Task-based modeling and optimization of a cone-beam CT scanner for musculoskeletal imaging. Med. Phys. 38(10), 5612–5629 (2011).
- Zbijewski W, De Jean P, Prakash P et al. A dedicated cone-beam CT system for musculoskeletal extremities imaging: design, optimization, and initial performance characterization. Med. Phys. 38(8), 4700–4713 (2011).
- Roza MR, Silva LA, Barriviera M, Januario AL, Bezerra AC, Fioravanti MC. Cone beam computed tomography and intraoral radiography for diagnosis of dental abnormalities in dogs and cats. J. Vet. Sci. 12(4), 387–392 (2011).
- Roza MR, Silva LA, Januario AL, Fioravanti MC, Barriviera M. Cone beam computed tomography in the diagnosis of temporomandibular joint alterations in cats. J. Feline Med. Surg. 13(6), 393–398 (2011).
- Weitkamp T, Diaz A, David C et al. X-ray phase imaging with a grating interferometer. Opt. Express. 13(16), 6296–6304 (2005).
- Pfeiffer F, Weitkamp T, Bunk O, David C. Phase retrieval and differential phase-contrast imaging with low-brilliance x-ray sources. Nat. Phys. 2(4), 258–261 (2006).
- Pfeiffer F, David C, Bunk O et al. Region-ofinterest tomography for grating-based X-Ray differential phase-contrast imaging. Phys. Rev. Lett. 101(16), 168101 (2008).
- European Commission. Cone beam CT for dental and maxillofacial radiology: evidence based guidelines. Radiation Protection Publication 172 (2012). http://ec.europa.eu/energy/nuclear/ radiation_protection/doc/publication/172.pdf
• • Overview of current intra- and extra-oral dental imaging techniques.
• • Overview of dental cone beam CT technology, equipment and image interpretation aspects.
• Potential of automatic exposure control for dose reduction, with a specific focus on pediatric applications.
• Large-scale study estimating the effective dose for dental cone beam CT devices using an anthropomorphic phantom, showing a 20-fold dose range.
• Quantitative and qualitative evaluation of the potential of iterative reconstruction in CT scanning of the head.
• Study showing evidence-based limitations of the use of cone beam CT gray values.
■ Website