Review Article - Interventional Cardiology (2010) Volume 2, Issue 1
Effect of drug-eluting stents on endothelial dysfunction
- Corresponding Author:
- Mehmet Cilingiroglu
Department of Internal Medicine, Division of Cardiovascular Diseases
University of Cincinnati College of Medicine, 231 Albert Sabin Way
ML0542, Cincinnati, OH 45267-0542, USA
Tel: +1 513 417 3889
Fax: +1 513 558 6899
E-mail: mehmet.cilingiroglu@uc.edu
Abstract
Keywords
drug-eluting stent, endothelial cell dysfunction, stent thrombosis, vasomotor reactivity
Widespread use of drug-eluting stents (DES) in clinical practice has had a tremendous impact in reducing the clinical events and angiographic restenosis after percutaneous coronary intervention [1–3]. However in early 2004, reports began to suggest an increased incidence of early, late and very late stent thrombosis (ST) after first-generation DES implantation, especially after discontinuation of dual antiplatelet therapy [4]. One of the primary mechanisms proposed for subacute thrombosis has been delayed or incomplete endothelialization of the vessel wall at the site of stent deployment because of the presence of the drug, the polymer, or both, leading to thrombus formation [5]. Thus, prolonged dual antiplatelet therapy has been recommended to reduce late ST, a rare but potentially life-threatening complication of DES use that has become an increasingly controversial issue in interventional cardiology [6–8]. In recent years, several studies have reported longterm endothelium-related vascular dysfunction in the nonstented segments of the coronary arteries following DES implantation [9–11]. Although the concept of DES-induced endothelial dysfunction leading to late ST is hypothetical, without studies showing causal association, previous studies have demonstrated that coronary endothelial vasodilator dysfunction is an independent predictor of atherosclerotic disease progression and cardiovascular event rates [12]. We review the currently available data with use of different DES types and their effects on endothelial dysfunction.
Endothelial function & dysfunction
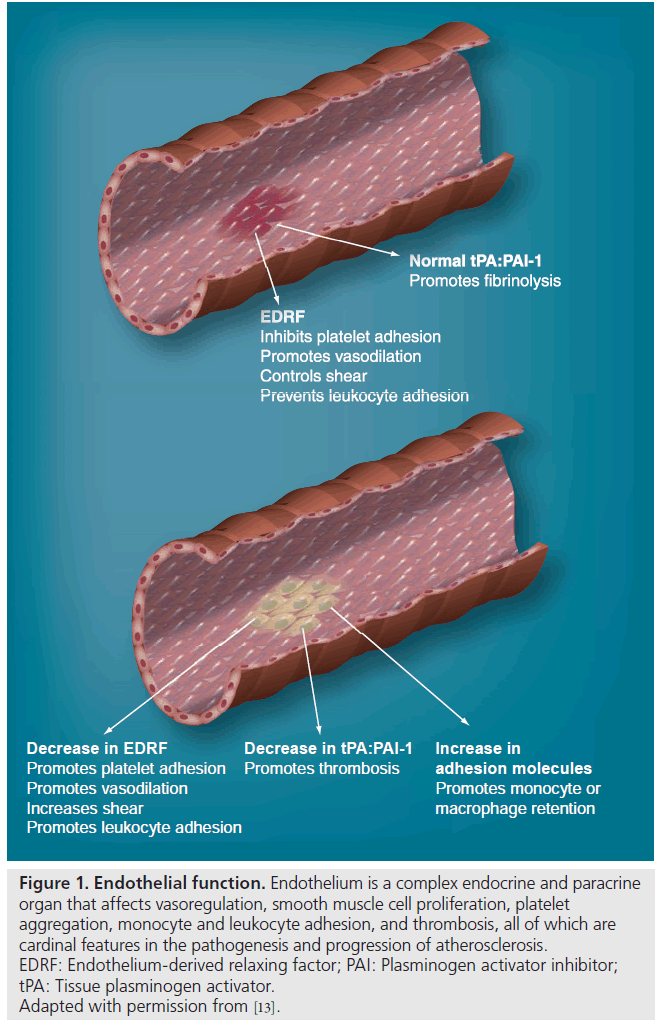
Endothelium is a single layer of cells that has a multitude of functions, including vasomotor regulation, maintaining the balance between coagulation and fibrinolysis, being the mediator of inflammation by allowing leukodiapethesis, and being one source promoting vasculogenesis (Figure 1) [13].
Figure 1: Endothelial function. Endothelium is a complex endocrine and paracrine organ that affects vasoregulation, smooth muscle cell proliferation, platelet aggregation, monocyte and leukocyte adhesion, and thrombosis, all of which are cardinal features in the pathogenesis and progression of atherosclerosis. EDRF: Endothelium-derived relaxing factor; PAI: Plasminogen activator inhibitor; tPA: Tissue plasminogen activator. Adapted with permission from [13].
Nitric oxide (NO) is primarily secreted by the endothelial cells and plays a key role in coronary vasomotor function, inflammation, thrombosis, atherosclerosis and arterial neointima formation [14]. Endothelial dysfunction is commonly defined as a state of reduced NO availability. This can be attributed to decreased availability of l-arginine or impaired expression of endothelial NO synthase (eNOS), or increased metabolism of NO itself.
Clinically, endothelial function is most often assessed as a vasodilator response to pharmacological or mechanical stimuli [15,16]. Under basal conditions, there is no or minimal resting sympathetic tone in the heart vasculature and, thus, there is no effect of denervation on resting perfusion. During sympathetic activation such as exercise, coronary tone is modulated by norepinephrine released from myocardial sympathetic nerves, as well as by circulating norepinephrine and epinephrine [17]. In conduit arteries, sympathetic stimulation leads to α1 constriction as well as β2-mediated vasodilatation. The net effect is to dilate the pericardial coronary arteries. This dilation is potentiated by concomitant flow-mediated vasodilatation from metabolic vasodilation of coronary resistance vessels. When NO-mediated vasodilation is impaired, α1 constriction predominates and can dynamically increase stenosis severity in asymmetrical lesions where the vessel is compliant. This is one of the mechanisms that can provoke ischemia during sympathetic activation from cold pressor testing or exercise [12,17].
Beside incomplete endothelialization or dysfunctional endothelium, there are other proposed mechanisms leading to paradoxical vasoconstriction in diseased coronary arteries as normal coronary arteries dilate in response to exercise [12]. The paradoxical response of the stenotic arteries during exercise has been attributed to a variety of potential mechanisms including: reduced NO availability at the site of the stenosis (endothelial dysfunction); enhanced vasoconstrictor response due to an increase in circulating catecholamines (enhanced sympathetic stimulation); enhanced platelet aggregation due to turbulent blood flow through the stenotic lesion with release of thromboxane A2 and serotonin; and flowinduced (passive) collapse of the normal vessel segment within the stenosis (Venturi effect).
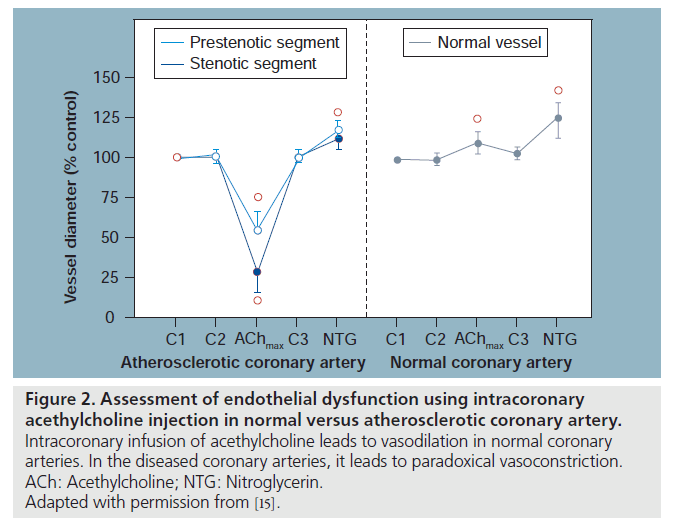
Sympathetic and vagal nerves innervate coronary conduit arteries and segments of the resistance vasculature. Neural stimulation affects vascular tone through mechanisms that alter vascular smooth muscle cells as well as by stimulating the release of NO from the endothelium. Diametrically opposite effects can occur in the presence of risk factors that impair endotheliumdependent vasodilation. Resistance arteries dilate in response to acethylcholine (ACh), resulting in increased coronary flow [12]. In conduit arteries, ACh normally causes mild coronary vasodilatation (Figure 2). This reflects the net action of a direct muscarinic constriction of vascular smooth muscle counterbalanced by an endothelium-dependent vasodilatation caused by direct stimulation of NOS and an increased flow-mediated dilation from concomitant resistance vessel vasodilatation. The response in humans with clinical or subclinical atherosclerosis is distinctly different. The resistance vessel dilation to ACh is attenuated and the reduction in flow-mediated NO production leads to net paradoxical epicardial conduit artery vasoconstriction, which is particularly prominent in stenotic segments (Figure 2) [12]. In the cardiac catheterization laboratory, endothelial function can be estimated by measuring the coronary vasoreactivity in response to titrating doses of intracoronary infusion of ACh.
Figure 2: Assessment of endothelial dysfunction using intracoronary acethylcholine injection in normal versus atherosclerotic coronary artery. Intracoronary infusion of acethylcholine leads to vasodilation in normal coronary arteries. In the diseased coronary arteries, it leads to paradoxical vasoconstriction. ACh: Acethylcholine; NTG: Nitroglycerin. Adapted with permission from [15].
In contrast to ACh, nitroglycerin (NTG) is an endothelium-independent vasodilator. It dilates epicardial conduit arteries and small coronary resistance arteries but does not increase coronary blood flow in the normal heart. Since it is an endothelium-independent vasodilator, it can induce coronary vasodilatation even in the presence of atherosclerosis and associated risk factors [18].
Coronary resistance arteries and arterioles also regulate their diameter in response to changes in local shear stress [19]. This is found to be endothelium- dependent and NO-mediated. Flowmediated vasodilation leads to vasodilatation in normal coronary arteries, but by contrast, it causes paradoxical vasoconstriction in coronary artery disease or in the presence of associated risk factors. Dynamic bicycle exercise or rapid atrial pacing has been used to induce flow-mediated coronary vasodilation to assess endothelial function in the cardiac catheterization laboratory [12].
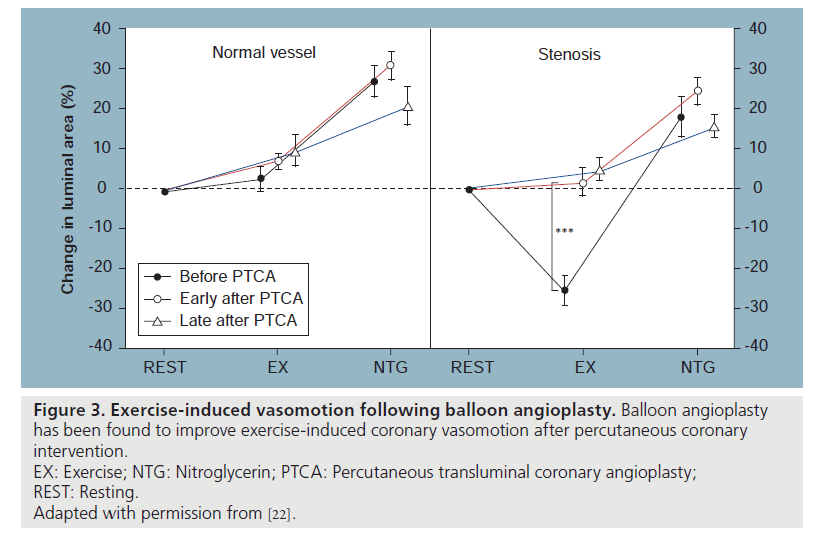
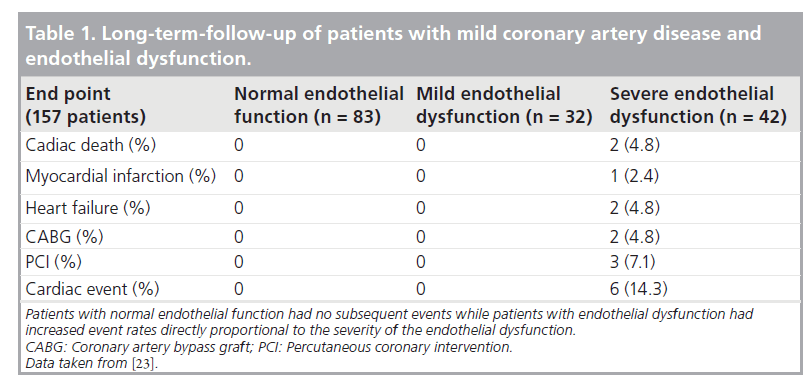
Investigation of endothelial function was performed early in the balloon angioplasty era. As a response to healing after the barotrauma of balloon angioplasty alone, the residual endothelial cells are dysfunctional and remain so for a variable period of time (Figure 3) [20,21]. However, the healing of an injured vessel segment after percutaneous transluminal balloon angioplasty is associated with re-endothelialization and improvement of endothelial function or even normalization of response at follow-up when assessed months later. It was also shown that successful angioplasty reverses the vasoconstrictive response of stenotic arteries during exercise [20]. The mechanism by which balloon angioplasty improves or restores coronary vasomotion is attributed mainly to an increase in endothelial cell number and function with enhanced NO release during exercise [21,22]. Clinical outcomes are associated with coronary vasoreactivity. Suwaidi et al. have reported that in a series of patients with nonobstructive coronary lesions stratified by endothelial dysfunction, those who had normal function had no subsequent cardiac events while those who had severe dysfunction had a 14% (six out of 42) cardiac event rate, including two deaths and one myocardial infarction (Table 1) [23].
Figure 3: Exercise-induced vasomotion following balloon angioplasty. Balloon angioplasty
has been found to improve exercise-induced coronary vasomotion after percutaneous coronary
intervention.
EX: Exercise; NTG: Nitroglycerin; PTCA: Percutaneous transluminal coronary angioplasty;
REST: Resting.
Adapted with permission from [22].
Table 1: Long-term-follow-up of patients with mild coronary artery disease and endothelial dysfunction.
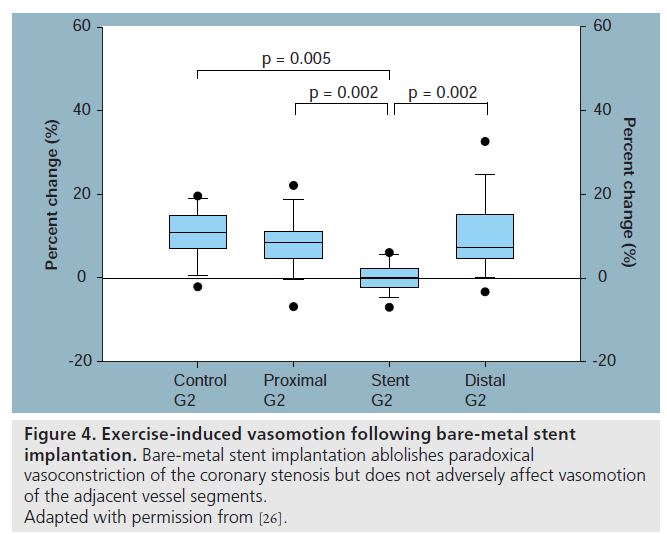
Implantation of balloon expandable stents is associated with deep vessel wall injury as well as trauma to the coronary endothelium. This trauma sets in motion the inflammatory reaction and a healing response that eventually leads to re-endothelialization during follow-up. In the first month after the implantation of a baremetal stent (BMS), a new immature endothelial layer covers the stent struts, re-establishing an endothelial lining of the coronary vessel wall. Abnormal and immature endothelial cells have a thrombogenic surface, promote adherence of various circulating monocytes and platelets, and facilitate platelet aggregation, leukocyte infiltration and vascular smooth muscle cell proliferation. Endothelial dysfunction is even more pronounced after BMS compared with balloon angioplasty [24,25]. BMS implantation abolishes paradoxical vasoconstriction of coronary stenosis in the stented segment and renders a previous vasoresponsive vessel to a rigid tube. Although initial vasomotor studies using intracoronary infusion of ACh suggested possible long-term impaired endothelial function in the proximal and distal adjacent segments after BMS implantation, Maier et al. showed maintenance of nearnormal vasomotor response to exercise at the proximal and distal vessel segments (Figure 4) [26].
Several landmark studies using DES have demonstrated an impressive reduction in the rates of angiographic restenosis and associated clinical events [1,3]. However, long-term results of DES implantation with regard to vascular integrity and coronary endothelial function were not studied in detail until recently.
First-generation drug-eluting stents
The sirolimus-eluting stent (SES; Cypher™, Cordis Corporation, FL, USA) is one of the first-generation DES. Sirolimus inhibits smooth muscle cells as well as endothelial cell proliferation via cell cycle arrest in the late G1 phase by forming a complex with FK506-binding protein (FKBP12) that inhibits the protein Ser- Thr kinase mammalian target of rapamycin (mTOR), with mTOR being a central element in signaling pathways involved in the control of cell growth and proliferation [27,28]. Recently, concerns have been raised that SES use could be associated with increased rates of ST owing to delayed or absent endothelialization [4,6]. Long et al. showed that acute in vitro sirolimus treatment as well as genetic deletion of the sirolimusreceptor isoform FKBP12.6 increased PKCmediated eNOS threonine 495 phosphorylation, which could result in decreased vascular NO production and subsequent endothelial dysfunction [29]. Impairment of endothelial recovery may theoretically predispose the patient to poor longterm effects such as ST, progression of coronary lesions and negative vascular remodeling [5,7].
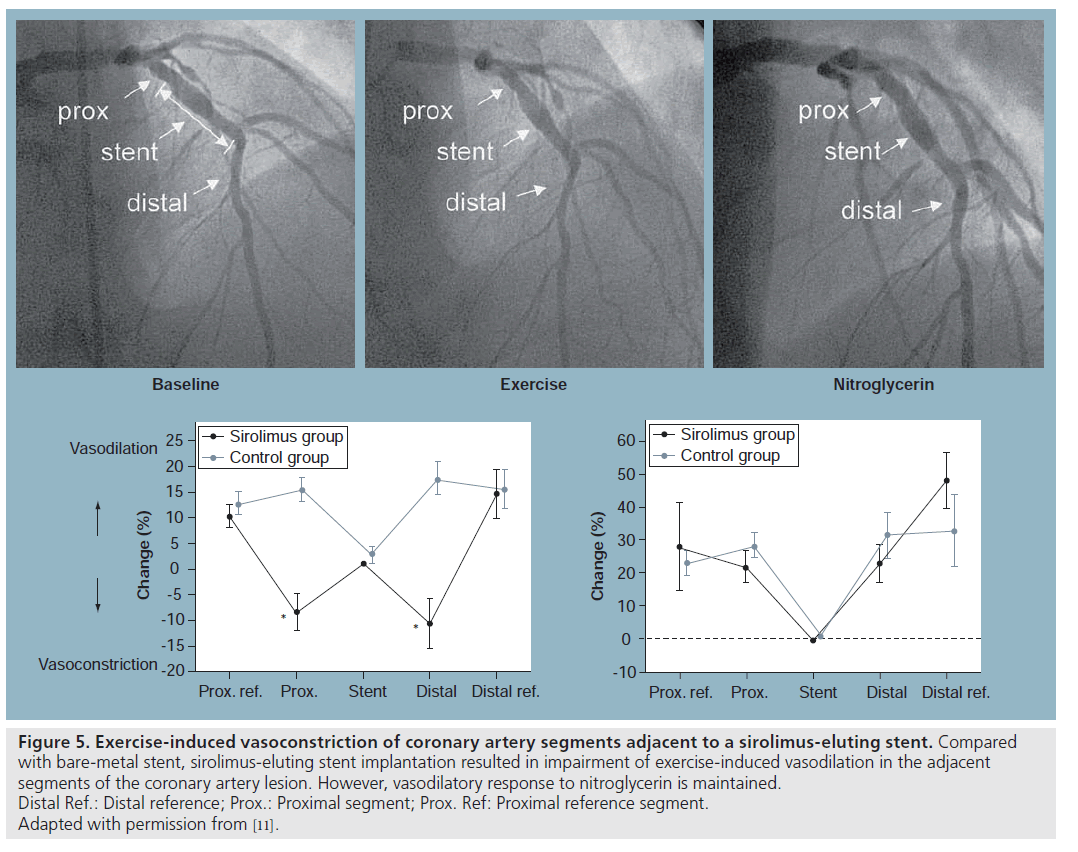
Togni et al. studied coronary vasomotor response to bicycle exercise after SES versus BMS in 24 patients with de novo lesions [11]. All the baseline characteristics and procedural data were comparable with regards to stented vessel, stent length and diameter between the two groups.
Stent implantations were performed according to standard guidelines. Vasoactive medications were discontinued at least 48 h before catheterization. Diagnostic catheterization was performed with standard techniques at baseline. At the end of diagnostic catheterization, biplane coronary angiography was performed with the patient’s feet attached to the supine bicycle ergometer. Exercise began at 50 or 75 W and workload was increased every 2 min in increments of 25 or 50 W. The catheter was left in place during exercise. Coronary angiography was carried out at the end of each exercise level and at maximal exercise in deep inspiration. At the end of the exercise test, all patients received 1.6 mg NTG sublingually, and 5 min later coronary angiography was repeated. NTG was administered routinely to assess endothelium-independent vasodilatation. Both groups were studied at 6 months (± 1 month) after the intervention. Minimal luminal diameter, stent diameter, and proximal, distal and reference vessel diameter were determined using quantitative coronary angiography. Angiographic as well as intravascular ultrasound (IVUS) data indicated normal structural appearance of the target vessel after SES. These findings, however, could not exclude functional abnormalities of peri-stent and in-stent segments after SES implantation. This study revealed exercise-induced vasoconstriction of coronary artery segments adjacent to SES (i.e., within a range of 5–10 mm proximal and distal to the stent edges [peristent region]) (Figure 5). Outside this region (i.e., 10–20 mm proximal and distal to the stent edges) exercise-induced vasomotion was normal, comparable to patients with BMS, suggesting that sirolimus may induce vascular damage with endothelial dysfunction, thereby reducing NO availability in the peristent region. Paradoxical vasoconstriction in a vessel segment not subjected to percutaneous transluminal coronary angioplasty barotraumas may be due to diffusion of the antiproliferative drug from the stent to peri-stent region, inducing endothelial dysfunction. This hypothesis remains to be tested in vivo. Similarly, Hofma et al. demonstrated endothelial dysfunction in the peristent region after SES with ACh testing [10].
Figure 5: Exercise-induced vasoconstriction of coronary artery segments adjacent to a sirolimus-eluting stent. Compared
with bare-metal stent, sirolimus-eluting stent implantation resulted in impairment of exercise-induced vasodilation in the adjacent
segments of the coronary artery lesion. However, vasodilatory response to nitroglycerin is maintained.
Distal Ref.: Distal reference; Prox.: Proximal segment; Prox. Ref: Proximal reference segment.
Adapted with permission from [11].
Cypher stents used in this study contained a 5‑μm-thick coating of the drug sirolimus mixed with nonerodable polymers, topped with a layer of drug-free polymer to allow the release of approximately 80% of the drug within 30 days after implantation [30]. Since most of the drug sirolimus is eluted from the polymer coating of the stent by 28 days [31] and is reportedly fully eluted by 60 days [32], abnormal vasomotion observed at 6 months in this study should not be the result of a direct effect of sirolimus itself. However, the induction of a persistent abnormality in intact or regenerating endothelium occurring while sirolimus was present cannot be excluded either. Alternatively, the polymer from which the drug elutes, which may have contributed to a case of a marked hypersensitivity reaction [32], could be involved in the abnormal vasomotion observed. In addition, the finding of paradoxical vasoconstriction could be the result of delayed endothelialization, with inadequate endothelial coverage leading to insufficient NO release to promote normal vasodilation with exercise. Vasomotion within the stented segment was abolished as expected. Sublingual NTG was associated with maximal vasodilatation of the proximal and distal vessel segments regardless of stent type.
Jabs et al. further studied the possible molecular mechanisms that mediate endothelial dysfunction induced by sirolimus [33]. To mimic the continuous sirolimus exposure of a stented vessel, they used Wistar rats that underwent drug infusion with an osmotic pump for 7 days. Both endothelium- dependent as well as endothelium-independent vasodilator responses were significantly impaired after 7 days of sirolimus treatment [33]. There was a 40% reduction in NO bioavailability. There was also increased transmural reactive oxygen species (ROS) production. This ROS increase was triggered by NADPH oxidase expression and membrane association as well as stimulation of mitochondrial ROS release. The systemic polymer containing the drug may also be the trigger of local inflammation. Coronary inflammation is responsible for delayed re-endothelialization of the stent and vessel wall, which eventually results in a delayed vascular healing response. Sirolimus impairs normal healing processes of the injured arterial wall by inhibiting endothelial cell proliferation. In addition, sirolimus was demonstrated to enhance tissue factor expression in human endothelial cells [34], and inhibit the migration and proliferation of endothelium progenitor cells, which may play an important role in endothelium regeneration [26]. These findings suggest that SES may impair both the endothelial regeneration and the ability of endothelial cells to exert their normal function. Poor endothelialization means that stent struts are in direct contact with blood and its elements. Human autopsies showed insufficient endothelial coverage and delayed arterial healing with SES, regardless of the delay from implantation [35]. These findings are complemented by angioscopy [36] and optical coherence tomography (OCT) imaging [37], which showed incomplete endothelialization and uncovered stent struts 3–6 months after SES implantation. Complete or partial lack of re-endothelialization of the stent and vessel surface favoring platelet adhesion and aggregation may eventually cause thrombus formation.
These findings of incomplete endothelial coverage within the stented segment and dysfunctional endothelium proximal and distal to the stent suggest that in a subset of patients, SES may be vulnerable for late-ST and peristent restenosis.
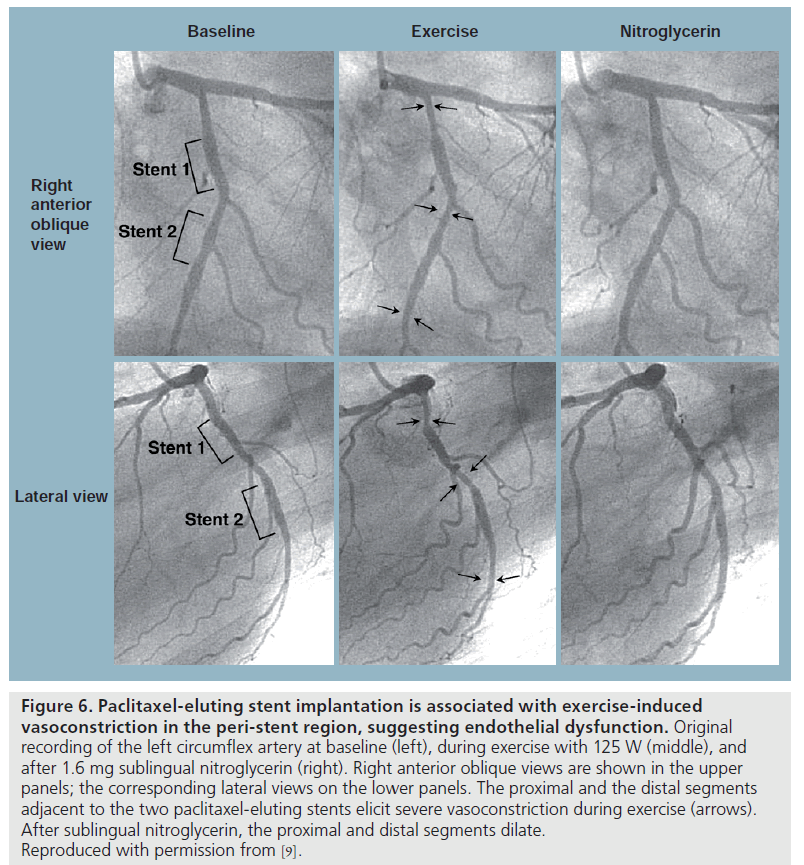
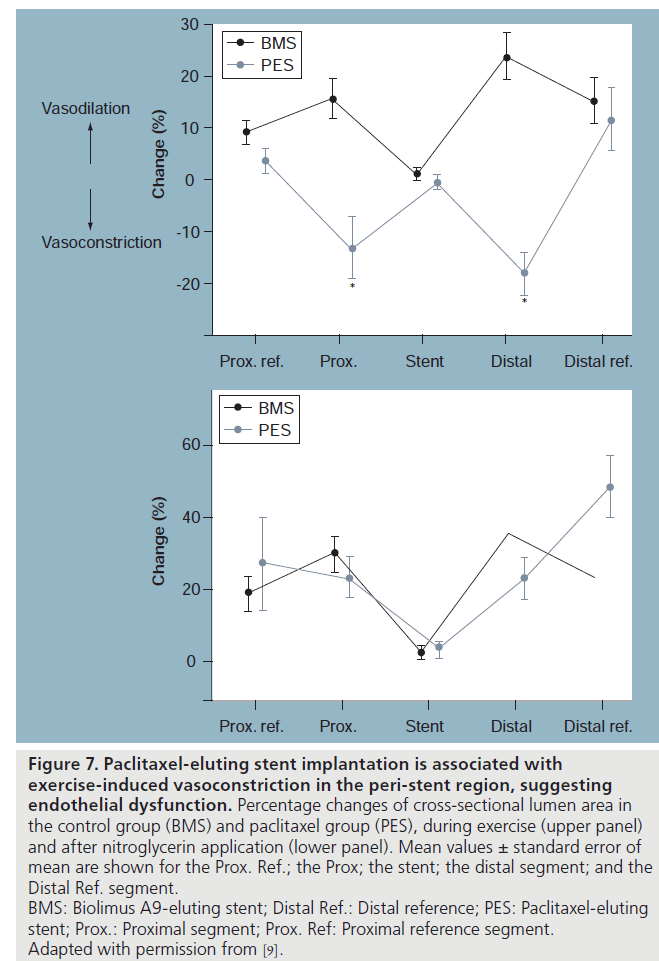
Paclitaxel is an antimicrotubule agent that interferes with neointimal vascular smooth muscle cell accumulation and proliferation [38]. Togni et al. also studied long-term effects (2–12 months) of paclitaxel-eluting stents (PES; TaxusTM, Boston Scientific Corporation, MA, USA) on vascular function compared with its BMS counterparts [9]. Coronary vasomotion was evaluated by biplane quantitative coronary angiography at rest and during supine bicycle exercise according to the previous protocol in 27 patients with significant coronary artery disease. Reference vessels showed exercise-induced vasodilatation in both groups. Vasomotor function within the stented segment was again abolished as expected. In the BMS group, the adjacent segments proximal and distal to the stent showed exercise-induced vasodilatation. By contrast, there was exercise-induced vasoconstriction of the proximal and distal vessel segments adjacent to the PES (Figures 6 & 7). After sublingual NTG, both the proximal and distal segments dilated in both groups. In addition, exercise-induced vasoconstriction adjacent to PES correlated inversely with the time interval after stent implantation; suggesting improvement in vascular function over time, indicating delayed vascular healing.
Figure 6: Paclitaxel-eluting stent implantation is associated with exercise-induced
vasoconstriction in the peri-stent region, suggesting endothelial dysfunction. Original
recording of the left circumflex artery at baseline (left), during exercise with 125 W (middle), and
after 1.6 mg sublingual nitroglycerin (right). Right anterior oblique views are shown in the upper
panels; the corresponding lateral views on the lower panels. The proximal and the distal segments
adjacent to the two paclitaxel-eluting stents elicit severe vasoconstriction during exercise (arrows).
After sublingual nitroglycerin, the proximal and distal segments dilate.
Reproduced with permission from [9].
Figure 7: Paclitaxel-eluting stent implantation is associated with
exercise‑induced vasoconstriction in the peri-stent region, suggesting
endothelial dysfunction. Percentage changes of cross-sectional lumen area in
the control group (BMS) and paclitaxel group (PES), during exercise (upper panel)
and after nitroglycerin application (lower panel). Mean values ± standard error of
mean are shown for the Prox. Ref.; the Prox; the stent; the distal segment; and the
Distal Ref. segment.
BMS: Biolimus A9-eluting stent; Distal Ref.: Distal reference; PES: Paclitaxel-eluting
stent; Prox.: Proximal segment; Prox. Ref: Proximal reference segment.
Adapted with permission from [9].
Finn et al. reported that sirolimus or paclitaxel released from a DES impaired the normal healing process of the injured arterial wall, even over a period of 40 months after implantation, and the heterogeneity of healing in the stents was associated with late-stent thrombi [35]. Kim et al. showed in vivo that both SES and PES can equally impair endothelial function and that their effects were demonstrable 6 months after implantation, especially in the arterial segments distal to the DES 6 months after stenting [39].
Second-generation drug-eluting stents
Second-generation DES have been developed in order to improve the biologic healing process. These stents employ newer formulations of biodegradable polymers and drugs specifically developed for local applications.
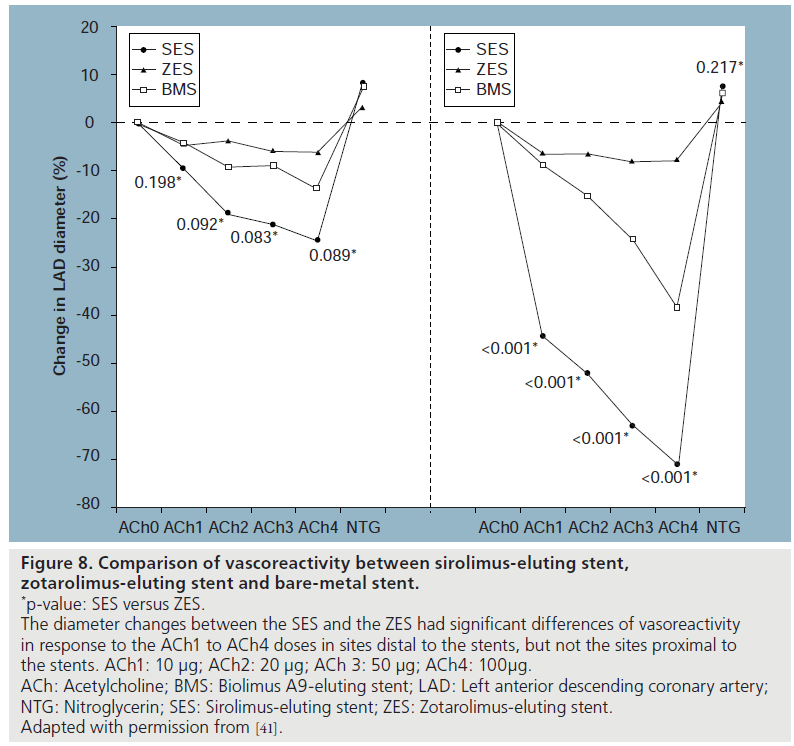
The zotarolimus-eluting stent (ZES; EndaveavorTM, Medtronic Vascular, Inc., CA, USA) has been reported to promote rapid and uniform healing of endothelium [40]. Recently, Kim et al. prospectively compared coronary endothelial function by measuring coronary vasoreactivity in response to titrated doses of intracoronary ACh and NTG in patients with ZES versus SES versus BMS implantation at 6‑month follow-up [41]. A total of 55 patients who were diagnosed with stable angina and treated with a single stent for a de novo lesion of the left anterior descending artery were enrolled. Two segments in the study vessel were chosen for analysis, specifically, 5‑mm proximal and distal to the site of stenting. Between groups, more intense vasoconstriction to incremental doses of ACh was observed in the DES groups at 6‑month follow-up compared with BMS counterparts. Vasoconstriction to ACh was more prominent in the distal segments than the proximal segments in both ZES and SES groups. On the other hand, endothelial function associated with the ZES was more preserved at 6‑month follow-up compared with the SES (Figure 8). Endotheliumindependent vasodilation to NTG did not differ significantly among study groups.
Figure 8: Comparison of vascoreactivity between sirolimus-eluting stent,
zotarolimus‑eluting stent and bare-metal stent.
*p-value: SES versus ZES.
The diameter changes between the SES and the ZES had significant differences of vasoreactivity
in response to the ACh1 to ACh4 doses in sites distal to the stents, but not the sites proximal to
the stents. ACh1: 10 μg; ACh2: 20 μg; ACh 3: 50 μg; ACh4: 100μg.
ACh: Acetylcholine; BMS: Biolimus A9-eluting stent; LAD: Left anterior descending coronary artery;
NTG: Nitroglycerin; SES: Sirolimus-eluting stent; ZES: Zotarolimus-eluting stent.
Adapted with permission from [41].
These observed differences between ZES versus SES in endothelial dysfunction could be due to several reasons. Obata et al. demonstrated that sirolimus released from SES reduced the level of VEGF in the coronary circulation 2 weeks after implantation [42]. An in vitro study by Jabs et al. reported that continuous sirolimus exposure causes impaired endothelium-dependent vascular relaxation by stimulation of mitochondrial ROS release [43]. Another mechanism might be earlier restoration of endothelial function after ZES implantation related to the more rapid elution of the active drug. Whereas in SES the loaded drug is released from the stent up to 60 days after stent implantation, ZES provides delivery of drug to the arterial wall only during the first 2 weeks of elution from the stent [44], and thus local toxicity is minimized. In addition, unique characteristics of the phosphorylcholine (PC) polymer could contribute to the differential effects of DES [41]. The PC polymer of ZES has, despite the permanent polymer, hydrophilic properties that are expected to generate less interfacial tension in the aqueous body environment and is thus more highly biocompatible compared with the hydrophobic polymer of SES, which has been shown to resist fibrinogen adsorption and cause less platelet and monocyte activation. Furthermore, the struts of ZES are thinner than those of the SES platform and previous reports demonstrated that reduced arterial injury and restenosis are associated with thinner struts in humans [45]. The primary association has been the ability of endothelial cells to migrate across the thinner struts improving the rate of endothelialization in vitro [46]. In humans, ZES is associated with a greater amount of neointimal hyperplasia by IVUS at 8 months [47] and a homogeneous complete healing by OCT at 6 months compared with SES [48]. It can be speculated that, on the basis of OCT data and this study result, the more preserved endothelial function at 6 months in ZES could be associated with more complete endothelial coverage compared with SES. Whether this contributes to an improved safety profile in terms of late thrombosis is awaiting confirmation. Specific data regarding the direct relationship between endothelial function and healing over stent struts or clinical outcomes are lacking at this time. There is compelling clinical outcomes data that ZES carries an extremely low risk of late-ST [49].
Thus, despite the greater in-stent late loss, taken together, it is conceivable that a secondgeneration DES (ZES) could be more beneficial in specific clinical situations in which it is needed, for example, in patients who inevitably discontinue dual antiplatelet agents within 12 months as guidelines recommend due to scheduled surgery, or patients with questionable compliance for dual antiplatelet agents. However, these concepts will require further studies before implementation to daily clinical practice.
The Nobori stent™ (Terumo Corporation, Tokyo, Japan), is a different kind of second-generation DES that uses a bioresorbable polymer (polylactic acid) from which biolimus A9, an analog of sirolimus, is eluted. Biolimus A9 is a new sirolimus derivative that binds to cyctosolic immunophilin FK binding protein 12. The formed complex binds to mTOR and inhibits growth-factor-driven cell proliferation. Its enhanced lipophilicity enables an increased uptake in local target tissues and reduced presence in areas surrounding the stented segment or even in the systemic circulation.
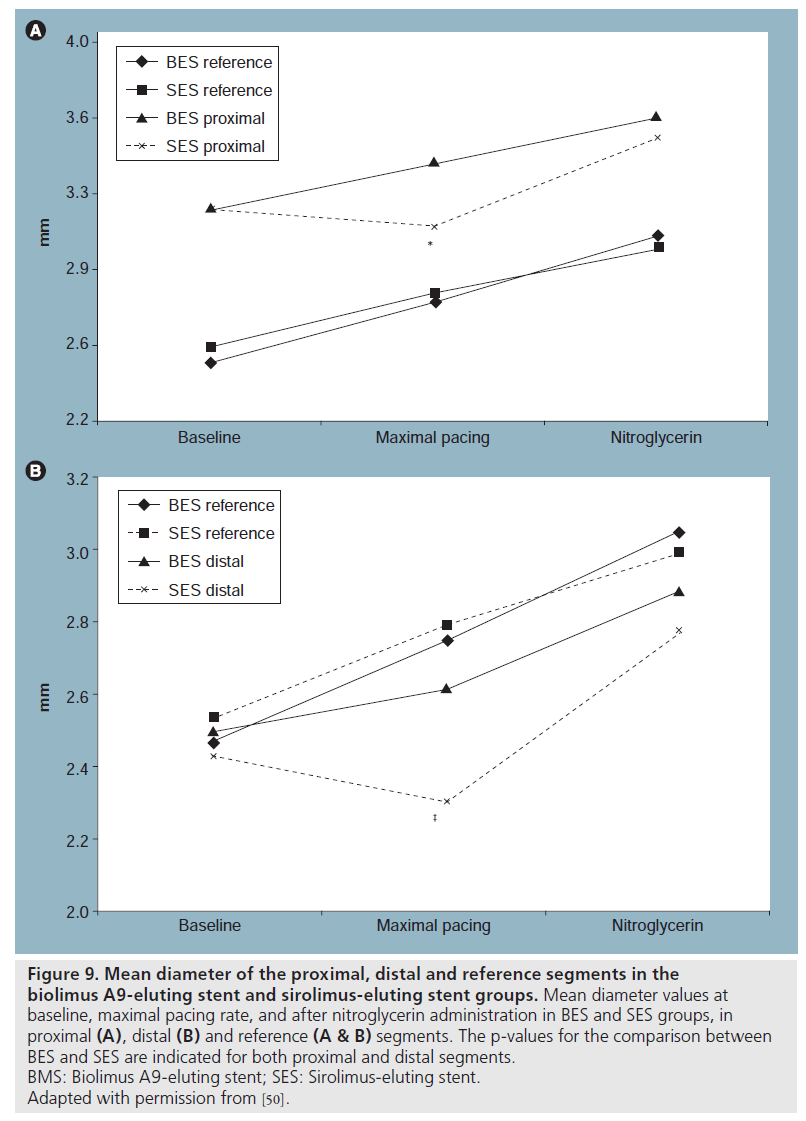
Hamilos et al. reported assessment of the endothelium-dependent as well as endothelium-independent coronary vasomotion 9 months after either biolimus A9-eluting stent (BES) or SES implantation, using right atrial pacing at increasing rate steps [50]. They found that unlike the case with SES, endothelium-dependent vasomotion at the adjacent stent segments appears to be well preserved after BES implantation (Figure 9). There can be several reasons why BES demonstrated better vasomotion response compared with SES. Biolimus A9 is more lipophilic than sirolimus, and upon release would quickly bind to the target lipid-rich tissue. The drug is present only on the vessel side (abluminally), and as such enters into peripheral circulation in only minimal quantities [51]. This results in a more localized effect and less systemic drug exposure. Animal studies showed that after BES implantation, the tissue concentration of the drug in segments 5 mm proximal and distal to the stent edges is almost nonmeasurable [51] and stent endothelialization is more complete than with either SES or PES [52]. SES and BES have different drug-release kinetics; total drug content is released from SES within 60 days with more than 60% released shortly after stent implantation [51,52], versus a small initial burst and sustained simultaneous drug release and polymer degradation taking place over 6 months in BES [51], exposing the surrounding tissue at any given time to a lower amount of drug. Finally, the polymer used with BES is absorbed over a few months. Since durable polymers have been held responsible for some of the late adverse events related to SES, it is expected that the degradation of polymer will improve arterial healing and long-term safety of BES.
Figure 9: Mean diameter of the proximal, distal and reference segments in the biolimus A9-eluting stent and sirolimus-eluting stent groups. Mean diameter values at baseline, maximal pacing rate, and after nitroglycerin administration in BES and SES groups, in proximal (A), distal (B) and reference (A & B) segments. The p-values for the comparison between BES and SES are indicated for both proximal and distal segments. BMS: Biolimus A9-eluting stent; SES: Sirolimus-eluting stent. Adapted with permission from [50].
The fact that the majority of patients with BES had a vasodilatory response to pacing is a robust indication that functional endothelium regrowth is almost complete 9 months after implantation. The findings give promise that newer-generation DES with different drug and polymer platforms may be more respectful of the vessel healing while remaining at least as effective [53].
Future perspective
Regardless of which mechanism(s) of the reduction of vascular function after DES deployment predominate, the following questions remain: what is the clinical relevance of these findings to late events and what can be done about it? In the literature, reduced endothelial function has been shown to correlate with increased atherogenesis, as well as cardiovascular risk [12]. Medical therapy including statins and ACE inhibitors have been demonstrated to improve endothelial dysfunction in patients with risk factors. Whether DES-induced endothelial dysfunction produces a similar risk is not known. However, in most longterm follow-up studies comparing the use of DES versus BMS, there is general lack of improvement in the hard outcomes of death and myocardial infarction [54]. Indeed, in several studies, the risk of death is increased with the use of DES over and above what might be expected from documented risk of ST [55]. It is possible that an increased cardiovascular risk from DES-induced endothelial dysfunction may help to explain these findings. In addition, it is possible that DES-associated endothelial dysfunction may be generalized to other coronary arteries aside from the specificone where the DES was placed. Finally, if DESassociated endothelial dysfunction is a real and harmful phenomenon, what can we do about it?
Conclusion
In summary, DES-associated endothelial dysfunction is reproducible and persists for an extended period of time after the stent implantation. Further studies are indicated to clarify its clinical significance and potential correlation to late events.
Executive summary
▪ First- and second-generation drug-eluting stents (DES) are widely used in interventional cardiology and they significantly reduce the rate of target vessel revascularization.
▪ Despite their widespread use, there is limited information regarding the long-term effects of DES on endothelial function and vascular healing.
▪ Here we review several studies in humans in the cardiac catheterization laboratory following DES implantation that show impaired endothelial functioning in both the proximal as well as distal stent segments.
▪ Although long-term effects of DES-induced endothelial dysfunction are unknown, they require further investigation for their potentially adverse vascular and possible clinical events.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- Windecker S, Remondino A, Eberli FR et al.: Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N. Engl. J. Med. 353, 653–662 (2005).
- Spaulding C, Daemen J, Boersma E et al.: A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356, 989–997 (2007).
- Stone GW, Moses JW, Ellis SG et al.: Safety and efficacy of sirolimus and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356, 998–1008 (2007).
- McFadden EP, Stabile E, Regar E et al.: Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 364, 1519–1521 (2004).
- Luscher TF, Steffel J, Eberli FR et al.: Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 115, 1051–1058 (2007).
- Iakovou I, Schmidt T, Bonizzoni E et al.: Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- Moreno R, Fernández C, Hernández R et al.: Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J. Am. Coll. Cardiol. 45, 954–959 (2005).
- Ong AT, Hoye A, Aoki J et al.: Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J. Am. Coll. Cardiol. 45, 947–953 (2005).
- Togni M, Raber L, Cocchia R et al.: Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int. J. Cardiol. 120, 212–220 (2007).
- Hofma S, Van der Giessen W, Van Dalen B et al.: Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur. Heart J. 27, 166–170 (2006).
- Togni M, Windecker S, Cocchia R et al.: Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol. 46, 231–236 (2005).
- Schachinger V, Britten MB, Zeiher AM et al.: Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101, 1899–1906 (2000).
- Anderson TJ: Assessment and treatment of endothelial dysfunction in humans. J. Am. Coll. Cardiol. 34, 631–638 (1999).
- Radomski MW, Moncada S: Regulation of vascular homeostasis by nitric oxide. Thromb. Haemost. 70, 36–41 (1993).
- Ludmer PL, Selwyn AP, Shook TL et al.: Paradoxical vasoconstriction induced by acethylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 315, 1046–1051 (1986).
- Gordon JB, Ganz P, Nabel E et al.: Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J. Clin. Invest. 83, 1946–1952 (1989).
- Heusch G, Baumgart D, Camici P et al.: a‑1 Adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 101, 689 (2000).
- Jones CJH, Kuo L, Davis MJ et al.: In vivo and in vitro vasoactive reactions of coronary arteriolar microvessels to nitroglycerin. Am. J. Physiol. 271, H461 (1996).
- Kuo L, Davis MJ, Chilian WM: Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation 92, 518 (1995).
- Suter TM, Hess OM, Bortone A et al.: Coronary stenosis vasomotion during dynamic exercise before and after PTCA. Eur. Heart J. 10(Suppl. G), 58–63 (1989).
- Lazzam C, Forster C, Gotlieb A et al.: Impaired vascular reactivity following angioplasty is mainly due to endothelial injury. Exp. Mol. Pathol. 56, 153–162 (1992).
- Suter TM, Buechi M, Hess OM et al.: Normalization of coronary vasomotion after percutaneous transluminal coronary angioplasty? Circulation 85, 86–92 (1992).
- Suwaidi JA, Hamasaki S, Higano ST et al.: Long-term-follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101, 948–954 (2000).
- Van Beusekom HMM, Whelan DM, Hofma SH et al.: Long-term endothelial dysfunction is more pronounced after stenting than after balloon angioplasty in porcine coronary arteries. J. Am. Coll. Cardiol. 32, 1109–1117 (1998).
- Caramori PRA, Lima VC, Seidelin PH et al.: Long-term endothelial dysfunction after coronary artery stenting. J. Am. Coll. Cardiol. 34, 1675–1679 (1999).
- Maier W, Windecker S, Kung A et al.: Exercise-induced coronary artery vasodilation is not impaired by stent placement. Circulation 105, 2373–2377 (2002).
- Fukuda D, Sata M, Tanaka K, Nagri R: Potent inhibitory effect of the sirolimus on the circulating vascular progenitor cells. Circulation 111, 926–931 (2005).
- Marx SO, Jayaraman T, Go LO et al.: Rapamycin-FKBP inhibits the cell cycle regulators of proliferation in the vascular smooth muscle cells. Circ. Res. 111, 926–931 (1995).
- Long C, Cook LG, Wu GY et al.: Removal of FKBP12/12.6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arterioscler. Throm. Vasc. Biol. 27, 1580–1586 (2007).
- Morice MC, Serruys PW, Sousa JE et al.: A randomized comparison of a sirolimus eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346, 1773–1780 (2002).
- Klugherz BD, Llanos G, Lieuallen W et al.: Twenty-eight-day efficacy and pharmacokinetics of the sirolimus-eluting stent. Coron. Artery Dis. 13, 183–188 (2002).
- Virmani R, Guagluimi G, Farb A et al.: Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent should we be cautious? Circulation 109, 701–705 (2004).
- Jabs A, Gobel S, Wenzel P et al.: Sirolimus-induced vascular dysfunction. J. Am. Coll. Cardiol. 51, 2130–2138 (2008).
- Steffel J, Latini RA, Akhmedow A et al.: Rapamycin, but not FK-506, increases endothelial tissue factor expression: implications for drug-eluting stent design. Circulation 112, 2002–2011 (2005).
- Finn AV, Joner M, Nakazawa G et al.: Pathological correlates of late drug-eluting stent thrombosis. Strut coverage as a marker of endothelialization. Circulation 115, 2435–2441 (2007).
- Kotani J, Awata M, Nanto S et al.: Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J. Am. Coll. Cardiol. 47, 2108–2111 (2006).
- Matsumoto D, Shite J, Shinke T et al.: Neointimal coverage of sirolimus-eluting stents at 6‑month follow up: evaluated by optical coherence tomography. Eur. Heart J. 28, 961–967 (2007).
- Sollott SJ, Cheng L, Pauly RR et al.: Taxol inhibits neointimal smooth muscle cell accumulation after angioplasty in the rat. J. Clin. Invest. 95, 1869–1876 (1995).
- Kim JW, Suh SY, Choi CU et al.: Six-month comparison of coronary endothelial dysfunction associated with sirolimus-eluting stent versus paclitaxel-eluting stent. J. Am. Coll. Cardiol. Interv. 1, 65–71 (2008).
- Whelan DM, van der Giessen WJ, Krabbendam SC et al.: Biocompatibility of phosphorylcholine coated stents in normal porcine coronary arteries. Heart 83, 338–345 (2000).
- Kim JW, Seo HS, Park JH et al.: A prospective, randomized, 6‑month comparison of the coronary vasomotor response associated with a zotarolimus-versus a sirolimus-eluting stent. J. Am. Coll. Cardiol. 53, 1653–1659 (2009).
- Obata J, Kitta Y, Takano H et al.: Sirolimuseluting stent implantation aggravates endothelial vasomotor dysfunction in the infarct-related artery in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 50, 1305–1309 (2007).
- Jabs A, Gobel S, Wenzel P et al.: Sirolimusinduced vascular dysfunction. J. Am. Coll. Cardiol. 51, 2130–2138 (2008).
- Kandzari DE, Leon MB: Overview of pharmacology and clinical trials program with the zotarolimus-eluting endeavor stent. J. Interv. Cardiol. 19, 405–413 (2006).
- Kastrati A, Mehilli J, Dirschinger J et al.: Intracoronary Stenting and Angiographic Results: Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO) trial. Circulation 103, 2816–2821 (2001).
- Spragues E: Influence of topography on endothelialization of stents: clues for new designs. J. Long Term Eff. Med. Implants. 10(1-2), 143–151 (2000).
- Miyazawa A, Ako J, Hongo Y et al.: Comparison of vascular response to zotarolimus-eluting stent versus sirolimuseluting stent: intravascular ultrasound results from ENDEAVOR III. Am. Heart J. 155, 108–113 (2008).
- Guagliumi G: Optical coherence tomography for drug eluting stent safety (ODESSA). Presented at: Annual Meeting of Transcatheter Cardiovascular Therapeutics. Washington, DC, USA, 22 October (2008).
- Gershlick A, Kandzari DE, Leon MB et al.: Zotarolimus-eluting stents in patients with native coronary artery disease: clinical and angiographic outcomes in 1,317 patients. Am. J. Cardiol. 100, 45–55 (2007).
- Hamilos MI, Ostojic M, Beleslin B et al.: Differential effects of drug-eluting stents on local endothelium-dependent coronary vasomotion. J. Am. Coll. Cardiol. 51, 2123–2129 (2008).
- Vetrovec G, Rizik D, Williard C et al.: Sirolimus PK trial: a pharmacokinetic study of the sirolimus-eluting BX velocity stent in patients with de novo coronary lesions. Catheter Cardiovasc. Interv. 67, 32–37 (2006).
- Chevalier B, Serruys PW, Silber S et al.: Randomized comparison of Nobori®, biolimus A9-eluting coronary stent with a Taxus®, paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: The Nobori 1 trial. Eurointerv. 2, 426–434 (2007).
- Ostojic M, Sagic D, Beleslin B et al.: First clinical comparison of Nobori® – biolimus A9 drug eluting stent with Cypher® – sirolimus eluting stent: NOBORI CORE nine months and one year clinical outcomes. Eurointerv. 3, 574–579 (2008).
- Stettler C, Wandel S, Allelmann S et al.: Outcomes associated with drug-eluting versus bare-metal stents: a collaborative network meta-analysis. Lancet 370, 937–948 (2007).
- Lagerqvist B, James SK, Stenestrand U et al.; SCAAR Study Group: Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 356, 1009–1019 (2007).
▪ Demonstrates the paclitaxel-eluting stent-associated endothelial dysfunction in humans after drug-eluting stent (DES) implantation.
▪ Outlines the importance of endothelial dysfunction and its long-term adverse cardiovascular outcomes.
▪ Outlines the physiological assessments of endothelial dysfunction in the cardiac catheterization laboratory in humans.
▪ Demonstrates the more favorable endothelial effect of newer second-generation DES compared with first-generation DES.