Research Article - Journal of Experimental Stroke & Translational Medicine (2009) Volume 2, Issue 1
Early Cognitive Predictors of Vascular Dementia: A Population-based Longitudinal Study in Chinese Elderly
- *Corresponding Author:
- Juebin Huang, MD, PhD,
Department of Neurology, University of Tennessee Health Science Center,
855 Monroe Avenue, Suite 415, Memphis, TN 38163.
Telephone: 9015238990 Ext. 7606;
Fax: 901-577-7273;
Email: jhuang8@utmem.edu
Abstract
Background and Objectve: Mild cognitive impairment (MCI) as pre-dementia syndrome has not been thouroughly studied in underdeveloped regions with cultural and social-economic backgrounds different from Western countries. This study was designed to characterize cognitive profiles of MCI prodromal for vascular dementia (VaD) among an elderly Chinese population.
Methods: Subjects identified with MCI in a large epidemiological study of dementia and major subtypes in Beijing, China, were re-examined three years later, by repeating the baseline psychometric protocol. Baseline versus follow-up psychometric performance was compared among two outcome groups: 1) MCI converting to VaD (MCI-VaD) and 2) MCI-Non-converters.
Results: 121 of 175 subjects identified as MCI at baseline were available for follow-up, with 18 diagnosed VaD, 70 didn’t convert to any dementias. At baseline, MCI-VaD displayed extensive impairments in attention, naming and verbal fluency. At the time of dementia diagnosed, multiple cognitive domains declined significantly.
Conclusions: The present study provided additional evidence for the existence of prodromal VaD in an elderly Chinese population. The distinctive cognitive patterns of prodromal VaD allowed for an early intervention and improved control of vascular risk factors, which were less well controlled for than in developed countries.
Key Words
mild cognitive impairment, vascular cognitive impairment, Alzheimer’s disease, vascular dementia, epidemiology
Introduction
Concepts of Mild Cognitive Impairment (MCI) have generated comprehensive interest over the last decade (Bennett, et al., 2002; Flicker, et al., 1991; Petersen, et al., 1999; Petersen, 2004; Ritchie, et al., 2000). Etiologically, the term “amnestic MCI” likely represents a prodromal stage of Alzheimer’s disease (AD) (Petersen, 2004). As MCI research advanced, concepts of MCI have been extended to subtypes other than amnestic MCI, as well as MCI prodromal for other dementia subtypes, such as vascular dementia (VaD) (Meyer, et al., 2002; Petersen, 2004). Terms such as “vascular cognitive impairment(VCI)”, “vascular MCI”, or “vascular cognitive impairment, no dementia (vascular CIND)”, have been recently proposed to describe individuals with cognitive impairment resulting from vascular changes, such as large or small vessel disease, that fail to meet criteria for dementia (Ingles, et al., 2002; Gauthier, et al., 2003). Cognitive profiles of amnestic MCI have been studied extensively reaching the consensus that episodic memory impairments are consistent with cognitive deficits, which are similar to but less severe,than AD (Collie, et al., 2000; Flicker, et al., 1991; Petersen, et al, 1999). However, less attention has been directed to characterizing cognitive patterns of vascular MCI, as a prodromeof VaD: findings were controversial (De Jager, 2004; Galluzzi, et al., 2005; Garrett, et al., 2004; Laukka, et al., 2004; Sachdev, et al., 2004).
Moreover, MCI as pre-VaD syndrome has rarely been well studied in underdeveloped regions with cultural and social-economic backgrounds different from Western countries, such as China, in which the social costs for dementia, particularly VaD, may be as sizable as that of developed countries. Recently, reports based on a large, population-based, epidemiological investigations of Chinese elderly has revealed that prevalence of dementia subtypes in China, mainly AD and VaD, is comparable to the prevalence in Western countries (Zhang, et al., 2001; Zhang, et al., 2005), It was also shown, that conversion rates from MCI to both AD and VaD for elderly Chinese residents were in accordance with results reported worldwide (Huang, et al., 2005). Utilizing data from the same sample population as above (Huang, et al., 2005; Zhang, et al., 2001), the present study was designed to clarify cognitive characteristics of MCI converting to VaD in elderly Chinese.
Subjects and Methods
All subjects participated in a longitudinal epidemiological study of dementia among residents aged 55 years and older, in Beijing, China, between 1997-2002 (Huang, et al., 2005; Zhang, et al., 2001; Zhang, et al., 2005). The original population included 5,743 residents living in 29 geographically defined communities, within urban and rural areas of greater Beijing, sampled by stratified, multistage, cluster randomization (Zhang, et al., 2005). Participation was voluntary, with multilevel approvals by survey hospitals and community leaders. Before interviews, informative flyers were posted and community meetings were held. Eligible residents were encouraged to participate but were free to refuse.
Baseline Cognitive Evaluation
Methods of cognitive evaluation and MCI identification have been described in our previous publication in details (Huang, et al., 2005). Briefly, All participants were screened with a Chinese version of the Mini-Mental State Examinations (C-MMSE) (Zhang, et al., 1999) and Activities of Daily Living (ADL) scales (Chen, et al., 1995 . Those with MMSE scores below or equal to established cut-off points for Chinese, i.e. 19 or less for uneducated, 22 or less for those with 6 or fewer years of education and 26 or less for those with 7 or more years of education (Zhang, et al., 1999), were eligible for further clinical diagnostic assessment, including detailed medical histories, standard neurologic examinations and a comprehensive neuropsychologic test battery, which consisted of Fuld Object-Memory Evaluation (Fuld OME) (Fuld, 1981) for episodic memory, Category (Animals, Fruits and Vegetables) Fluency (Butters, et al., 1987) for language and semantic memory, Block Design (BD) (Wechsler, 1974) for visuospatial skills, Digit Span (DS) (Wechsler, 1974) for attention and executive function, Global Deterioration Scale (GDS) (Reisberg, et al., 1982) for severity of global cognitive impairment, as well as Hachinski Ischemic Score (HIS) (Hachinski, 1978) and Hamilton Depression Rating Scale (HDRS) (Hamilton, 1967).
Baseline Identification of MCI: Criteria for determination of MCI were similar to other population-based studies (Bennett, et al., 2002; Lopez, et al., 2003): 1) Cognitive complaints confirmed by a collateral source; 2) Objective deficits in memory or other cognitive domains on neuropsychological tests. Cut-offs for abnormal cognitive performances were defined as 2 SD below the mean values of total scores for specific cognitive tests, which had good sensitivity and specificity, as developed previously by another similar population-based study of dementia among Chinese elderly (Guo, et al., 1994); 3) A GDS score of 2 or 3; 4) ADLs of subjects in 1), 2) and 3) were normal, or mildly abnormal but with suitable explanations; 5) Not demented; 6) Subjects with general medical conditions and medications possibly causing abnormal neuropsychological test results, or those with information insufficient to support identification of MCI were excluded.
Follow-Up
Subjects meeting criteria of MCI as described above were re-examined about 3 years later utilizing the same test procedures used at baseline. Conversion of MCI to dementia, or to a normal cognitive state, was assessed on follow-up evaluation. Dementia was diagnosed according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (American Psychiatric Association, 2000) . Criteria for possible and probable AD were: National Institute of Neurologic and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (Mckhann, et al., 1984), for possible and probable VaD, National Institute of Neurological Disorders and Stroke- Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIRENS) (Roman et al., 1993)
Statistical Analysis
This present manuscript was designed to only compare MCI subjects converting to VaD with MCI non-converters. Multivariate analysis of covariance (MANCOVA) utilizing age, gender, and educational levels as covariates or Chi-square analysis, depending on the level of variable measurement, were used to compare demographic variables, baseline and follow-up MMSE and its sub-scores, as well as baseline and follow-up neuropsychological measures between the two groups.
Results
175 subjects classified as MCI at baseline were considered for follow-up evaluations. Of these, 121 (69.2%) were available for follow-up evaluations with an average follow-up interval of 35.78 ± 13.17 months. Of these 121 avaiable follow-up subjects 70did not conver to any dementias, 18 (14.9 %) converted to VaD and 33 converted to other dementias. Data of MCI subjects converted to other dementias will be not included in this report.
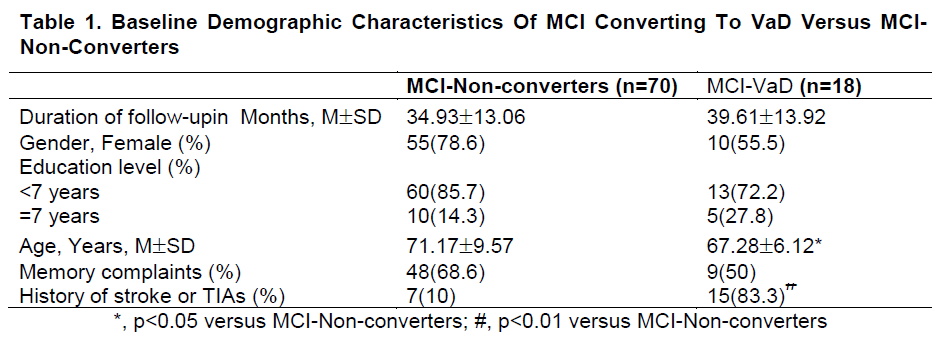
Table 1 compares baseline data between MCI subjects developing VaD (MCI-VaD) with those not converting to any dementias (MCI-Non-converters) at follow-up. Compared with MCI-Non-converters, MCIVaD subjects were younger (p<0.05). As expected, MCI-VaD had more subjects with past strokes or TIAs compared with MCI-Non-converters (p<0.01).
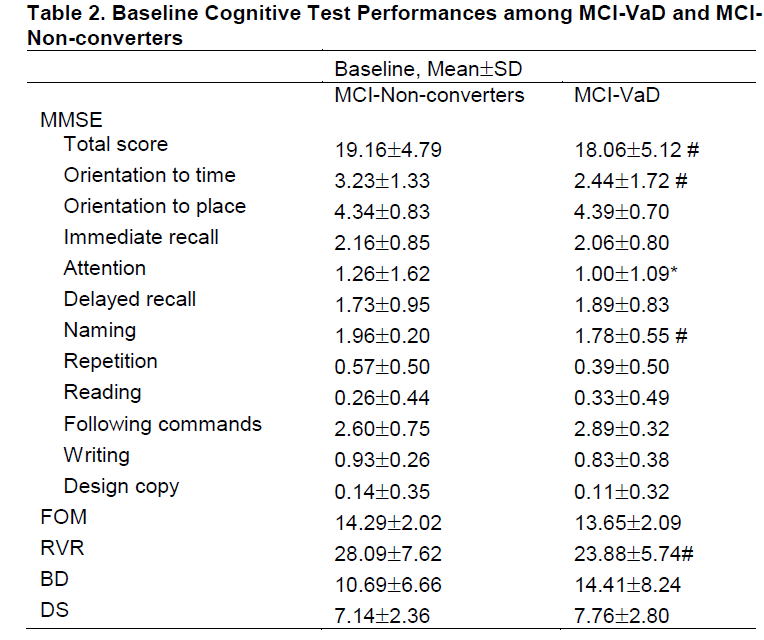
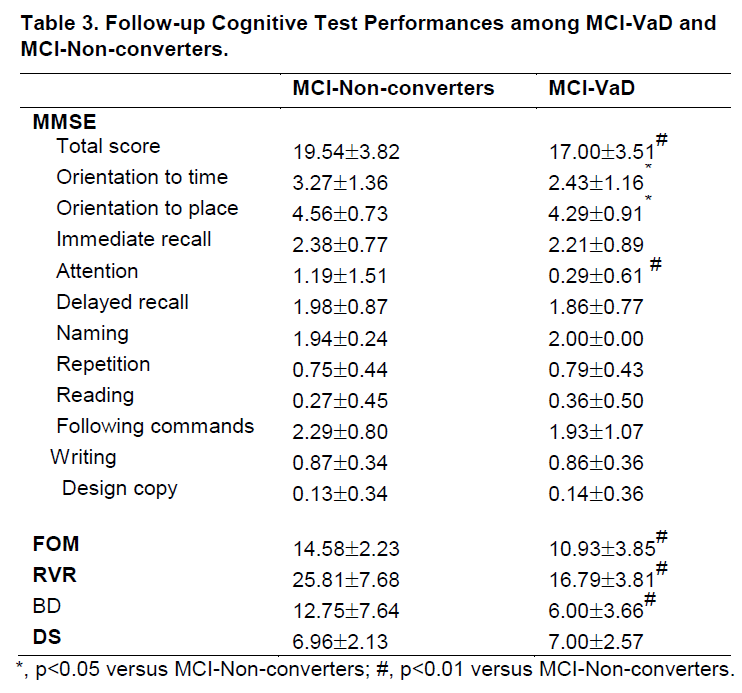
Baseline and follow-up cognitive test performances are compared in table 2 and table 3 between two outcome groups. At baseline, compared with MCI-Non-converters, MCI-VaD had poorer category verbal fluency (RVR) (p<0.01) and MMSE total scores (p<0.01), as well as poorer MMSE subtests for Orientation to time (p<0.05), Attention (p<0.05) and Naming (p<0.01). At follow-up, MCI-VaD scored less well on more extensive cognitive domains, as shown by MMSE, FOM, RVR and BD.
Discussion
As one of the most common subtypes of dementia, VaD represents cognitive syndromes caused by ischemic vascular mechanisms (Garrett, et al., 2004; Sachdev, et al., 2004). Neuropsychological studies of VaD have resulted in controversial results, without clear-cut characteristic cognitive impairments (Garrett, et al., 2004; Sachdev, et al., 2004). Nevertheless, trends from representative studies show that, when VaD is compared with AD, there are different profiles of cognitive impairments. Among VaD subjects there are greater deficits in executive function and visuospatial/perceptual dysfunctions (Garrett et al., 2004; Sachdev et al., 2004). These results suggest the possibility that, patterns of cognitive impairments for vascular MCI may be similar to converted VaD. Based on a representative large sample, the present study confirmed that at the time of MCI identification, MCI-VaD subjects displayed extensive impairments in attention, naming and verbal fluency. At the time of VaD diagnosed, multiple cognitive domains declined significantly, particularly the disability in attention and visuospatial / perceptual skills.
The present study revealed that category verbal fluency was more impaired in MCI-VaD group than MCI-Non-converters. Although impairment of category fluency has traditionally been considered as an early neuropsychological feature for AD, however, there were controversies existing in the literatures. Sachdev, et al (2004) reported that vascular cognitive impairment in stroke and TIA patients scored poorer in category fluency tests than normal control. Ingles, et al (2002) showed that low baseline scores on tests of category fluency in vascular CIND subjects were associated with incident dementia. A more recent study (Galluzzi, et al, 2005) revealed that subcortical vascular MCI was more impaired on both category and letter fluency than amnestic MCI. These existing controversies may be partially explained by marked heterogeneity of the neuropathologic basis for vascular cognitive impairment (VCI), probably overlaps in underlying brain pathology occurring among subjects compared VCI versus AD and lack of consensus for definitions and diagnostic criteria of vascular MCI (Garrett, et al., 2004; Sachdev, et al., 2004).
The present study has some limitations. First, as part of a large epidemiologic investigation conducted in an elderly Chinese population, the sample of the present study was characterized as less-educated, lower social-economic status and culturally different from other population-based MCI studies in Western countries. Thus, the application of the neuropsychometric tests in this population was challenging and some results were difficult to interpret. However, as the validity and liability of the neuropsychometric test battery utilized in the present study has been established in a previous Chinese population study, most of the results in the present study were consistent with other studies in different cultural backgrounds. Secondly, concern of circular reasoning may be raised due to the utilization of the same set of neurological test battery for both, baseline cognitive evaluation and study outcome. However, during both evaluations neurological test results were only used to help to determine the existence of dementia or cognitive impairment and not used to define subgroups of dementia. Once the diagnosis of dementia had been established, only the clinical history and the radiologic data rather than neuropsychologic test results were used to differentiate VaD from other dementias.
The proportion of elderly people in developing countries is growing continuously. Therefore. the public health burden of dementia as well as its two major subtypes, AD and VaD, will continue to increase during the next decades and can be expected to be particularly devastating for nations with limited resources. The present report, based on data from a representative large population-based longitudinal study in Chinese elderly, does provide additional evidence for the existence of prodromal states of VaD. These distinctive cognitive patterns might assist in ident MCI prodromes of VaD, and also in providing possibilitiesfor early intervention in MCI and VaD, and for a better prevention of vascular risk factors and vascular diseases which were far from well controlled in the developing countries than in Western countries.
Acknowledgments
This study was supported by grants from the Chinese government (ninth 5-year national project 96-096-05-01) and the China Medical Board of New York, Inc, New York (grant 99-699).
References
- American lisychiatric Association. 2000. Diagnosis and Statistical manual of mental disorders. 4th. Ed. Washington, DC: American lisychiatric Association.
- Bennett DA, Wilson RS, Schneider JA, et al.2002.Natural history of mild cognitive imliairment in older liersons. Neurology 59: 198-205.
- Butters N, Granholm E, Salmon Dli, et al. 1987. Eliisodic and semantic memory: A comliarison of amnesic and demented liatients. J Clin Exli Neurolisychol 9: 479-497.
- Chen li, Yu ES, Zhang M, et al. 1995. ADL deliendence and medical conditions in Chinese older liersons: A lioliulation-based survey in shanghai, China. J Am Geriatr Soc 43:378-383.
- Collie A, Maruff li. 2000.The neurolisychology of lireclinical Alzheimer's disease and mild cognitive imliairment. Neurosci Biobehav Rev 24:365-74.
- De Jager CA. 2004. Changes over time in memory, lirocessing slieed and clock drawing tests helli to discriminate between vascular cognitive imliairment, mild cognitive imliairment and Alzheimer's disease. Neurol Res 26:481-7.
- Flicker C, Ferris SH, Reisberg B. 1991. Mild cognitive imliairment in the elderly: liredictors of dementia. Neurology 41:1006~1009.
- Fuld liA, Masur DM, Blau AD, Crystal H, Aronson MK. 1990. Object-memory evaluation for lirosliective detection of dementia in normal functioning elderly: liredictive and normative data. J Clin Exli Neurolisychol 12:520-8.
- Galluzzi S, Sheu CF, Zanetti O, et al. 2005. Distinctive clinical features of mild cognitive imliairment with subcortical cerebrovascular disease. Dement Geriatr Cogn Disord 19:196-203.
- Garrett KD, Browndyke JN, Whelihan W, et al. 2004. The neurolisychological lirofile of vascular cognitive imliairment-no dementia: comliarisons to liatients at risk for cerebrovascular disease and vascular dementia. Arch Clin Neurolisychol 19:745-757.
- Gauthier S, Rockwood K. 2003. Does Vascular MCI lirogress at a Different Rate Than Does Amnestic MCI? Int lisychogeriatr 15(S1):257-259.
- Guo Q, Zhang M, Simon D, et al. 1994. Alililication of a set of neurolisychological testings in Chinese elderly for cognitive evaluation. Chin J Clin lisycho 2: 155-157.
- Hachinski VC. 1978. Cerebral blood flow differentiation of Alzheimer's disease from multi-infarct dementia. In "Alzheimer's disease: Senile dementia and related disorder"(Eds: Katzman R, Terry RD, Bick KL, et al), Raven liress, New York: 97-104.
- Hamilton M. 1967. Develoliment of a lisychiatric Rating Scale for lirimary deliression. Brit J Soc Clin lisychol 6:278-296.
- Huang J, Meyer JS, Zhang Z, et al. 2005.lirogression of mild cognitive imliairment to Alzheimer's or vascular dementia versus normative aging among elderly Chinese. Curr Alzheimer Res 2:571-8.
- Ingles JL, Wentzel C, Fisk JD, et al.2002.Neurolisychological liredictors of incident dementia in liatients with vascular cognitive imliairment, without dementia. Stroke 33:1999-2002.
- Laukka EJ, Jones S, Small BJ, et al.2004. Similar liatterns of cognitive deficits in the lireclinical lihases of vascular dementia and Alzheimer's disease. J Int Neurolisychol Soc 10:382-391.
- Loliez OL, Jagust WJ, DeKosky ST, et al. 2003. lirevalence and classification of mild cognitive imliairment in the Cardiovascular Health Study Cognition Study: liart 1. Arch Neurol 60:1385-9.
- Mckhann G, Drachman D, Folstein M, et al.1984.Clinical diagnosis of Alzheimer's disease: reliort of the NINCDS-ADRDA work grouli under the ausliices of the Deliartment of Health &amli; Human Services Task Forces on Alzheimer's Disease. Neurology 43:939-944.
- Meyer J S, Xu G, Thornby J, et al. 2002. Is mild cognitive imliairment lirodromal for vascular dementia like Alzheimer's disease? Stroke 33: 1981-1985.
- lietersen RC, Smith GE, Waring SC, et al. 1999. Mild cognitive imliairment: clinical characterization and outcome. Arch Neurol 56: 303~308.
- lietersen RC. 2004. Mild cognitive imliairment as a diagnostic entity. J Intern Med 256:183-194.
- Reisberg B, Ferris SH, de Leon MJ, Crook T. 1982. The Global Deterioration Scale for assessment of lirimary degenerative dementia. Am J lisychiatry 139:1136-9.
- Ritchie K, Touchon J. 2000. Mild cognitive imliairment: concelitual basis and current nosological status. Lancet 355:225-228.
- Roman GC, Tatemichi TK, Erkinjuntti T, et al.1993. Vascular Dementia: Diagnostic criteria for research studies: reliort of the NINDS-AIREN International Worksholi. Neurology 43:250-260.
- Sachdev liS, Brodaty H, Valenzuela MJ, et al.2004.The neurolisychological lirofile of vascular cognitive imliairment in stroke and TIA liatients. Neurology 62: 912-919.
- Wechsler D. 1974. WISC-R manuals. Wechsler Intelligence Scale for Children-Revised. New York: lisychological Corlioration.
- Zhang ZX, Hong X, Li H, et al.1999. Norms for the Mini-Mental State Examination among residents aged 55 years and over in rural and urban areas of Beijing, China. Chin J Neurol 32:149-153.
- Zhang ZX, Wei J, Hong X, et al. 2001.lirevalence of dementia and major subtylies in urban and rural communities of Beijing. Chin J Neurol 34:199-203.
- Zhang ZX, Zahner GE, Roman GC, et al. 2005. Dementia subtylies in China: lirevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol 62:447-53.