Case Report - Diabetes Management (2017) Volume 7, Issue 4
Developing diabetic ketoalkalosis in a patient with Cushing disease
- *Corresponding Author:
- Nobutaka Hirooka
Department of General Internal Medicine
Saitama Medical University, Japan
E-mail: nkaorohi@gmail.com
Abstract
Diabetic Ketoacidosis (DKA) is the most common metabolic complications of diabetes mellitus. In some situations, other acid-base disorders coexist with DKA including metabolic alkalosis. There is several underlying etiology for the DKA with metabolic alkalosis such as extracellular volume loss secondary to vomiting or diuretics, alkali ingestion, and corticosteroid excess. We report herein the first case of DKA with metabolic alkalosis secondary to autopsy-confirmed Cushing disease. The case report elucidates clinically important lessons through its management as well as a future guide for scientific investigation on this combined acid-base disorder.
Keywords
diabetic ketoalkalosis, cushing disease, acid-base disorder
Introduction
Diabetic Ketoacidosis (DKA) is the most common metabolic complications of diabetes mellitus. While case fatality rate of DKA has been decreasing, the rate is still around 5% or more in the modern medical era [1]. Thus, DKA must be recognized appropriately leading to prompt diagnosis and treatment.
In some situations, another acid-base disorder coexists with DKA and the usual definition of DKA might not be met. Thus, coexisting acid-base disorders hinder prompt recognition of DKA, which may affect outcome of the treatment. Among the combined acid-base disorders in DKA, a metabolic alkalosis normalizing or obscuring the blood pH and serum bicarbonate has been reported as diabetic ketoalkalosis (DKALK) or originally diabetic ketoacidosis with metabolic alkalosis [2,3].
Since the first publication for this condition, more than 30 cases have been published in the English literature [2-4]. There is several underlying etiology for this condition such as extracellular volume loss secondary to vomiting or diuretics, alkali ingestion, and corticosteroid excess. Although there are handful reported cases of corticosteroid excess in the past, this case report is the first report of DKALK secondary to Cushing disease for the etiology of corticosteroid excess.
Case presentation
An 82-year-old female with a history of multiple episodes of stroke and aspiration pneumonia, hypertension, and diabetic mellitus brought by ambulance because of fever and respiratory distress. She also has been followed by an endocrinologist for her potential Cushing syndrome, while she was on no treatment for the suspected disorder. Cushing syndrome was suspected because she had some of the clinical features of Cushing syndrome. She had diabetes, hypertension and occasional high level of both cortisol and ACTH. However, pituitary and adrenal MRI (non-enhanced) did not show any abnormality. She did not want to have dexamethasone suppression test or other hormonal tests. Her activities in daily life were restricted to mostly bed-ridden and occasionally sit in a wheel chair when eating because of the previous stroke. Her medical history included frequent aspiration episodes per caring daughter at home, some of which required inpatient care to the aspiration pneumonia. When presented to the emergency room, she was febrile (38.9°C) and Tachypneic (respiration rate 24/min) with 93% of pulse oximetry on room air. Her blood pressure was 80/52 mmHg with heart rate being 98 beat per minute. Her chest exam was normal, although the chest CT showed consolidation in bilateral lower lobe and high white cell counts with left sided shift. Laboratory results are shown in Table 1.
| Normal Range | ||
|---|---|---|
| CBC | ||
| WBCs (µl) | 33,100 | (4,000-8,000) |
| Neutrophil (%) | 89.5 | (44-74) |
| Lymphocyte (%) | 7 | (22-50) |
| Others (%) | 3.5 | (0-20) |
| RBCs (x104/µl) | 450 | (420-500) |
| Hemoglobin (g/dl) | 11.7 | (13.0-17.0) |
| Hematocrit (%) | 34.3 | (40.0-50.0) |
| Platelet (x104/µl) | 25.5 | (15.0-40.0) |
| Chemistry | ||
| Total bilirubin (mg/dl) | 0.5 | (0.2-1.0) |
| AST (IU/l) | 15 | (8-30) |
| ALT (IU/l) | 12 | (5-35) |
| LDH (IU/l) | 198 | (100-225) |
| ALP (IU/l) | 152 | (104-338) |
| Protein (g/dl) | 5.4 | (6.5-8.2) |
| Albumin (g/dl) | 2.7 | (3.8-5.2) |
| Glucose (mg/dl) | 100 | (65-110) |
| Urea Nitrogen (mg/dl) | 23 | (8-20) |
| Creatinine (mg/dl) | 0.39 | (0.6-1.2) |
| Amylase (IU/l) | 67 | (41–112) |
| Na (mEq/l) | 127 | (135-147) |
| K (mEq/l) | 4.5 | (3.5-5.0) |
| Cl (mEq/l) | 95 | (98-108) |
| Ca (mg/dl) | 8.8 | (8.8-10.2) |
| P (mg/dl) | 2.9 | (2.5-4.5) |
| CRP (mg/dl) | 2.45 | (<0.3) |
| CK (IU/l) | 25 | (43–165) |
| HbA1c (%) | 6.9 | (4.6-6.2) |
| Coagulation | ||
| PT-INR | 1.09 | (0.9-1.1) |
| APTT (s) | 27.4 | (24-34) |
| Fibrinogen (mg/dl) | 21 | (150-350) |
| D-dimer (μg/ml) | 10 | (<1.0) |
| Urinalysis | ||
| Gravity | 1.012 | (1.010-1.030) |
| PH | 8 | (5-8) |
| Protein | - | (-) |
| Glucose | - | (-) |
| Ketone | - | (-) |
| Bilirubin | - | (-) |
| RBCs | - | (-) |
| WBCs | 2+ | (-) |
| Nitrate | - | (-) |
Table 1. Laboratory findings.
The patients were admitted for her pneumonia probably due to aspiration. An Intravenous (IV) antibiotic (piperacillin/sulbactam) was initiated and she was kept nothing by mouth initially. She responded to the IV antibiotics well. While kept nothing by mouth, her glucose was monitored because of the diabetes. Control of glucose level was well with sliding scale ranging from 130 to 190 mg/dl. After the swallowing test, she resumed oral intake from hospital day 10. Her pneumonia and general condition continued to improve.
On hospital day 15, she developed fever again which required investigating focus of infection. Sputum, blood, and urine culture was obtained. The chest X-ray was also ordered. Because of positive nitrates, WBCs, bacteria in urine and clear on chest X-ray, we diagnosed her with urinary tract infection and began to administer intravenous ceftazidime. Her mental status deteriorated from her baseline requiring further work-up. In the blood chemistry at that time, her serum glucose was 585 mg/dl. Arterial blood gas analysis showed combined acid-base abnormalities. It included primary metabolic acidosis and primary metabolic alkalosis (Table 2).
| Normal Range | ||
|---|---|---|
| ABG | ||
| PH | 7.5 | (4,000-8,000) |
| PaCO2 (mmHg) | 39.2 | (44-74) |
| PaO2 (mmHg) | 97.2 | (22-50) |
| HCO3- (mmol/l) | 29.9 | (0-20) |
| BE (mmol/l) | 6.3 | (420-500) |
| Sat O2 (%) | 97.5 | (13.0-17.0) |
| Chemistry | ||
| Na (mEq/l) | 152 | (135-147) |
| K (mEq/l) | 3.1 | (3.5-5.0) |
| Cl (mEq/l) | 110 | (98-108) |
| Glucose (mg/dl) | 585 | (65-110) |
| Total ketone (μmol/l) | 2723 | (<30) |
| β-hydroxybutyrate (μmol/l) | 1940 | (<85) |
| Acetoacetate (μmol/l) | 783 | (<55) |
Table 2. Laboratory findings for primary metabolic acidosis and primary metabolic alkalosis.
We sent her serum sample to an outside laboratory requesting measuring ketone level which revealed high beta-hydroxybutyrate level (1940 μMol/l). Thus, we confirmed she developed diabetic ketoacidosis, which was treated with IV hydration and regular insulin to correct serum glucose while carefully monitoring electrolytes and bicarbonate level. A relatively rare complication, metabolic alkalosis, was also found in the blood gas analysis as described previously (Table 2). We looked back her medical record for previous blood gas analysis which showed metabolic alkalosis even in the unstressed condition. We thought she had underlying chronic metabolic alkalosis which masked acidosis in diabetic ketoacidosis.
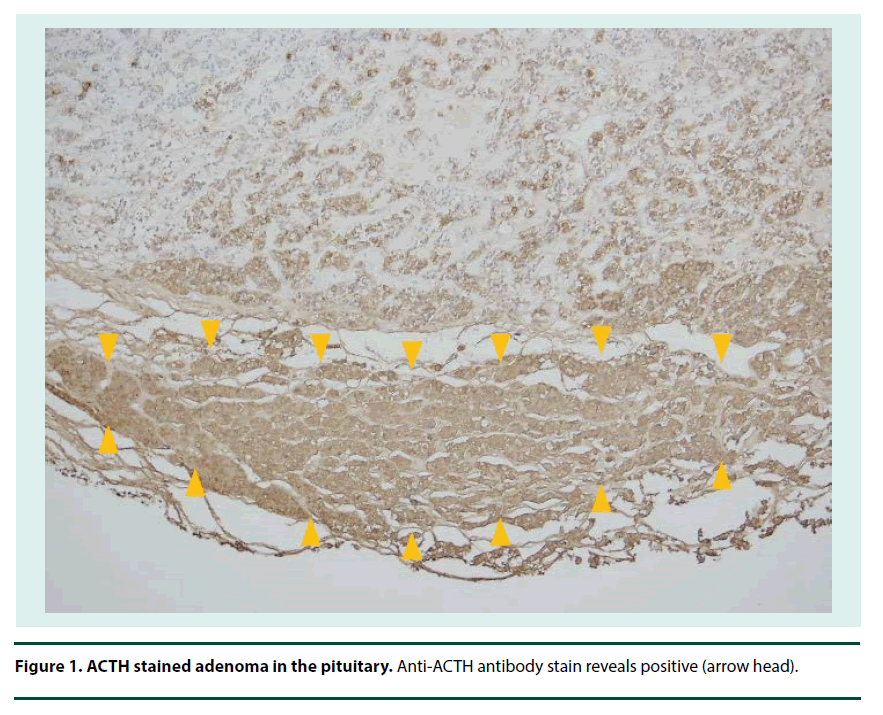
After the treatment, she became more alert within a few days. Her metabolic acidosis, blood glucose and insulin dosage returned to the previous level. However, she developed another episode of aspiration a week from the DKA episode; she became in septic shock state probably secondary to aspiration pneumonia and died a week later after the aspiration. Postmortem examination was permitted. We requested pathology if she had Cushing syndrome. The autopsy pathology confirmed adenoma in the pituitary gland (Figure 1).
Discussion
This is the first report, to our knowledge, of diabetic ketoacidosis with metabolic alkalosis (or DKALK) secondary to autopsy-confirmed Cushing disease. Some authors have objected to the term DKALK since alkalosis is not directly from diabetes, rather other causes [5]. Regardless of the term, this combined acid-base disorder does not seem to be familiar to most of the practicing physicians. Thus, little investigation has been performed on etiology and/or prognosis.
Once this acid-base disorder is recognized in DKA, diagnosis can be relatively straightforward with blood gas analysis. High delta ratio ([Na – (Cl+HCO3-)] > 1.2) or serum bicarbonate >24 mEq/dL indicates combined metabolic alkalosis and suspect DKALK. However, failure to recognize the disorder may hinder prompt diagnosis of DKA as well as investigation for the cause of metabolic alkalosis. This failure may adversely influence patient outcome. Since the case fatality rate of DKA still remains high [6,7], clinician need to appreciate this disorder adequately.
There are several publications reporting the prevalence of combined acid-base disorders in DKA. Elisaf et al. reported that 47.5% of DKA patients showed other acid-base disturbance [8]. Tanahashi et al. also studied acid-base disorders in DKA patients resulting 57% of the patients had mixed acid-base disorders [9]. Among the acid-base disorders in DKA, most prevalent one is DKA with metabolic alkalosis. This was originally described in 1959 [2], then Bleicher described the disorder as DKALK [3]. Since then more than 30 cases has been published in the English literature. Huggins et al. showed 21% and 35% of DKA cases fulfilled DKALK in adults and children, respectively [4]. In Tanahashi’s report, 6 out of 21 (28.6%) cases showed DKALK. It seems this combined acid-base disorder is more prevalent than expected [9].
To our knowledge, this is the first case of DKALK due to Cushing disease. Several underlying diseases have been shown to cause DKALK in series of case reports. Published DKALK cases were mainly DKA in cases with dehydration such as vomiting or diuretic use. Then, a few of corticosteroid excess and alkali ingestion [10] were reported. Handful cases of DKALK secondary to Cushing syndrome have been published [2,11-14]. Cushing syndrome by lung or bronchogenic cancer, prostate cancer with adrenal metastasis, pancreatic cancer with lung metastasis [2,11-14]. No report so far has been published describing DKALK secondary to Cushing disease.
Considering both Cushing disease as well as previous Cushing syndrome causing this acid-base disorder, hypothetically any conditions where cortisol is excess might cause this combined acid-base disorder. Ikema et al. reported steroid-induced Diabetes Mellitus (DM) complicated with DKALK. The case was also on diuretics. No case has been reported that a diabetic patient on steroid developed this condition or that steroid induced DM purely caused DKALK. There are many occasions where diabetic patient should receive steroid for some medical reasons. In addition, as we previously discussed that combined acid-base disorders in DKA are more prevalent than we expect, this phenomenon needs to be further investigated for elucidating its mechanism and clinical implication.
We present the case report to shed light on several important lessons in recognition and management of DKA. First, we need to appreciate this coexisting acid-base disorder to achieve prompt diagnosis and treatment since it seems prevalent among DKA. Second, diagnostic approach specific to DKALK which can be performed by thorough acid-base analysis also need to be well recognized. Third, in case of DKA with normal to high pH or abnormal delta ratio, active sought for the cause of alkalosis is required, while the cause may not be apparent initially.
Conclusion
We have experienced the first case of diabetic ketoacidosis with metabolic alkalosis secondary to Cushing disease. In real-world clinical practice, diabetic ketoacidosis may not always shows acidemia secondary to underlying alkalosis and corticosteroid excess in Cushing disease can cause DKALK. Index of suspicion may improve the outcome in patients with diabetic ketoacidosis.
References
- Chen HF, Wang CY, Lee HY et al. Short-term case fatality rate and associated factors among inpatients with diabetic ketoacidosis and hyperglycemic hyperosmolar state: a hospital-based analysis over a 15-year period. Intern. Med. 49(8), 729–737 (2010).
- Webster GD, Touchstone JC, Suzuki M. Adrenocortical hyperplasia occurring with metastatic carcinoma of prostate: report of a case exhibiting increased urinary aldosterone and glucocorticoid excretion. J. Clin. Endocr. Metab. 19(8), 967–979 (1959).
- Bleicher S. Ketosis not always acidosis: “heartburn” can be relevant. Diabetes Outlook. 2, 3–4, (1967).
- Huggins EA, Chillag SA, Rizvi AA, Moran RR, Durkin MW. Diabetic ketoalkalosis in children and adults. South. Med. J. 107(1), 6–10 (2014).
- Iqbal SJ, Walsh DB. Diabetic ketoalkalosis: a readily diagnosed non-entity. Br. Med. J. 2(6048), 1389 (1976).
- Delaney MF, Zisman A, Kettyle WM. Diabetic ketoacidosis and hyperglycemic hyperosmolar non-ketotic syndrome. Endocrinol. Metab. Clin. North Am. 29(4), 683–705 (2000).
- Ko SH, Lee WY, Lee JH et al. Clinical characteristics of diabetic ketoacidosis in Korea over past two decades. Diabet. Med. 22(4), 466–469 (2005).
- Elisaf MS, Tsatsoulis AA, Katopodis KP, Siamonpoulos KC. Acid-base and electrolyte disturbances in patients with diabetic ketoacidosis. Diabet. Res. Clin. Pract. 34(1), 23–27 (1996).
- Tanahashi H, Yasuda K, Hayashi M et al. Acid-base disturbances in Japanese patients with diabetic ketoacidosis. J. Jpn. Diabetes Soc. 49, 259–265 (2006).
- Zonszein J, Baylor P. Diabetic ketoacidosis with alkalemia-a review. West. J. Med. 149(2), 217–219 (1988).
- Greco AV, Bertoli A, Caputo S et al. Ketoalkalosis as a result of triple derangement of acid-base equilibrium in a diabetic patient. Acta Diabetol. Lat. 22(1), 73–77 (1985).
- Hudson B, Evans J. Adrenocortical hyperplasia associated with bronchogenic carcinoma. J. Clin. Endocr. Metab. 22(5), 494–500 (1962).
- Pearson DW, Thompson JA, Kennedy AC, Toner PG, Ratcliffe JG. Diabetic ketoalkalosis due to ectopic ACTH production from an oat cell carcinoma. Postgrad. Med. J. 57(669), 455–456 (1981).
- O’Reilly DS, Delamere JP. Cause of alkalosis in “diabetic ketoalkalosis”. Clin. Chem. 26(1), 171–172 (1980).