Review Article - Imaging in Medicine (2011) Volume 3, Issue 1
Developing a quality control program for digital mammography: achievements so far and challenges to come
Martin J Yaffe*Sunnybrook Health Sciences Centre, 2075 Bayview Avenue, Toronto, ON, M4N 3M5, Canada
- Corresponding Author:
- Martin J Yaffe
Sunnybrook Health Sciences Centre
2075 Bayview Avenue, Toronto
ON, M4N 3M5, Canada
Tel: +1 416 480 5715
Fax: +1 416 480 5714
E-mail: martin.yaffe@sunnybrook.ca
Abstract
Detection of breast cancers with mammography is a challenging task. Images must be of high quality if cancers are to be found at the earliest possible time. This motivates the need for a quality assurance program. It has long been recognized that the performance of a complex imaging system such as mammography can drift over time and, therefore, quality control procedures must be in place to ensure that all components of the imaging chain are operating properly. While digital mammography overcomes many of the technical limitations of screen-film mammography, its performance can easily be diminished if it is carried out in a suboptimal manner. Routine quality control is equally important for digital mammography as it was for screen-film imaging. While the need to monitor film processing generally disappears when digital imaging is employed, there are new requirements for quality control related to the display workstation and imaging software. Furthermore, to aid in controlling radiation dose to the breast, it is important to establish a linkage between digital signal value and dose and to monitor signal levels over time. The availability of images in a digital format offers an opportunity to improve efficiency in that it permits automated testing to be performed.
Keywords
digital mammography • quality control
Mammography has been demonstrated to be invaluable in the detection of breast cancers before they cause symptoms or can be found by physical examination. Employed in routine screening programs of women over 40 years of age, mammography has been demonstrated to contribute to a reduction in mortality due to breast cancer of 25% or more [1–4].
The radiologic signs of breast cancer include mass lesions, microcalcifications, asymmetries between images of the two breasts, and architectural distortion. In order for breast cancer to be detected accurately at the earliest possible opportunity, all factors influencing the acquisition, display and interpretation of the mammogram must be optimized and those optimum conditions must be maintained over time. This process, referred to as quality assurance, requires the cooperative efforts of several individuals: the technologist (radiographer), the radiologist, the equipment manufacturer and the medical physicist.
Detection of breast cancer requires that the breast be properly positioned in the mammography system and appropriately compressed by the technologist (radiographer). The exposure factors must be properly selected. Furthermore, the equipment used to acquire and display the image must be properly designed to facilitate including as much breast tissue as possible in the image and producing an image with excellent contrast and spatial resolution, with minimum distortion or artifact. This should be accomplished at the lowest radiation dose to the breast, compatible with these aspects of image quality.
Quality assurance embraces a wide range of activities, including the training, evaluation and continuing education of radiologic technologists and radiologists, the selection of equipment, reporting, dissemination of results and maintenance of records. The article focuses on quality control (QC), a term used to describe the program of testing the technology used in mammography to ensure that it is operating within an acceptable range of its optimum performance. I will consider the changes in the QC program that should occur as digital mammography replaces screenfilm technology, concentrating on the philosophy and principles of testing and on what needs to be tested. Detailed procedures for carrying out the tests have been described elsewhere [5–9]. Where possible, I have based the commentary in this article on material in the scientific literature and references are given where available. However, I have also included my observations that derive from working in the laboratory with digital mammography systems for over 15 years leading a large team of medical physicists who since 1990 have developed QC procedures and used them for testing film and digital (since 2005) equipment in The Ontario Breast Screening Program.
The principles of modern QC came from earlier work in general radiology and mammography [10–16,101]. Both in Europe and North America there was considerable effort in establishing recommendations for organized QC programs for mammography [17,102]. In North America, much of the momentum toward implementing routine QC in mammography came from the American College of Radiology (ACR) who began developing QC recommendations for mammography in 1987 and published comprehensive QC manuals in 1992, 1994 and 1999 [18].
A QC program will ensure that the tools used for mammography are operating properly. It is essential, however, to keep in mind that the skills and knowledge of the people who use these tools, the technologists who acquire the mammograms and the radiologists who interpret them, are absolutely critical in establishing high quality in detection and radiological diagnosis of breast cancer. The 1999 Quality Control Manual published by the ACR [18], which has sections for the radiologist and radiologic technologist, continues to be an excellent reference for this purpose, although hands-on continuing education courses provided by experts are probably the best resource for maintaining skills at the state-of-the art.
Digital mammography
Digital mammography was introduced in 2000 and has been shown in several studies to provide superior performance in screening [19–23] for breast cancer, particularly in women with dense breasts and those who are under 50 years of age or are pre- or peri-menopausal [19]. Overviews of these and other studies evaluating the performance of digital mammography are given in [24,25]. Digital mammography also has potential for increased efficiency in image archiving and retrieval, the possibility of avoiding the costs, complexity and waste disposal problems associated with chemical processing of film. In addition there is enhanced ability to perform quantitative imaging (e.g., computeraided diagnosis), contrast subtraction studies and 3D mammography (tomosynthesis). These factors have driven a steady replacement of film mammography by digital systems. This presents both opportunities and challenges to those involved in delivering mammography services. One of the important challenges is to have in place, in a timely fashion, an appropriate framework of quality assurance for digital mammography systems.
Approach to QC
A QC program for digital mammography should monitor all aspects of the image acquisition, archiving and display operations that affect clinical image quality or radiation dose. Clinical image quality refers to those factors that can affect the ability to make an accurate radiological diagnosis. Radiation dose is of concern because x-rays have carcinogenic potential. The guiding principle of radiological protection is that doses should be as low as reasonably achievable. Clinical image quality is, to some extent, related to radiation dose, and therefore, the word ‘reasonably’ implies that the dose should be high enough to provide the necessary clinical image quality.
The approach to QC is to establish appropriate baseline or reference values for the factors related to image quality and for the dose and to monitor on a routine basis that those factors and the dose remain within an acceptable range of the reference level. Technical QC is carried out primarily by two individuals, a QC radiological technologist and a medical physicist. Tests are assigned to each of these according to their background experience and their proximity to the facility; the radiological technologist working within the facility on a day-to-day basis carries out tests that must be performed daily, while the physicist, who may be available less frequently, performs those tests that require more expensive specialized equipment or a sophisticated understanding of physics and mathematics.
What requires monitoring?
Quality control programs were initially developed for screen-film mammography and the tests were based around those factors of image acquisition, archiving and display that had an important influence on image quality and that were likely to drift from their optimum setting or condition. The tests specified in the ACR program for screen-film mammography are listed in Box 1.
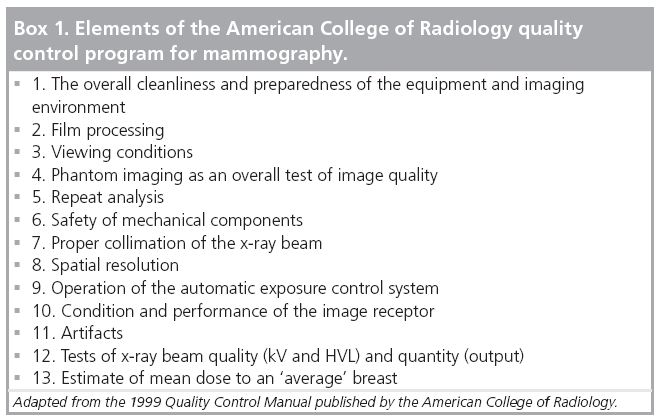
In the ACR program, the first five tests and test 11 are performed by the technologist while tests 6 –13 and modified versions of tests 3 and 4 are performed by the medical physicist. In addition, the physicist provides oversight of the technologist’s testing program as well as advice in problem solving.
Special considerations for digital mammography
Digital mammography differs from screen-film imaging in that the x-ray detector has a linear (or in some cases logarithmic) response to x-rays over a very wide range of exposure levels. Furthermore, because the detector signal is digitized and stored in computer memory, the image can be displayed and manipulated independent of the acquisition process. Unlike the case in film mammography, the brightness and contrast of digital images can be adjusted independent of x-ray exposure while they are being viewed on a high-resolution monitor. Image processing can be used to increase sharpness and alter the display characteristics of the image, for example to magnify (zoom) the images, or to compensate for changes in thickness of the breast at the periphery.
There is more variability in the technology used for digital mammography in that there are several different types of detectors. While most systems acquire ‘snapshot’ radiographs, some systems acquire the image by scanning a slotshaped beam of x-rays across the breast while recording the transmitted x-ray pattern with a corresponding slot-shape detector. In some systems the x-ray detector is a fixed integral part of the system, while in so-called ‘CR’ systems, the detectors are thin plates of a photostimulable phosphor that reside in lightproof cassettes during image acquisition, and a cassette is transferred after each x-ray exposure to a separate reading device.
Despite the improvements that have occurred with the introduction of digital mammography, routine QC testing is still necessary, but the testing requirements are somewhat different. Several jurisdictions have been active in developing QC programs tailored to the needs of digital mammography. These include The European Community [5], The National Health Services in the UK [6], Norway [7], Belgium, The International Atomic Energy Agency [8] and The American College of Radiology. This is not only due to the differences associated with digital imaging, but also due to the maturing of x-ray generation and control technology that has occurred over the past 25–30 years. Many of the tests remain the same, but some of the tests that were necessary for film mammography can now be eliminated and some new ones are required for digital mammography [9].
The purpose of QC testing is to be able to predict departures in the performance of components of the imaging system that could lead to degradation in clinical imaging performance and to correct them before such degradation occurs. The availability of images in digital form provides an enormous advantage in that it facilitates the introduction of tests that provide objective and quantitative measures of imaging performance to replace those that required subjective evaluation used in QC for screen-film mammography. These tests should provide more reliable results, free from observer variability, allow simplification of the testing procedure in some cases, as well as automatic logging of test results. It will be necessary, however, on the basis of experience, to validate these tests in terms of their sensitivity and relevance in predicting failures.
In addition to the purpose of QC, testing of equipment is carried out when the imaging equipment is installed or commissioned. In this case, a more comprehensive set of performance tests is usually conducted. Some of these tests are intended to ensure that the equipment complies with applicable regulatory requirements or meets the specifications guaranteed by the manufacturer in the purchase agreement, but the tests that form the routine QC program should also be performed. In many cases the results of these tests comprise the baseline or reference values about which the tolerances for the ongoing QC tests are set.
Aside from the physical factors underlying image quality in mammography there are also practical considerations related to the consistency of formatting and scaling of image data and the manner in which they are presented at the display workstation. The formatting of digital mammograms is prescribed by the Digital Imaging and Communications in Medicine (DICOM) standard [103]. There are two basic versions of digital mammograms, referred to as DICOM ‘for processing’ and DICOM ‘for presentation’. The former consists of an image whose pixels are nominally linearly (or in the case of CR images, logarithmically) related to the number or intensity of x-rays transmitted by the breast. As such, these images are useful for quantitative evaluation of the image and particularly the performance of the x-ray detector. Certain operations may have been performed on the original detector signal in producing this image. These include flat-field correction and possibly a sharpening function to restore spatial resolution. The ‘for presentation’ version of the image has been further processed to enhance contrast, adapt the dynamic range of the image to the display device and compensate for large changes in attenuation by the breast at its periphery. In most cases, tests of the image display system should be performed using the ‘for presentation’ image.
The DICOM standard was developed to facilitate image communication between different devices and image storage systems. Additional efforts have been made to strengthen the compatibility between image acquisition systems and display workstations among a multiplicity of vendors (e.g., multiple acquisition systems whose images are sent to one display workstation or shipment of images to a facility that uses another vendor’s display system). Notwithstanding these attempts, inconsistencies remain among systems, meaning that it is important to test the intercompatibility of components when they are put in place.
In addition, an image formatted by a vendor’s software for display on that vendor’s workstation may display on another vendor’s workstation, but the contrast characteristics or even the arrangement in which individual images in an examination are ‘hung’ may be other than what is expected. Needless to say, this is very frustrating for clinical users, contributing to inefficiency and possibly errors. To address issues of this type, the Integrating the Healthcare Enterprise (IHE) Committee has worked to develop ‘profiles’ for ensuring greater compatibility [104]. It would be of value to require in a purchase specification document that the components of a digital mammography system be compliant with the most recent IHE profile for digital mammography.
It is recognized that the capacity for testing and the ability to maintain a specified level of imaging performance will vary with economic conditions and availability of trained personnel. Therefore, the tests have been classified into two types – essential and desirable, with respect to their importance in influencing image quality and dose. The performance of the first category of tests is considered indispensable; however, it is recommended that the tests in the second category be carried out if adequate human resources and equipment can be made available. In a similar manner, the frequency at which testing is performed can be classified in terms of an essential minimum level and a desirable more frequent level.
Following the practice in the QC program developed by the European Community [5] test specifications can also be defined at two levels, a minimum acceptable level below which imaging should not be performed and a higher, achievable (referred to in the European program as ‘desirable’) level, which is considered as the goal for excellence. A facility should strive to ensure that equipment operates at the achievable level of performance, as this will produce the highest image quality and the most appropriate dose performance. It is recognized, however, that limited resources, uncorrectable environmental factors and other factors may prevent the achievable levels from being obtained. In no case should the facility continue to perform mammography if the equipment does not meet the acceptable standard of operation because, below this level, the value of the procedure and/or its safety is considered unacceptable.
Many of the tests for digital mammography systems employ algorithms that evaluate characteristics of the digital data. If the analysis of images is carried out with the intention of evaluating the characteristics of the acquired image (i.e., factors related to the x-ray beam and detector), it is desirable that test images be in ‘for processing’ DICOM format as this most directly reflects those characteristics. Similarly. if the analysis of the acquired image is to be carried out on a separate computer the image should be exported in ‘for processing’ DICOM format. A user-friendly interface for exporting images is a highly desirable feature.
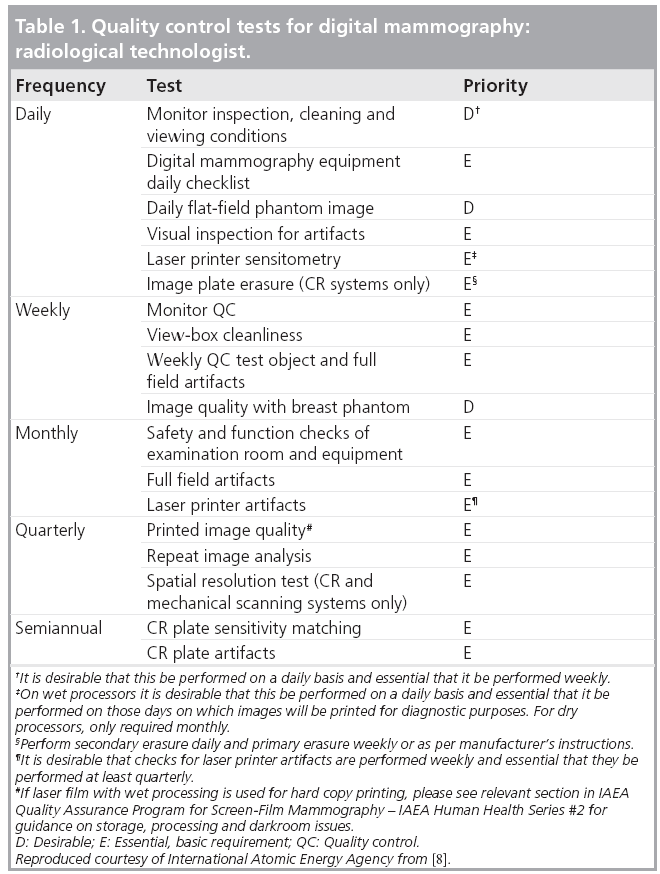
The International Atomic Energy Agency has developed a Harmonized Quality Control Program for Digital Mammography. The elements of this program have been based on the practical experience in QC of imaging physicists and clinical professionals from several countries. They have brought that experience and, in some cases, have borrowed from the strengths and attempted to avoid the perceived weaknesses of existing QC programs to develop what is hoped to be a practical set of tests. The tests required to be performed by technologists are given in Table 1, while those normally performed by a medical physicist are listed in Table 2. The explanation of the rationale for each test and details for carrying it out are given in [8].
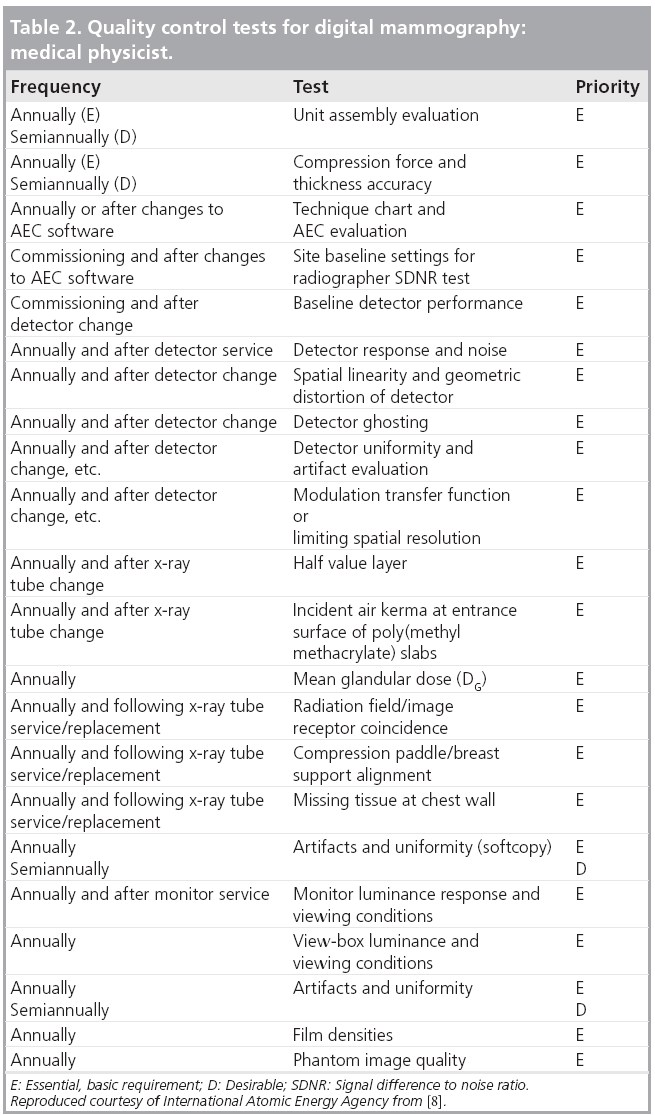
Differences between tests for digital and screen-film mammography
The principles that govern image quality in digital and screen-film mammography remain essentially the same. Nevertheless, the design and operation of digital mammography differs from those of screen-film mammography in several important ways. These motivate differences in the needs for QC testing and the manner in which the tests are conducted. Some of these are discussed here with reference to the different components of the imaging process.
X-ray generation
As in screen-film mammography, it is important that the quantity and quality of x-rays produced in each exposure is predictable and accurate. This is one of the areas where changes from historical QC programs are justified. While, at one time, intensive testing of all aspects of generator performance was necessary, x-ray generators have become much more sophisticated and reliable over the past 25 years. Modern generators are based on a high-frequency design providing automatic compensation for line voltage variations and internal feedback systems to maintain accurate kilovoltage and tube current. Digital exposure timers are extremely precise and reliable. Typically, when a generator fails, it ceases to operate rather than drifting away from proper calibration. Therefore, it is my opinion that routine monitoring of kilovoltage calibration is no longer necessary. Instead, a simplified QC procedure is recommended where the quantity of x-rays emitted (x-ray output) is monitored and the quality of the x-ray beam is measured in terms of the half-value layer. Only if the half-value layer and/or output depart from their normal values is it necessary to perform more detailed tests such as measurement of kilovoltage to diagnose a potential problem.
Automatic exposure control
Virtually all digital mammography is performed using automatic exposure control, in which the exposure time is determined by monitoring a signal related to transmission of x-rays by the breast. In addition, the choice of x-ray target material and kilovoltage are also performed automatically. As in film imaging these systems can go out of proper operation and, therefore, as in screen-film imaging they should be monitored as part of QC testing. This is accomplished by imaging slabs of uniform x-ray attenuating material and monitoring the ability of the system to maintain a particular image parameter, typically signal level or signal-to-noise ratio (SNR), within a specified range of acceptability.
Flat field (gain & offset) correction
Digital mammography systems, with the exception of those employing photostimulable phosphors in cassettes, have the advantage that spatial variations in sensitivity of the detector can be easily corrected. This is carried out by exposing the digital x-ray detector to x-rays transmitted through a slab of uniform thickness, and recording the resultant image. A second, ‘dark’, image is acquired without x-rays. The data from these two images can be used to produce a pixel-by-pixel correction for variations in detector sensitivity, offset (signal produced in the absence of radiation), as well as spatial variation in the x-ray beam itself due to such phenomena as the heel effect or spatial variation in attenuation of beam filters.
While flat field correction is a powerful tool that improves image quality by reducing what is referred to as ‘fixed pattern noise’, drifts in detector sensitivity or temporal changes in the x-ray field can cause the correction to become less effective. Therefore, as part of the QC program for digital mammography, a test of the flat-field correction is required. While full flatfield correction is not typically performed for the detector plates of CR systems (because each of several detector plates would have to be corrected individually, possibly for each of the multiple x-ray systems), the spatial uniformity of the plate reader can vary over time and this should be tested routinely so that it can be corrected if necessary.
Overall image quality
Historically, overall image quality in mammography has been evaluated by radiographing phantoms, containing objects that mimic structures of interest in the breast. Evaluation of such images is subject to both intra- and interobserver variability. An objective test that was introduced for digital mammography as a possible replacement is the measurement of signal difference to noise ratio (SDNR) as illustrated in Figure 1. The phantom is simply a slab of x-ray attenuating material with a small (~1 cm2) circular or square area that represents a slightly different attenuation. In the digital image, two regions of interest (ROIs) are selected, one corresponding to the square or disc and the other of similar area immediately adjacent to it. The difference between the mean signal levels measured in the ROIs (the signal difference) is divided by the standard deviation in the background ROI (the noise) to obtain the SDNR. This test incorporates a measure of image contrast, but also includes the undesirable influence of random fluctuation or noise in the image signal. Contrast alone is not a very useful measure of quality in digital imaging in that it can easily be amplified to any desired level at the display system. However, when such amplification is performed, the apparent image noise also increases, so that when the signal difference (related to contrast) is compared with the noise the measure should be more relevant.
Spatial resolution
With digital mammography, it is possible, using a simple test object and a software algorithm, to make a quantitative measurement of the spatial resolution characteristics of the imaging system in terms of the modulation transfer function, something that is very difficult to do with film imaging, particularly as a field test. This greatly facilitates tracking any changes in spatial resolution that may occur over time.
Dose
For QC in screen-film mammography, measurements of x-ray output are made routinely and they allow estimation of the ‘mean glandular dose’ that would be received by a breast of ‘average thickness and composition’ on the system. Knowledge of the doses that are being delivered is one of the factors that helps those providing mammography to determine that the equipment is being operated appropriately.
For screen-film mammography there is some degree of safeguard that doses are within an acceptable range because a shift to higher or lower dose is likely to cause the optical density to increase or decrease. These darker or lighter films will quickly be noticed and the unacceptable optical density and loss of proper contrast will trigger complaints and remedial action. With digital mammography the image brightness and contrast are controlled at the display workstation and, therefore, are largely independent of x-ray exposure. As such, changes in radiation exposure are much less likely to be noticed. For this reason, measurement of dose and provision of information to the technologists that allows them to infer dose and dose changes from signal levels on phantom images is even more important with digital mammography. In fact, some systems provide an indication of estimated dose as part of the image DICOM header. This can be valuable, although in some cases problems with the accuracy of such estimates have been noted in field testing by members of my group and, therefore, the validity of the reported doses should be confirmed as part of QC testing.
Image display system
Conversion to digital mammography eliminates the need for chemical processing of film and with it the cumbersome QC tasks of film sensitometry, processor and darkroom, and also the large number of artifacts associated with film and film processing.
Most digital mammography is carried out with images viewed on a high-resolution computer monitor. Proper performance of the display is critical in ensuring diagnostic image quality, and QC of the display workstation and monitor can be thought of as analogous to the testing previously performed on film and processors. QC testing can be carried out by loading an image of a test pattern onto the display workstation and making various objective measurements with a photometer and conducting a set of visual tests. Ideally, the test patterns would have the same overall format as the digital mammograms from the system. A Task Group of The American Association of Physicists in Medicine has created an excellent set of test patterns, which can be modified for use in digital mammography [105]. Tests can be conducted of image uniformity, contrast resolution and conformance to a standard grayscale display function [106]. To facilitate testing of the softcopy and hardcopy displays, it is desirable to be able to import test images to the digital mammography system in a convenient manner. This is difficult with some systems currently in use.
One valuable test is for the system to automatically present an image to the radiologist where they are required to discern a random low-contrast text string in the image [9]. This test could be performed daily and would only require a few seconds. A DICOM server can send these images to each workstation, and if the radiologist enters the correct sequence of letters, then the workstation is validated, and they can continue to review images. If the challenge fails, then imaging display conditions (e.g., intensity calibration and room lighting) are not suitable, and recalibration is indicated.
In cases, where digital images are printed on film for viewing, it will still be necessary to conduct QC on the film printing process, although modern printers are typically equipped with internal test patterns and use a dry development process.
Image processing software
In digital mammography it is possible to render significant changes to the appearance of the acquired image through image processing. This can be valuable in contributing to diagnostic quality and viewer esthetic impressions of the image.
One important consideration is the transformation that is often applied to an image so that it will fit onto a specific display system (i.e., the native format of the image usually has more pixels than can be accommodated by the display device and some type of image resampling or interpolation must be performed to reduce the size). Images are also manipulated to provide magnification of a specific ROI. Although the image is acquired at a certain spatial resolution, it has been noted in the course of our field testing of systems as part of the Ontario Breast Screening Program Physics QC protocol, that the interpolation operations applied to adjust the image size can degrade spatial resolution markedly. It is important to test that this does not occur as the displayed ‘magnification factor’ is varied.
Vendors may upgrade or modify image manipulation software and often this is caried out without communication of these changes to the personnel at the imaging facility. Changes can also be inadvertent; for example, date and time information for examinations or other default imaging parameters that had been set at the facility can become incorrect after software updates. These changes can have undesirable consequences, particularly if personnel are not aware that they have occurred. While it is difficult to recommend specific test procedures, evaluation by the technologist or medical physicist that the software is performing in an expected and appropriate manner is an important aspect of QC. This is an area where clear communication between the facility and service personnel as to expectations is of great importance.
Harmonized testing
Quality control programs for screen-film mammography generally can be applied universally to all x-ray units and screen-film products used for mammography. This has been less the case in digital mammography, particularly in the USA where each manufacturer was required to develop and submit to the US FDA a QC program specific to its digital mammography product as part of the regulatory approval process. Furthermore, once approved, changes in that program (e.g., improvements that would occur as additional knowledge was accumulated regarding performance) would again require government review at a cost of time and money. There are now systems from multiple vendors in the field, as well as multiple systems from particular vendors. If it were required to test equipment according to the different QC programs for each product (as has been the case in the USA), field testing within facilities and screening programs would be complicated and cumbersome and it would be difficult to compare the performance of different systems.
Some vendors of digital mammography equipment have introduced automated QC programs based on specialized test objects that are imaged by the technologist at regular intervals. The associated tests evaluate and track over time factors such as spatial resolution, SNR, defective pixels in the detector and response of the automatic exposure control system. While these programs can be quite effective, they are not standardized among vendors.
A harmonized QC program would, therefore, be highly desirable and some progress has been made in this direction [5–9].
Remote QC monitoring for digital mammography
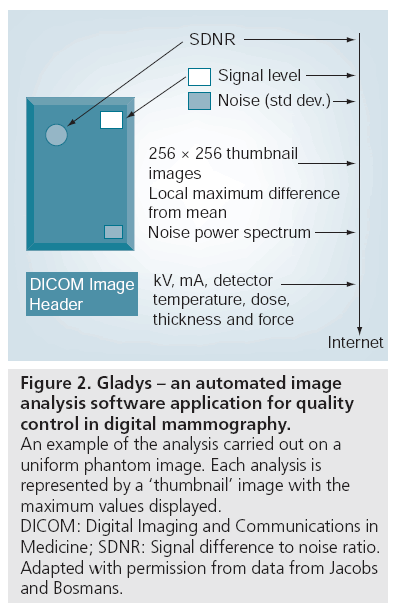
The personnel working at imaging facilities are trained for and are primarily involved with performing imaging of patients. Testing of equipment is often seen as displacing personnel from patient care and reducing productivity. Furthermore, some radiological technologists are not comfortable with the measurement and analysis required in the QC program. Therefore, particularly in multifacility screening programs, it may be valuable to implement a centralized QC program in which image data obtained on phantoms and test objects are automatically analyzed and results transmitted to a central location for oversight. Such a program has been implemented in Belgium (Figure 2) where it has been in place for several years [9,26]. In this program, images of test objects are analyzed with respect to constancy of signal response, SNR and other important parameters and the results are automatically transmitted by email to the analysis center where they are compared with previous values and compliance with the range of acceptability of each parameter is evaluated. Reports are sent to the facility to guide remedial action if required.
Figure 2: Gladys – an automated image analysis software application for quality control in digital mammography. An example of the analysis carried out on a uniform phantom image. Each analysis is represented by a ‘thumbnail’ image with the maximum values displayed. DICOM: Digital Imaging and Communications in Medicine; SDNR: Signal difference to noise ratio. Adapted with permission from data from Jacobs and Bosmans.
Future perspective
As the prevalence in use of digital mammography continues to rise both for screening and for assessment of symptoms or suspicious findings, we are gaining valuable experience with this modality in terms of what aspects of the systems fail and how frequently. We are also gathering data that will better inform new standards for acceptable and optimal performance of the imaging systems and their components. In the next 5 years I expect that these standards will solidify. I also expect that the use of automated QC testing programs will become standard. With increasing pressures on healthcare resources it would only seem logical to implement the sort of automated process control that is already pervasive across most industries. A uniform set of tests applied across all types of equipment would be most desirable, particularly for institutions or screening programs that employ a variety of different digital mammographic systems from different vendors. Furthermore, it would be most desirable for the QC tests to be easily interoperable with the digital mammography systems, either by being directly integrated into them or through a userfriendly interface.
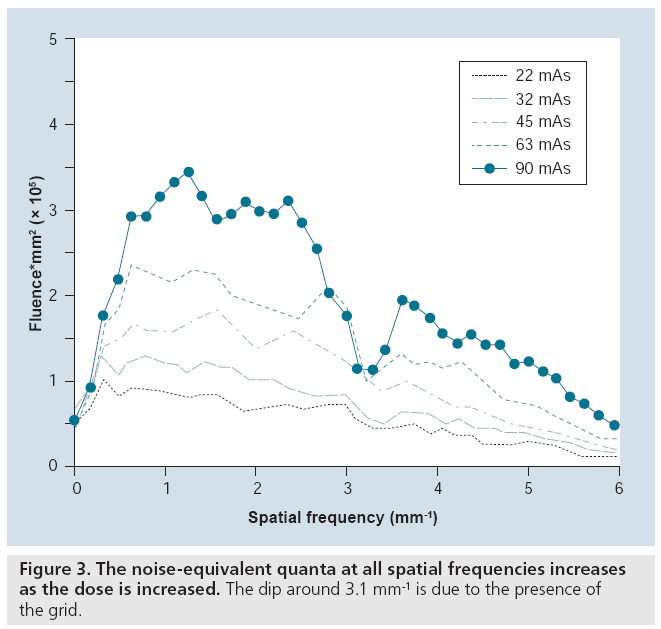
Quality control measurements will also become more sophisticated. For example, as discussed previously, a simple measure that incorporates image contrast and noise, to SDNR (akin to the contrast ratio, which is also used), is widely employed for QC testing. This measure is of some value, but does not take the spatial frequency or resolution aspects of imaging into account and can, therefore, be misleading. For example, simply blurring an image will cause the noise measurement to decrease, thereby elevating the SDNR. Conversely, sharpening an image (normally a desirable action) can cause a drop in SDNR. A potentially more informative test, measurement of the number of noise-equivalent quanta (NEQ) versus spatial frequency, includes spatial information, possibly giving a more relevant index of image quality. The NEQ is simply a graph of the square of the SNR at each spatial frequency and reflects the number of x-rays that appear to be used in forming the image as deduced from its noise characteristics. NEQ will increase as the number of x-rays actually used increases, as shown in Figure 3. NEQ also increases as the efficiency of the imaging system increases and as extraneous sources of image noise are eliminated. Evaluation of this measure for digital mammography is currently underway.
The quest for metrics that can be demonstrated to correlate more closely with clinical imaging performance will continue. Ideally, such metrics will be able to be evaluated objectively, for example from images acquired on phantoms or test objects. These measures will closely simulate the process of a particular clinical imaging task, such as detection of masses or microcalcifications and characterization of the morphology of lesions, among others. Considerable work is already underway in the development and testing of such ‘task-based’ metrics.
In addition, it is almost certain that the platform of digital mammography will evolve with the addition of quantitative tools such as measurement of breast density, 3D capability (tomosynthesis) and the capability of supporting contrast-enhanced studies. Each of these will require accompanying tests to ensure that quality is maintained.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Tabar L, Yen M-F, Vitak B, Chen H-HT, Smith RA, Duffy SW: Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 361, 1405–1410 (2003).
- Coldman A, Phillips N, Warren L, Kan L: Breast cancer mortality after screening mammography in British Columbia women. Int. J. Cancer. 120, 1076–1080 (2006).
- Moss SM, Cuckle H, Evans A et al.: Effect of mammographic screening from age 40 years on breast cancer mortality. Lancet 368, 2053–2060 (2006).
- Numan B, Duffy SW, Abdsaleh S et al.: Effectiveness of population-based service screening with mammography for women ages 40 to 49 years evaluation of the Swedish Mammography Screening in Young Women (SCR Y) cohort. Cancer DOI: 10.1002/ cncr.25650 (2010) (Epub ahead of print).
- van Engen R, Young K, Bosmans H: EUREF, European Protocol for the Quality Control of the Physical and Technical Aspects of Mammography Screening: Digital Mammography. In: European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, 4th Edition. Perry N, Broeders M, de Wolf C, Tornberg S, Holland R, von Karsa L (Eds). Luxembourg Office for Official Publications of the European Communities, 105–150 (2006).
- NHS Breast Screening Programme: Routine quality control tests for full field digital mammography systems: NHSBSP Equipment Report 0702. NHS Cancer Screening Programmes, Sheffield, UK (2007).
- Pedersen K, Landmark ID: Trial of a proposed protocol for constancy control of digital mammography systems. Med. Phys. 36(12), 5537–5546 (2009).
- Quality Assurance Programme for Digital Mammography. IAEA Human Health Series International Atomic Energy Agency (2011) (In press).
- Bosmans H, Carton A-K, Rogge F et al.: Image quality measurements and metrics in full field digital mammography: an overview. Radiat. Prot. Dosimetry 117(1–3), 120–130 (2005).
- Gray JE: Photographic quality assurance in diagnostic radiology, nuclear medicine and radiation therapy. Volume 1: The basic principles of daily photographic quality assurance. HEW Pub. (FDA) 76–8043 (1976).
- Hendee WR, Rossi RP: Quality assurance for radiographic x-ray units and associated equipment. HEW Pub. (FDA) 80–8094 (1979).
- Egan RL, Fenn JO: Phantoms for evaluating mammography techniques and roentgenographic detail. Am. J. Roentgenol. 102, 936–940 (1968).
- Tonge KA, Davis R: A phantom designed to compare the quality of various mammographic images. Br. J. Radiol. 51, 731–733 (1978).
- Yaffe MJ, Mawdsley GE, Nishikawa RM: Quality Assurance in a National Breast Screening Study. Proc. SPIE 419, 23–30 (1983).
- Screen Film Mammography: Imaging Considerations and Medical Physics Responsibilities. Barnes GT, Frey GD (Eds). Medical Physics Publishing, Madison, WI, USA (1991).
- British Institute of Radiology: Assurance of Quality in the Diagnostic X-ray Department: Quality Assurance Working Group of the Diagnostic Methods Committee of the British Institute of Radiology. British Institute of Radiology, London, UK (1988).
- Fitzgerald M, Dance DR, Fisher K, Lawinski CP, Ramsdale ML: Commissioning and Routine Testing of Mammographic X Ray Systems. Report No. 59. The Institute of Physical Sciences in Medicine, York, UK (1989).
- American College of Radiology: Mammography Quality Control Manual. American College of Radiology, Reston, VA, USA (1999).
- Pisano ED, Gatsonis C, Hendrick E et al.: Diagnostic accuracy of digital versus film mammography for breast cancer screening. The results of the American College of Radiology Imaging Network (ACRIN) Digital Mammographic Imaging Screening Trial (DMIST). N. Engl. J. Med. 353, 1773–1783 (2005).
- Skaane P, Hofvind S, Skjennald A: Randomized trial of screen-film versus full-field digital mammography with soft-copy reading in population-based screening program: follow-up and final results of Oslo II study. Radiology, 244, 708–717 (2007).
- Del Turco MR, Mantellini P, Ciatto S et al.: Full-field digital versus screen-film mammography: comparative accuracy in concurrent screening cohorts. AJR Am. J. Roentgenol. 189, 860–866 (2007).
- Hambly NM, McNicholas MM, Phelan N et al.: Comparison of digital mammography and screen-film mammography in breast cancer screening: a review in the Irish Breast Screening Program. AJR Am. J. Roentgenol. 193, 1010–1018 (2009).
- Vigeland E, Klaasen H, Klingen TA et al.: Full-field digital mammography compared to screen film mammography in the prevalent round of a population-based screening programme: the Vestfold County Study. Eur. Radiol. 18(1), 183–191 (2008).
- Lewin JM: Digital mammography clinical trials: The North American Experience. In: Digital Mammography. Bick U, Diekmann F (Eds). Springer, Heidelberg, Germany, 146–154 (2010).
- Skaane P: Digital mammography in European population-based screening programs. In: Digital Mammography. Bick U, Diekmann F (Eds). Springer, Heidelberg, Germany, 155–173 (2010).
- Jacobs J, Lemmens K, Nens J et al.: One year of experience with remote quality assurance of digital mammography systems in the Flemish Breast Cancer Screening Program. In: IWDM 2008. Krupinski EA (Ed.). Springer-Verlag, Berlin, Gemany, LNCS 5116, 703–710 (2008).
- AAPM Report No, 29, Equipment Requirements and Quality Control for Mammography, American Association of Physicists in Medicine (1990) www.aapm.org/pubs/reports/RPT_29.pdf (Accessed 24 January 2011)
- The European Protocol for the Quality Control of the Physical and Technical Aspects of Mammography Screening. CEC-Report EUR 14821 (1993) www.euref.org
- Digital Imaging and Communications in Medicine (DICOM): DICOM STANDARD http://medical.nema.org.
- Integrating the Healthcare Enterprise (IHE): IHE Radiology: Mammography User’s Handbook (2007) www.ihe.net/Resources/upload/IHE_ Mammo_Handbook_rev1.pdf
- Samei E, Badano A, Chakraborty D et al.: Assessment of Display Performance for Medical Imaging Systems. Report of the American Association of Physicists in Medicine (AAPM) Task Group 18. Medical Physics Publishing, Madison, WI, USA, AAPM On-Line Report No. 03 (2005) www.aapm.org/pubs/reports/OR_03.pdf (Accessed 24 January 2011)
- National Electrical Manufacturers Association Digital Imaging and Communications in Medicine (DICOM) Part 14: Grayscale Standard Display Function (2008) ftp://medical.nema.org/medical/ dicom/2008/08_14pu.pdf (Accessed 24 January 2011)
• Very complete program for quality control (QC) of digital mammography systems used as a template for the programs implemented primarily in Western European countries.
• Thoroughly documented program, well supported by experimental data.
• Describes a harmonized QC program for digital mammography with emphasis on jurisdictions having limited financial resources.
• Description of a remote automated QC program for digital mammography that has been implemented in the screening program in Belgium.
Websites
• Description of the Integrating the Healthcare Enterprise profiles for digital mammography, an invaluable resource for moving toward intercompatibility of systems.