Special Report - Imaging in Medicine (2013) Volume 5, Issue 4
CT-guided high dose rate brachytherapy ablation of liver metastases
Federico Collettini*1 and Bernhard Gebauer1
Department of Diagnostic& Interventional Radiology, Charité – Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany
- *Corresponding Author:
- Tel.: +49 30 450 627 117
Fax: +49 30 450 527 911
Email: :federico.collettini@charite.de
Abstract
Keywords
ablation ;CT-guided brachytherapy; CT-guided high dose rate brachytherapy; liver metastases ; liver tumors
With a central role in human metabolism and having an important portal filtering function for the GI tract, the liver is the most common target of hematogenous metastases from different primary cancers [1]. Metastatic spread to the liver is especially common in patients with gastrointestinal tumors, such as colorectal carcinoma, pancreatic carcinoma or neuroendocrine tumors. Primary cancers of the breast, stomach, esophagus and kidney, as well as less common tumors, tend to metastasize to the liver. The presence of liver metastases and their extent have a marked effect on survival [2].
State-of-the-art treatment of liver metastases is an interdisciplinary endeavor. All surgical, chemotherapeutic and interventional radiologic treatment options share one aim – to achieve local tumor control and improve patient survival.
Since the 1970s, surgical resection has been the standard treatment approach with curative intent for patients with liver metastases from colorectal cancer [3]. Nevertheless, the number and distribution of liver metastases may preclude surgical resection, and only 10–50% of patients have hepatic metastases amenable to surgery [4]. No agreement exists in the literature regarding the role of surgical resection in patients with noncolorectal and nonendocrine liver metastases. There is growing scientific evidence that resection of liver metastases is also beneficial in these patients. Although the patient populations reported in the literature are very heterogeneous, making it difficult to derive general recommendations, more and more patients with isolated noncolorectal, non- neuroendocrine hepatic metastases undergo surgical resection in the clinical setting [5,6]. Nevertheless, it must be borne in mind that primary surgical resection of liver metastases is only an option in approximately 25% of patients and that, despite considerable advances in the treatment of primarily unresectable liver metastases, the majority of patients are not candidates for surgical resection [7]. Moreover, 70% of patients will develop recurrent liver metastases after successful resection [8].
This situation has led to the development of various tumor ablation techniques for patients with unresectable or recurrent liver tumors. The most widely used and best understood alternatives are thermal ablation techniques, such as radiofrequency ablation (RFA) and laser-induced thermotherapy. Over the last decade, many retrospective and prospective studies have shown thermal ablation techniques to yield promising results in selected patients, and thermal ablation has become a valid treatment option for patients with inoperable liver disease [9,10]. However, these studies have also revealed some important limitations of thermal ablation, including large size, unfavorable location within the liver, nearby structures at risk (e.g., stomach, intestine and bile ducts) or nearby large vessels (heat sink effect) [11]. Here we present and review CT-guided high dose rate brachytherapy (CT-HDRBT) of secondary liver tumors – a nonthermal ablation procedure with some crucial advantages over thermal ablation.
CT-guided high dose rate brachytherapy
CT-HDRBT is an alternative radioablative technique that was introduced and implemented in clinical practice almost 20 years ago with an expanding role in the treatment of primary and secondary liver malignancies. CT-HDRBT was developed as a less invasive and more precise evolution of intraoperative radiotherapy [12,13]. Intraoperatively, interstitial HDRBT was used as early as the late 1980s; however, this intraoperative precursor was not only invasive, but also inaccurate in terms of dosimetry since catheter placement was guided by palpation or ultrasound. By contrast, the more recent, percutaneous technique of CT-HDRBT with iridium-192 enables accurate positioning of the afterloading catheters and exact 3D dosimetry based on CT data sets [14]. The procedure is performed with the patient under sedation and analgesia. With this technique, a radioactive radiation source is placed directly within the malignant lesion with CT or MR fluoroscopy guidance. A high dose rate is applied, which is defined as a dose over 12 Gy/h. With the radiation source within malignant lesion, precise radiation planning and a rapid drop in dose outside the target tissue, CT-HDRBT enables application of a very high radiation dose to the target volume (>50 Gy in the central tumor parts with high risk for hypoxic [radioresistant] cell clones), while at the same time sparing sensitive organs and structures outside the target lesion. Therefore, CT-HDRBT is superior to thermal ablation techniques in that it does not destroy adjacent healthy structures, such as bile ducts or blood vessels. As a result, CT-HDRBT can also be used safely for ablating subcapsular liver neoplasms, as well as lesions at the liver hilum or the hepatocaval confluence [15]. Limitations regarding the size and vascularization of potential target lesions are also overcome by CT-HDRBT [16]. The heat sink effect refers to the convective loss of heat near large vessels, which may result in incomplete destruction of malignant cells in vascularized lesions and in lesions near large vessels when a thermal ablation technique is used [17]. This dissipation of heat by flowing blood degrades the efficiency of thermal ablation techniques, but does not affect the outcome of CT-HDRBT.
Treatment planning, interventional technique & complications
General indications for CT-HDRBT comprise patients with up to five, unresectable liver metastases and no evidence of progressive extrahepatic disease (liver-dominant disease). There is no upper limit for CT-HDRBT concerning maximal tumor diameter and successful treatment of lesions up to 12 cm in diameter are reported in the literature. Further general indications comprise patients with limited chemorefractary disease or patients with progressive hepatic disease under systemic therapy.
CT-HDRBT of metastatic liver disease is most frequently planned on the basis of a MRI examination of the upper abdomen after administration of a hepatocyte-specific MR contrast agent (gadoxetic acid [Gd-EOB-DTPA]; Primovist ®, Bayer, Berlin, Germany) on the day before the planned procedure to assess technical feasibility and to obtain an accurate estimate of the extent of liver metastases.
While other groups perform direct puncture of the tumor with dedicated brachytherapy needles, at our institution the probe is placed within the tumor through closed-end brachytherapy catheters. The brachytherapy catheters are advanced into the metastatic lesions under CT fluoroscopy guidance (SOMATOM® Definition AS, Siemens, Erlangen, Germany). Following percutaneous puncture of the target lesion with a 17-G needle, a 270 mm 6-F sheath (Radiofocus®, Terumo, Tokyo, Japan) is introduced over a stiff angiographic guidewire (Amplatz, Boston Scientific, MA, USA). Next, the guidewire is withdrawn and a closed-end 350-mm 6-F afterloading catheter (Primed™, Halberstadt Medizintechnik GmbH, Halberstadt, Germany) is advanced through the sheath. Immediately after placement of the brachytherapy catheter, a contrast-enhanced CT scan of the upper abdomen is obtained during breath hold.
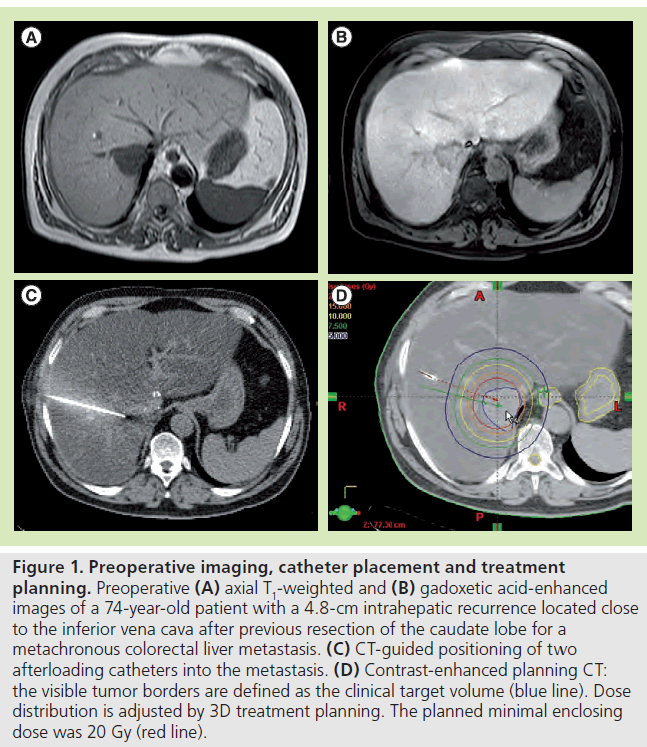
The CT data set is used for computer-assisted 3D irradiation planning using Brachyvision™ (Gammamed™, Varian, CA, USA). The positions of all afterloading catheters are marked from the tip to the body exit site (Figure 1). Next, the lesion is encircled and marked as clinical target volume, and all relevant structures at risk are also marked (e.g., stomach, intestine and gallbladder). The dwell times of the 192-iridum solid body source in the catheter are semiautomatically and manually optimized in order to ensure complete coverage of the target volume and sparing of risk structures. The minimum dose for coverage of the clinical target volume is 20 Gy. Maximum doses of >50 Gy are allowed in the tumor center. All treatments are performed as single-fraction irradiation using an after-loading system (GammaMed). The iridium-192 solid body permanent radiator has 10 Ci nominal activity and a diameter of less than 1 mm. Bleeding after removal of the brachytherapy catheter is controlled by insertion of a torpedo-shaped gelatin sponge (Gelfoam®, Pfizer Inc., NY, USA).
CT-HDRBT has a low complication rate. Complications related to catheter insertion are the same as in RFA. CT-guided puncture minimizes inadvertent damage and hemorrhage from adjacent organs (e.g., lung, stomach and intestine). Radiogenic complications include inflammation of the gastric mucosa or gastric ulcer.
Skin burns due to catheter dislocation are described in the literature, but are extremely rare. Intermittent or irreversible loss of liver function may occur when an excessive radiation dose is applied. This is taken into account by marking sensitive structures such as normal liver parenchyma, stomach, intestine, lung or spinal cord in the planning phase (Table 1). In the case of critical, but not excessive, radiation exposure of the stomach or small intestine, patients receive a prophylactic proton pump inhibitor for 6 weeks. When a very large tumor volume is irradiated, decay of the tumor induces an inflammatory reaction with cytokine release, presenting with fever, chills and nausea 4–6 h after treatment. These symptoms only persist for a few hours and can be treated symptomatically.
Results with CT-HDR BT of colorectal liver metastases
Liver metastasis continues to be a therapeutic challenge in patients with colorectal cancer. Most liver metastases are primarily unresectable or recur after surgery. Therefore, a variety of minimally invasive ablation techniques have been developed and tested over the last two decades. Many retrospective and prospective studies have shown promising outcomes for RFA in selected patients. Hence, RFA has become a valid treatment option for patients with inoperable liver metastases. Recently, the CLOCC study by Ruers and colleagues has shown that combined RFA and systemic therapy improve progression-free survival compared with systemic therapy alone (16.8 vs 9.9 months) [18].
Indications for CT-HDRBT of colorectal liver metastases comprise patients with limited nonresectable liver metastases or patients with hepatic recurrence after liver resection for colorectal liver metastases.
The first study of CT-HDRBT for colorectal liver metastases was published in 2010. Ricke et al. treated 73 patients with 199 colorectal liver metastases. Local progression was observed in 25.1% of lesions, and the authors found that the radiation dose was crucial for achieving local tumor control [19].
Figure 1. Preoperative imaging, catheter placement and treatment planning. Preoperative (A) axial T1-weighted and (B) gadoxetic acid-enhanced images of a 74-year-old patient with a 4.8-cm intrahepatic recurrence located close to the inferior vena cava after previous resection of the caudate lobe for a metachronous colorectal liver metastasis. (C) CT-guided positioning of two afterloading catheters into the metastasis. (D) Contrast-enhanced planning CT: the visible tumor borders are defined as the clinical target volume (blue line). Dose distribution is adjusted by 3D treatment planning. The planned minimal enclosing dose was 20 Gy (red line).
These findings suggest that the outcome of CT-HDRBT in terms of local tumor control is promising compared with RFA, especially in view of the fact that the metastases treated by CT-HDRBT are markedly larger than the lesions typically treated with RFA.
Results with CT-HDR BT of noncolorectal liver metastases
Although most experience with both RFA and CT-HDRBT has been gained in the treatment of hepatocellular carcinoma and liver metastases from colorectal cancer, some case series published in recent years report experience with the treatment of other tumor entities (Table 2). There are several rationales for also using CT-HDRBT in the treatment of liver metastases from primaries other than colorectal cancer: common indications for CT-HDRBT include recurrent liver malignancy after surgery, complete cytoreduction in nonoperable patients with limiting liver disease, palliative debulking for better quality of life or alleviation of tumor-related symptoms, for instance, in patients with neuroendocrine tumors.
Breast cancer liver metastases
Hormone therapy, chemotherapy and supportive care remain the therapies of choice in the management of patients with metastatic breast cancer. Local ablative techniques including CTHDRBT can be adopted in chemorefractory patients with isolated liver metastases and no extrahepatic progressive disease. At our institution stable skeletal metastases are regarded as a contraindication for CT-HDRBT.
Initial results of CT-HDRBT in the ablation of breast cancer liver metastases are very encouraging: both the group from Berlin (Germany) and the group from Magdeburg (Germany) have published promising results. In the first Phase II trial of 41 women with 115 liver metastases, Wieners et al. achieved a 93.5% local tumor control rate at 12 months and found a mean time to tumor progression of 8.1 months [20]. Our experience with breast cancer liver metastases is also very encouraging. Between January 2008 and December 2010, we treated 37 patients with 80 breast cancer liver metastases. The local tumor control rate was 97.4% at a mean followup of 11.6 months; only two metastases showed local progression. In both cases, repeat ablation was performed and further local progression was locally controlled [20].
Liver metastases from gastroesophageal cancer
Recently, Geisel et al. reported their initial experience with CT-HDRBT in patients with liver metastases from gastroesophageal adenocarcinoma [21]. None of the eight patients developed local recurrence, and no major complications associated with treatment were observed. Although the number of patients treated in this first pilot study is very low, CT-HDRBT was shown to be a feasible alternative to surgical resection of liver metastases from gastric or gastroesophageal adenocarcinoma in selected patients, and our preliminary data suggest that the outcome is comparable to the results achieved with surgical resection.
Liver metastases from ovarian cancer
Although some authors have proposed liver resection as part of cytoreduction, reluctance to practice upper abdominal surgery in patients with ovarian cancer remains and many patients with otherwise resectable disease are declared not to be candidates for cytoreduction. In a pilot study, Collettini et al. investigated the feasibility and clinical outcome of minimally invasive cytoreduction by means of CT-HDRBT in seven multiply pretreated patients with ovarian cancer and metachronous liver metastases [22]. Gd-EOB-DTPA-enhanced MRI obtained 6 weeks after CT-HDRBT demonstrated successful ablation of all liver metastases. There was no local progression after a mean followup of 15.4 months. This pilot study shows CT-HDRBT to be feasible and allow minimally invasive ablation of liver metastases in breast cancer patients. No minor or major complications were observed although all patients had a history of multiple abdominal interventions and were in a reduced condition. As we only treated seven patients, the only conclusion that can be drawn from this study is that CTHDRBT is reliable and feasible in this subset of patients. CT-HDRBT can hence be considered as an attractive alternative for cytoreduction of liver metastases from ovarian cancer. Moreover, the results achieved in the severely ill patients included in this small pilot study provide convincing evidence that CT-HDRBT is well tolerated.
Conclusion
The studies discussed above consistently show that CT-HDRBT is a reliable and effective minimally invasive treatment for local tumor ablation in the liver. It is superior to the widely used thermoablative methods in that there are no restrictions regarding lesion size or location within the liver (e.g., central, subcapsular or close to vessels).
Future perspective
Reported local control rates with CT-HDRBT are satisfactory; however, in order to obtain favorable results in terms of overall survival, in the coming years the association of effective systemic therapies to decrease the incidence of new hepatic metastases should be evaluated. Furthermore, as literature about the use of CT-HDRBT in the management of liver metastases is still scarce and composed mainly of retrospective or single-center studies with relatively small patients groups, larger trials are urgently needed to confirm that this highly effective local ablative treatment has an impact on overall survival.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Eder M, Weiss M. [Hematogenous liver metastases-human pathologic principles]. Chirurg. 62, 705–709 (1991).
- Manfredi S, Lepage C, Hatem C et al. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 244, 254–259 (2006).
- Ott R, Wein A, Hohenberger W. [Liver metastases–primary or multimodal therapy?] Chirurg. 72, 887–897 (2001).
- Mutsaerts EL, van Ruth S, Zoetmulder FA et al. Prognostic factors and evaluation of surgical management of hepatic metastases from colorectal origin: a 10-year single-institute experience. J. Gastrointest. Surg. 9, 178–186 (2005).
- Adam R, Chiche L, Aloia T et al. Hepatic resection for noncolorectal nonendocrineliver metastases: analysis of 1452 patients and development of a prognostic model. Ann. Surg. 244(4), 524–535 (2006).
- Selzner M, Morse MA, Vredenburgh JJ et al. Liver metastases from breast cancer: long-term survival after curative resection. Surgery 127, 83–89 (2000).
- Adam R, Avisar E, Ariche et al. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann. Surg. Oncol. 8, 347–353 (2001).
- Pasetto LM, Rossi E, Monfardini S. Liver metastases of colorectal cancer: medical treatments. Anticancer Res. 23, 4245–4256 (2003).
- Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur. J. Ultrasound 13, 129–147 (2001).
- Livraghi T, Goldberg SN, Monti F et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology 202, 205–210 (1997).
- Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann. Surg. 242(2), 158–171 (2005).
- Thomas DS, Nauta RJ, Rodjers JE et al. Intraoperative high-dose rate interstitial irradiation of hepatic metastases from colorectal carcinoma. Results of a Phase I–II trial. Cancer 71(6), 1977–1981 (1993).
- Griffin PC, Amin PA, Hughes P, Levine AM, Sewchand WW, Salazar OM. Pelvic mass: CT-guided interstitial catheter implantation with high-dose-rate remote afterloader. Radiology 191(2), 581–583 (1994). n Griffin et al. first described this technique in 1994.
- Ricke J, Wust P, Stohlmann A et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: Phase I-II results of a novel technique. Int. J. Radiat. Oncol. Biol. Phys. 58(5), 1496–1505 (2004).
- Ricke J, Wust P, Wieners G et al. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J. Vasc. Interv. Radiol. 15(11), 1279–1286 (2004). n Ricke and colleagues, who published a large patient series treated with CT-guided interstitial high dose rate brachytherapy for primary and secondary liver malignancies, first highlighted the advantages of CT-guided high dose rate brachytherapy over thermal ablation.
- Collettini F, Schnapauff D, Poellinger A et al. Hepatocellular carcinoma: computedtomography- guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5–7 cm) and very large (>7 cm) tumours. Eur. Radiol. 22(5), 1101–1109 (2012).
- Lu DS, Raman SS, Vodopich DJ et al. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the ‘heat sink’ effect. AJR Am. J. Roentgenol. 178(1), 47–51 (2002).
- Ruers T, Punt C, Van Coevorden F et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup Phase II study (EORTC 40004). Ann. Oncol. 23(10), 2619–2626 (2012).
- Ricke J, Mohnike K, Pech M et al. Local response and impact on survival after local ablation of liver metastases from colorectal carcinoma by computed tomography-guided high-dose-rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010 78(2), 479–485 (2010).
- Wieners G, Mohnike K, Peters N et al. Treatment of hepatic metastases of breast cancer with CT-guided interstitial brachytherapy – a Phase II-study. Radiother. Oncol. 100(2), 314–319 (2011).
- Geisel D, Denecke T, Collettini F et al. Treatment of hepatic metastases from gastric or gastroesophageal adenocarcinoma with computed tomography-guided high-dose-rate brachytherapy (CT-HDRBT). Anticancer Res. 32(12), 5453–5458 (2012).
- Collettini F, Poellinger A, Schnapauff D et al. CT-guided high-dose-rate brachytherapy of metachronous ovarian cancer metastasis to the liver: initial experience. Anticancer Res. 31(8), 2597–2602 (2011).
- Collettini F, Golenia M, Schnapauff D et al. Percutaneous computed tomography-guided high-dose-rate brachytherapy ablation of breast cancer liver metastases: initial experience with 80 lesions. J. Vasc. Interv. Radiol. 23(5), 618–626 (2012).