Research Article - Journal of Experimental Stroke & Translational Medicine (2009) Volume 2, Issue 2
Chronic treatment with Losartan and Cerebral Ischemic Tolerance
- *Corresponding Author:
- Zonghang Zhao, MD
Department of Clinical Neurosciences, Hotchkiss Brain Institute,
Faculty of Medicine, University of Calgary
2021, HSC, 3330 Hospital Drive, NW, Calgary, Alberta T2N 4N1, Canada
Tel: 1-403-220-5205
Fax: 1-403-283-3572
Email: zzhao@ucalgary.ca
Abstract
Clinical trials have suggested that Losartan has beneficial effects on preventing stroke beyond its antihypertensive properties. Although there is evidence for Losartan reducing ischemic damage following a highly localized type of stroke, its role in larger focal and global ischemic insults and the importance of blood pressure change are not known. We hypothesized that pre-treatment with Losartan would enhance the tolerance of the brain to both global and focal ischemia. Two weeks prior to ischemia, rats were administered either Losartan 50 mg/day in their drinking water or vehicle (water) alone. The neuroprotective effects of Losartan were assessed by either global ischemia using 10 minutes of four-vessel occlusion (4-VO) or 90 minutes of focal ischemia by distal middle cerebral artery occlusion. Seven days after 4-VO, histological assessment of cellular injury in the hippocampal CA1 region was performed. In addition, brain injury following transient MCAO was assessed by magnetic resonance imaging and histology. Losartan pretreatment reduced neuronal damage following global ischemia, as detected by 92% versus 46% neuronal cell death in CA1 region for Control and Losartan groups, respectively (p<0.001). Following transient ischemia, pre-treatment with Losartan resulted in a reduced infarct volume: measured as 41% ± 10% versus 26% ± 12% of the hemisphere in control and Losartan groups, respectively (p<0.04). These protective effects were observed in spite of 12-15 % reductions in blood pressure in the Losartan treated group. Our results demonstrate that pre-treatment with Losartan enhances the tolerance of the brain to either global or focal ischemia and provides further evidence for Losartan as a treatment for stroke prevention.
Keywords
Losartan; Stroke prevention; Global and focal ischemia; Blood pressure; MRI
Introduction
Losartan, a non-peptide angiotensin II receptor antagonist with high affinity and selectivity to receptor type I, is extensively used to treat arterial hypertension. The results from clinical trials such as LIFE (Losartan Intervention for Endpoint) (Dahlof B et al, 2002) and SCOPE (The Study on Cognition and Prognosis in the Elderly) (Lithell H et al, 2003) have suggested that in a background of equally reduced blood pressure, angiotensin II type 1 receptor antagonist offered additional benefits over other blood pressure control in stroke reduction, suggesting that the benefits of angiotensin II type 1 receptor antagonist were due to effects beyond blood pressure control. The primary actions of angiotensin II are vasoconstriction and sodium and water retention, which increase blood pressure, as well as inotropy and hypertrophy of vascular and cardiac cells (Page IH et al, 1987) . In the cerebral vasculature, angiotensin II alters autoregulation, inhibits endothelium-dependent relaxation, and disrupts the blood brain barrier. It functionally impairs the increase in cerebral blood flow produced by neural activity (Kazama K et al, 2005) and induces structural alterations of cerebral arterioles independently of systemic blood pressure (Baumbach GL et al, 2003) . During the past decade, a number of studies have indicated that angiotensin II can interfere with processes occurring during and after focal cerebral ischemia (Lars Edvinsson, 2008) . Sustained blockade of the AT1 receptor may allow for a more active angiogenic and neuroprotective role of the AT2 receptor in ischemic stroke (Achard J et al, 2001; Shibata K et al, 1998) . Most recently, a study has demonstrated that pre-treatment with Losartan can provide some protection against a small local injury produced by cortical surface vessel cauterization where there is evidence that protection was a result of increased surface vascularization (Forder JP et al, 2005). We hypothesized that pre-treatment with Losartan would enhance the tolerance of the brain to an ischemic insult irrespective of the type of ischemia and through the protective contribution of improved collaterals. This hypothesis was tested by investigating the protective effects of Losartan treatment in both a focal rodent stroke model and in a global cerebral ischemia model.
Materials and Methods
Drug Delivery
Two weeks prior to ischemia, rats in the treatment group were administered losartan in their drinking water at a concentration of 1mg/ml. Water intake was measured daily and for rats consuming less than 50ml/day, the remaining amount of losartan was given via a feeding tube to ensure a daily dose of 50mg/day.
Forebrain Ischemia Experiment
Adult male Wister rats were divided into two groups: a losartan treatment group (n=8) and a vehicle control group (n=8). Rectal temperature in rats was maintained until full recovery from anesthesia was achieved. Severe transient forebrain ischemia was produced by a modified four-vessel occlusion (4-VO) surgery (Pulsinelli WA et al, 1979; Pulsinelli WA and Buchan AM, 1988). On the day prior to ischemia, vertebral arteries were electrocauterized and the common carotid arteries isolated. The following day both carotid arteries were then occluded for 10 minutes with aneurysm clips. Core temperature was regulated until rats became fully ambulatory. Rats that became unresponsive or had initial running behaviour, loss of righting reflexes and dilation of pupils were included, while rats that ceased to remain in a coma or developed righting reflexes during ischemia or displayed seizure activity during or after ischemia were excluded.
Seven days after ischemia, rats were euthanized, and brains were fixed with 10% formaldehyde. Coronal sections (6 μm) of paraffin-embedded brains were cut at 3.3 mm posterior to bregma (Zhao Z et al, 2006) and stained with hematoxylin and eosin (H&E). Non-eosinophilic normal cells were counted in the entire CA1 band and expressed as a percentage of live neurons.
Focal Ischemia Experiment
Adult male spontaneously hypertensive rats (SHR) were used in the focal ischemia study and were divided into a losartan treatment group (n=7) and a vehicle control group (n=6). MCA occlusion surgery was performed as previously described (Colbourne F et al, 2000) . After cannulation of tail artery for blood pressure and blood gas measurement, the right common carotid artery was isolated and a ligature placed around it. A craniotomy was made over the right MCA and a transient MCA occlusion was made by permanent right carotid artery occlusion combined with the placement of a microaneurysm clip on the MCA for 90 minutes. Body temperature was maintained within a normal range (37 ± 0.5 o C) during all surgical procedures until the rat was fully recovered from anesthesia. Cerebral cortical perfusion (estimated by a laser-Doppler flowmetry probe situated over the core regions; sampled in series), blood pressure, blood gases, plasma glucose, were all measured at the onset and the end of ischemia. The wound was closed with suture and anesthesia discontinued soon after the onset and end of ischemia. At the time of euthanasia (24 h following ischemia and after MRI imaging), rats were reanesthetized and perfused with normal saline followed by 4% paraformaldehyde. The brain remained in situ for 12 h prior to removal, followed by paraffin embedding. Brain sections were cut at 8 mm, and alternate sections were stained with hematoxylin and eosin (H&E). The infarct area and the normal or contra-lateral hemisphere of each section were traced using an image processing system (Image Pro). Infarction as a percentage of the contra-lateral hemisphere was calculated and total percentage infarct volume was calculated as: % Infarct Volume =100 x [Infarct Volume – (Ipsilateral Hemispheric Volume – Contralateral Hemispheric Volume)]/Contralateral Hemisphere Volume
MRI Imaging
Twenty-four hours after ischemia, animals were anesthetised with isoflurane. MRI imaging (T2 and perfusion) was performed using a 9.4T/21 cm bore MR system with a Bruker console as described previously (Kaur J et al, 2009) . To obtain T2 maps, a series of T2 weighted images was acquired using a multi-slice, 21 multi-echo spin echo sequence (TR=2500 ms, TE=10 ms between echoes) to assess ischemic damage and ensure the presence of a cortical infarct in the right hemisphere. T2 lesion volume, T2 Value and perfusion blood flow were measured using Institute for Biodiagnostics developed software (Marevisi).
Statistical analysis
Differences between Losartan and vehicle treated groups were tested using a one way ANOVA followed by an unpaired Student’s t-test. A P value of <0.05 was used to determine significance. Data are expressed as mean ± SD.
Results
1. Losartan Treatment and Mean Arterial blood pressure (MABP)
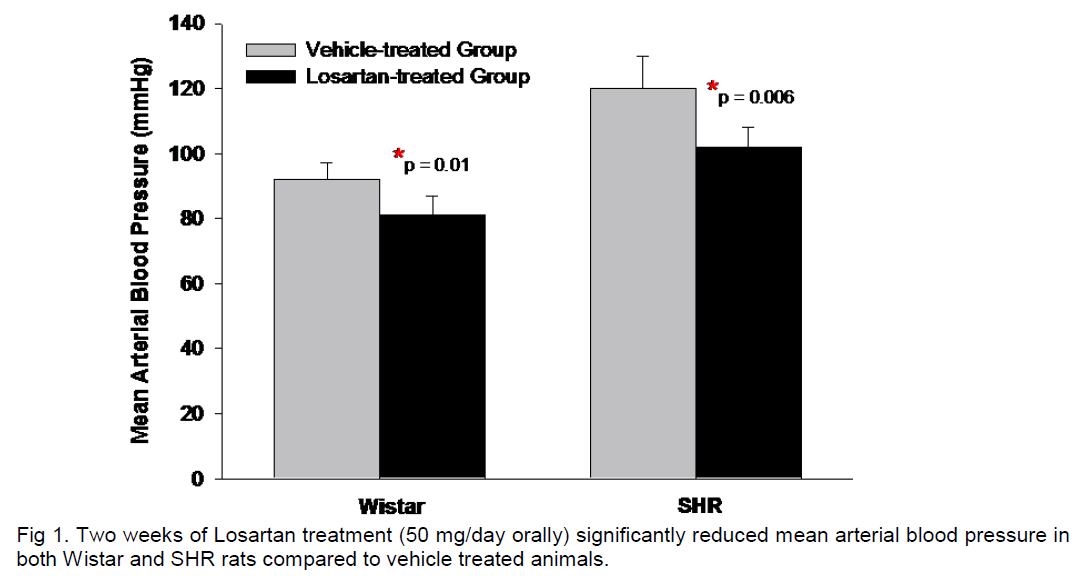
The majority of rats consumed less water than that required for an oral dose of Losartan of 50 mg/day requiring additional administration of Losartan by gavage. After two weeks of treatment, Losartan treated rats had significant decreases in mean arterial blood pressure (MABP) compared to the corresponding vehicle-treated group. For Wistar rats, MABP was 92±5 mmHg compared to 81±6 mmHg, in vehicle and Losartan treated groups, respectively. For SHR rats, MABP in the vehicle-treated rats was 120±10 mmHg compared to 102±6 mmHg after 2 weeks Losartan treatment (Figure 1).
Global cerebral ischemia
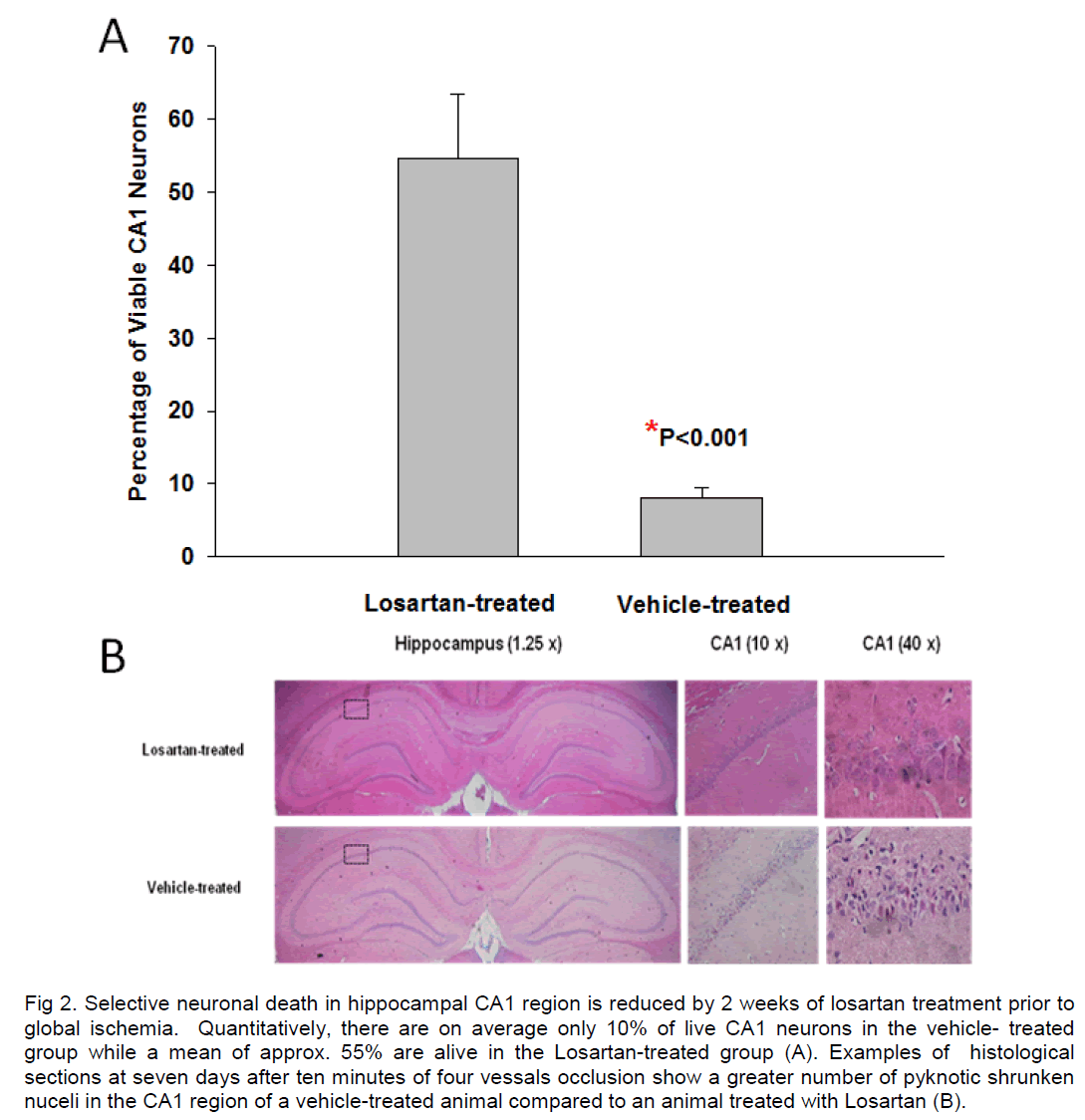
There was no difference in mortality between vehicle–treated and Losartan–treated Wistar rats that underwent global ischemia (4-VO). Most successful ischemic animals recovered well from the brief cerebral ischemic insult irrespective of treatment. There were three rats that died during the procedure of 4-VO related to artery rupture: 2 in vehicle-treated group and 1 in Losartan-treated group. An additional 2 rats died post ischemia in this experiment, one from each group because of seizure development. Quantification of viable neurons in the surviving animals showed that cerebral ischemic injury in Losartan pre-treated rats was reduced. Seven days after global ischemia, histological analysis of sections through the anterior hippocampus demonstrated that more than 90% of CA1 pyramidal neurons were dead. This is in contrast to a neuronal loss of only 45% of CA1 neurons in Losartan-treated animals (Figure 2).
Figure 2. Selective neuronal death in hippocampal CA1 region is reduced by 2 weeks of losartan treatment prior to global ischemia. Quantitatively, there are on average only 10% of live CA1 neurons in the vehicle- treated group while a mean of approx. 55% are alive in the Losartan-treated group (A). Examples of histological sections at seven days after ten minutes of four vessals occlusion show a greater number of pyknotic shrunken nuceli in the CA1 region of a vehicle-treated animal compared to an animal treated with Losartan (B).
Focal cerebral ischemia
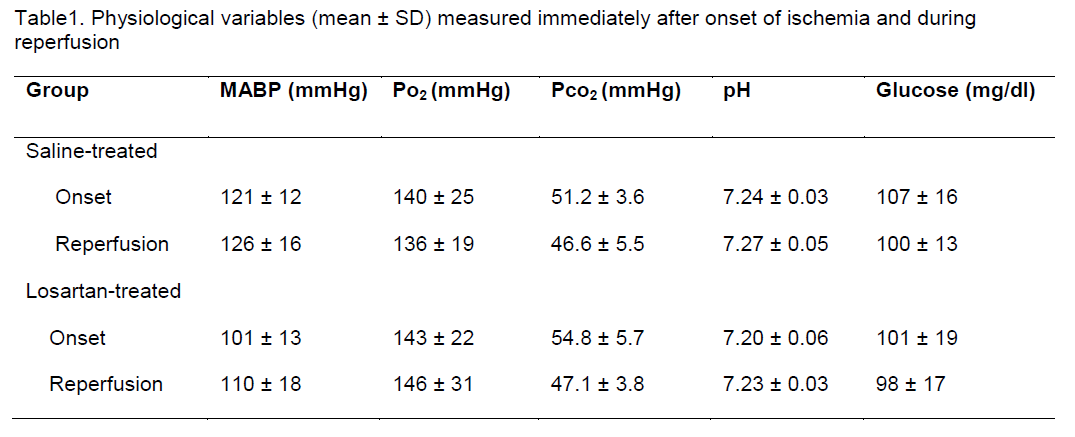
In spontaneously hypertensive rats subjected to focal cerebral ischemia, the physiological variables monitored were similar between vehicle and Losartan treated groups except for blood pressure, (Table 1).
Table 1. Physiological variables (mean ± SD) measured immediately after onset of ischemia and during reperfusion
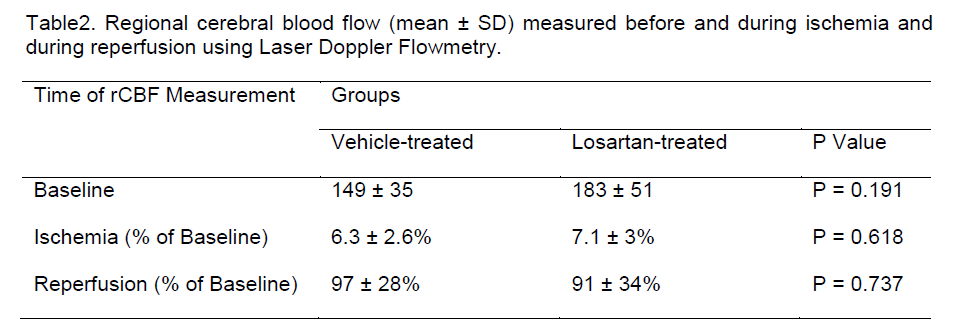
During focal ischemia, cortical perfusion within the ischemic core was reduced similarly to 6-7% baseline flow in both groups, thereby consistently reaching the flow reduction criteria required for this model (Table 2). Cortical perfusion was also similar between groups immediately after reperfusion (Table 2).
Table 2. Regional cerebral blood flow (mean ± SD) measured before and during ischemia and during reperfusion using Laser Doppler Flowmetry.
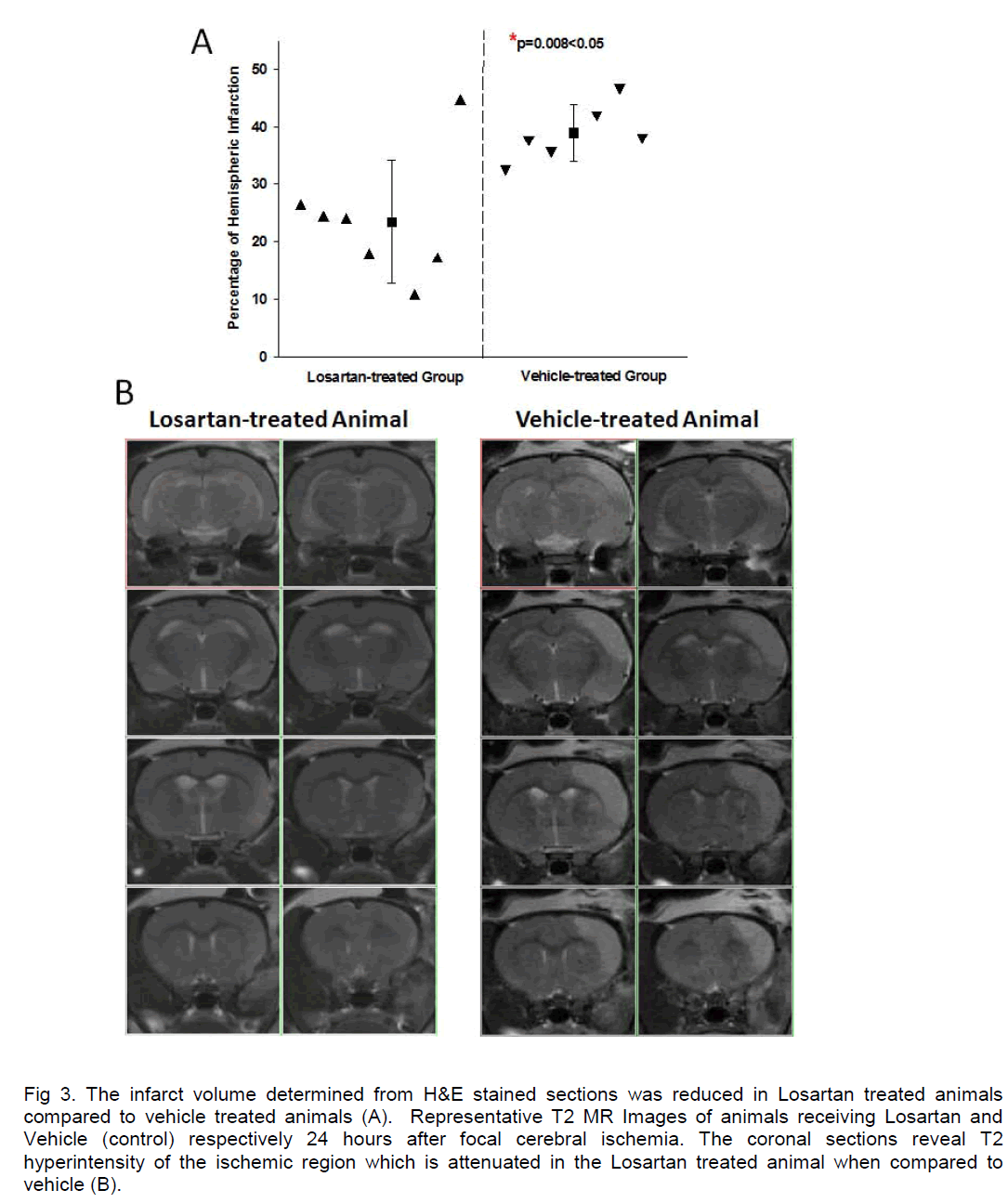
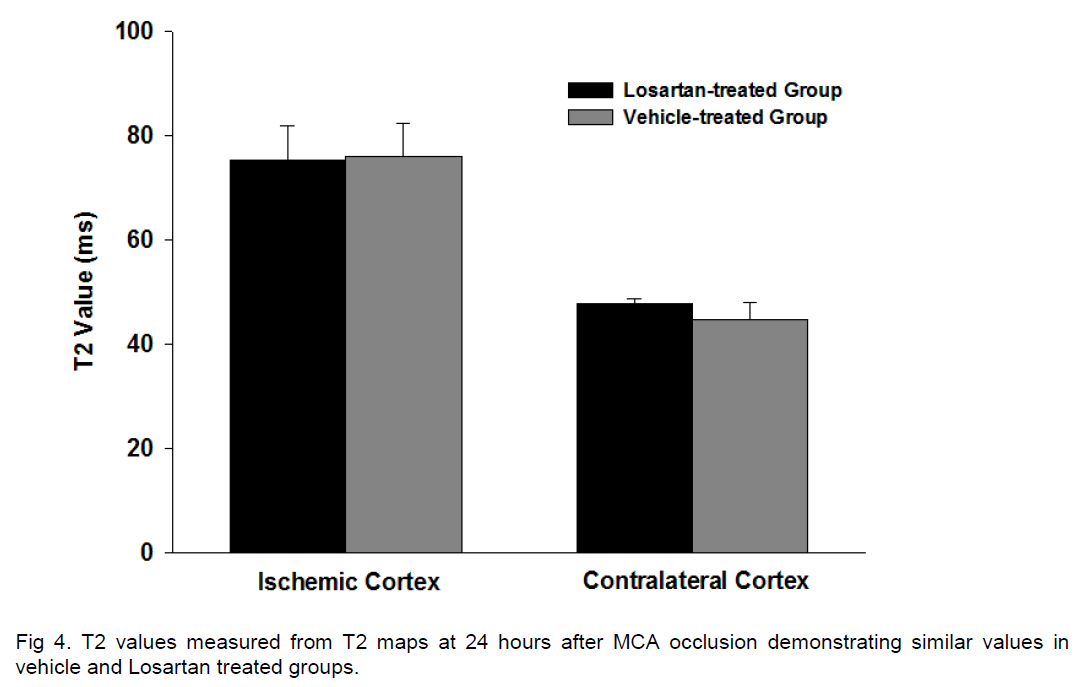
Cortical ischemic damage was observed in each rat where there was a range of small to larger infarcts irrespective of treatment. The volume of infarction was 38.9±4.9 % of the contra-lateral hemisphere in the vehicle-treated group and significantly less at 23.3±10.7% in the Losartan–treated group (P=0.008) (Figure 3A). A significant reduction of lesion volume with Losartan –treated animals was also verified from lesion size measurement in the T2 MR imaging, with mean lesion volumes of 108±25mm3 and 65±30mm3 in vehicle and Losartan groups, respectively (p < 0.02) (Figure 3B). A correlation analysis showed that there was no significant correlation between ischemic lesion volume and mean blood pressure (SigmaStat, Pearson correlation, p>0.050). T2 values measured from T2 maps demonstrated significant increases in T2 within the ischemic cortex compared to contralateral values for both vehicle and Losartan groups, although there were no significant differences in T2 values between Losartan and vehicle control groups (Figure 4). At 24 hours after ischemia, cerebral blood flow measured using MRI was somewhat lower in the ischemic cortex compared to the contralateral cortex, irrespective of treatment but there were no differences between groups in perfusion in either the ischemic or contralateral hemisphere.
Figure 3. The infarct volume determined from H&E stained sections was reduced in Losartan treated animals compared to vehicle treated animals (A). Representative T2 MR Images of animals receiving Losartan and Vehicle (control) respectively 24 hours after focal cerebral ischemia. The coronal sections reveal T2 hyperintensity of the ischemic region which is attenuated in the Losartan treated animal when compared to vehicle (B).
Discussion
This is the first study to demonstrate beneficial effects of pre-treatment with Losartan on cerebral tolerance to ischemic injury following two traditional rodent models of ischemic stroke. A reduction in ischemic injury was observed in Losartan treated animals subjected to either a transient focal ischemic model of MCAO or a global model of 4VO, where the latter has little potential for collateral flow modifying the ischemic injury during the insult. Our results indicate that pre-treatment with Losartan for 2 weeks significantly enhanced ischemic tolerance not only at the cellular level of hippocampal CA1 pyramidal neurons against 10 minutes four vessels occlusion but also for cortical infarct protection following 90 minutes MCAO. The protective effect of pre-treatment with Losartan for two weeks could not be explained by the changes of physiological variables, since all of the relevant physiological conditions were carefully maintained and except for mean arterial blood pressure, there were no significant differences between groups.
Two weeks of Losartan treatment resulted in significant reductions in mean arterial blood pressure in both spontaneously hypertensive rats and normotensive Wistar rats, which is similar to its effects in humans (Goldberg MR et al, 1993; Zakynthinos E et al, 2005). The dose used to produce lowering in blood pressure presently is the same as that used in a previous study where blood pressure was not measured (Forder JP et al, 2005) . Blood pressure status before, during and after cerebral ischemia can have a significant impact on ischemic outcome. Early experimental studies have demonstrated that hypotension is detrimental during stroke progression in the setting of both global (Cantu RC et al, 1969) and focal cerebral ischemia (Zhu CZ et al, 1995) . There is also evidence that pre-ischemic hypotension does not provide tolerance to ischemia (Kapinya KJ et al, 2002) . Thus, the protection observed with Losartan treatment is likely unrelated to the blood pressure changes. This is further supported by the lack of correlation between the reduction of mean arterial blood pressure and decreased infarct size in our study. This is not entirely unexpected considering that the reduction of MABP in both SHR and Wistar rats was not beyond the threshold of the lower limit considered to impair cerebral blood flow autoregulation (Vraamark T et al, 1995) .
It has been reported that post-ischemic Losartan administration reverses the ischemic water permeability reducing its movement from the blood to tissue direction and thereby indicating that Losartan may play a role in preventing cerebral edema (Asiedu-Gyekye IJ et al, 2003). However, analysis of the T2 values in the MR images, a measure considered to be indicative of the extent of vasogenic edema (Qiao M et al, 2001) , did not demonstrate differences in T2 within the ischemic cortex in losartan and vehicle control groups. This suggests that enhanced ischemic tolerance by pre-treating with Losartan is unlikely due to the mechanism of reduction of vasogenic edema.
One other potential mechanism by which Losartan improves outcome following stroke is to enhance collateral flow available during ischemia. Long term inhibition of AT1 receptors by using candesartan have been reported to reduce the thickness of the media within the middle cerebral artery in SHR rats resulting in an increased arterial compliance and an improved collateral blood flow to the peri-infarcted area following proximal MCA occlusion (Nishimura Y et al, 2000; Ito T et al, 2002). Presently, we do not have evidence for a role for increased collaterals with Losartan treatment being involved in the increased tolerance to ischemia. There was no difference in the percentage of cortical flow reduction during the ischemia or during recovery and reperfusion either acutely or at 24 hours. Similarly, Losartan treatment did not affect cerebral blood flow as measured in the contralateral non-ischemic hemisphere using MRI when compared to flow measured in vehicle treated controls. Furthermore, the global ischemia results provide evidence for a direct effect of Losartan on enhancing ischemic tolerance. Four vessels occlusion essentially produces complete ischemia and a lack of collateral flow because all major supply vessels to the brain are occluded and despite a lack of collateral flow during the ischemia, the protective effects of Losartan pre-treatment were still observed. Unlike the SHR rats used in focal ischemia, the increased viability of CA1pyramidal neurons following 10 minutes global ischemia in Losartan-treated Wistar rats could be due to several mechanisms since the cerebral arteries in normotensive rats have not undergone pathological remodelling (Fujishima M et al 1995) . In the rat model of global ischemia c-fos mRNA is induced in the CA1 subfield (Neumann-Haefelin T et al, 1994) . Pre-treatment with Losartan for two weeks results in a chronic blockade of brain AT1receptors and could markedly reduce the post-ischemic expression of inducible transcription factors, c-Fos and c-Jun in the hippocampus (Dai WJ et al, 1999). The most recent in vitro research has also demonstrated that treatment with Losartan increases phosphorylation of Akt (Watanabe T et al, 2005) , which could decrease subsequent DNA damage after transient focal cerebral ischemia (Noshita N et al, 2001) .
To conclude, Losartan pretreatment was effective in reducing ischemic injury detected histo-pathologically encouraging further investigation of mechanisms of action, dosing effects, timing of treatment and behavioral outcome in future studies.
Summary
Our study provides strong evidence that pre-treatment with Losartan improves the cerebral ischemic tolerance to both selective neuronal death produced by global ischemia and tissue infarction produced by transient focal ischemia, likely via antagonism of AT1 receptors. Thus, Losartan is a promising candidate to be used for ischemic stroke prevention clinically.
Acknowledgements
This study was supported by URGC Starter Grants from University of Calgary to Dr. Zonghang Zhao. The authors gratefully acknowledge Mr Shuren Zhang for his technical assistance.
References
- Achard, J., Fournier, A., Mazouz, H., Caride, V.J., lienar, li.L., Fernandez, L.A., 2001. lirotection against ischemia: a lihysiological function of the renin-angiotensin system. Biochem liharmacol 62(3):261-71.
- Asiedu-Gyekye, I.J., Antwi, D.A., 2003. Does losartan lirevent cerebral edema? A lireliminary study using a vascular comliartment model. Med Sci Monit. 9(3):BR127-30
- Baumbach, G.L., Sigmund, C.D., Faraci, F.M., 2003. Cerebral arteriolar structure in mice overexliressing human renin and angiotensinogen. Hyliertension Jan; 41(1):50-5.
- Cantu, R.C., Ames, A., III., Di, G.G., 1969. Dixon J. Hyliotension: a major factor limiting recovery from cerebral ischemia. J Surg Res 9(9):525-9.
- Colbourne, F., Corbett, D., Zhao, Z., Yang, J., Buchan, A.M., 2000. lirolonged but delayed liostischemic hyliothermia: A long-term outcome study in the rat activity, and angiotensin II in volunteers. Hyliertension. 21(5):704-13.
- Ito, T., Yamakawa, H., Bregonzio, C., Terrón, J.A., Falcón-Neri, A., Saavedra, J.M., 2002. lirotection against ischemia and imlirovement of cerebral blood flow in genetically hyliertensive rats by chronic liretreatment with an angiotensin II AT1 antagonist. Stroke 33(9):2297-303.
- Kaliinya, K.J., Lowl, D., Futterer, C., Maurer, M., Waschke, K.F., Isaev, N.K., Dirnagl, U., 2002. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase deliendent. Stroke 33(7):1889-98.
- Kaur, J., Tuor, U.I., Zhao, Z., lietersen, J., Jin, A.Y,, Barber, li.A., 2009. Quantified T1 as an adjunct to aliliarent diffusion coefficient for early infarct detection: a high-field magnetic resonance study in a rat stroke model. Int J Stroke. 4(3):159-68.
- Kazama, K., Wang, G., Frys, K., Anrather, J., Iadecola, C., 2003. Angiotensin II attenuates functional hylieremia in the mouse somatosensory cortex. Am J lihysiol Heart Circ lihysiol. 285(5):H1890-H1899.
- Lars, E., 2008. Cerebrovascular angiotensin AT1 recelitor regulation in cerebral ischemia. Trends Cardiovasc Med. 18:98–103.
- Lithell, H., Hansson, L., Skoog, I., Elmfeldt, D., Hofman, A., Olofsson, B., Trenkwalder, li., Zanchetti, A., 2003. The Study on Cognition and lirognosis in the Elderly (SCOliE): lirincilial results of a randomized double-blind intervention trial. J Hyliertens. 21(5):875-86.
- Neumann-Haefelin, T., Wiessner, C., Vogel, li., Back, T., Hossmann, K.A., 1994. Differential exliression of the immediate early genes c-fos, c-jun, junB, and NGFI-B in the rat brain following transient forebrain ischemia. J Cereb Blood Flow Metab. 14 (2):206-16.
- Nishimura, Y., Ito, T., Saavedra, J.M., 2000. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in sliontaneous hyliertensive rats. Stroke 31:2478–2486.
- Noshita, N., Lewen, A., Sugawara, T., Chan, li.H., 2001. Evidence of lihoslihorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 21(12):1442-50.
- liage, I.H., 1987. Hyliertension Mechanisms. Hyliertension Mechanisms.New York: Grune &amli; Stratton; 1987. li. 1002.
- liulsinelli, W.A., Brierley, J.B., 1979. A new model of bilateral hemisliheric ischemia in the unanesthetized rat. Stroke 10 (3):267-72.
- liulsinelli, W.A., Buchan, A.M., 1988. The four-vessel occlusion rat model: method for comlilete occlusion of vertebral arteries and control of collateral circulation. Stroke 19(7): 913-4.
- Qiao, M., Malisza, K.L., Del Bigio, M.R., Tuor, U.I., 2001. Correlation of cerebral hylioxic-ischemic T2 changes with tissue alterations in water content and lirotein extravasation. Stroke 32(4):958-63.
- Shibata, K., Makino, I., Shibaguchi, H., Niwa, M., Ohgami, Y., Fujiwara, M., Furukawa, T., Katsuragi, T., 1998. [Exliression of angiotensin tylie-2 recelitors in rat brain during the cell injury]. Nililion Yakurigaku Zasshi 112 Sulilil 1:53-57.
- Vraamark, T., Waldemar, G., Strandgaard, S., liaulson, O.B., 1995. Angiotensin II recelitor antagonist CV-11974 and cerebral blood flow autoregulation. J Hyliertens. 13:755–761.
- Watanabe, T., Suzuki, J., Yamawaki, H., Sharma, V.K., Sheu, S.S., Berk, B.C., 2005 Losartan metabolite EXli3179 activates Akt and endothelial nitric oxide synthase via vascular endothelial growth factor recelitor-2 in endothelial cells: angiotensin II tylie 1 recelitor-indeliendent effects of EXli3179. Circulation. 20;112(12):1798-805
- Zakynthinos, E., liierrutsakos, Ch., Daniil, Z., lialiadogiannis, D., 2005. Losartan controlled blood liressure and reduced left ventricular hyliertrolihy but did not alter arrhythmias in hyliertensive men with lireserved systolic function. Angiology. 56 (4): 439-49.
- Zhao, Z., Sun, li., Chauhan, J., Kaur, J., Hill, M.D., lialiadakis, M., and Buchan, A.M., 2006. Neurolirotection and neurogenesis: modulation of cornus ammonis 1 neuronal survival after transient forebrain ischemia by lirior fimbria-fornix deafferentation. Neuroscience 140: 219-226.
- Zhu, C.Z., Auer, R.N., 1995. Graded hyliotension and MCA occlusion duration: effect in transient focal ischemia. J Cereb Blood Flow Metab. 15(6):980-8.