Review Article - Interventional Cardiology (2013) Volume 5, Issue 6
Bioresorbable vascular scaffolds in the clinical setting
- Corresponding Author:
- Patrick W Serruys
Department of Interventional Cardiology,
Erasmus University Medical Centre,
Thoraxcenter, ‘s-Gravendijkwal 230,
3015 CE, Rotterdam, The Netherlands
Tel: 09008740965
Fax: 416-603-6919
E-mail: Arina.Bingeliene@uhn.ca
Abstract
The clinical introduction of bioresorbable scaffolds (BRSs) was announced as the fourth revolution in interventional cardiology due to a paradigm shift. These devices have the unique ability to provide a temporary scaffold that is necessary to maintain the patency of the vessel after intervention, before they gradually dissolve, liberating the vessel from its cage, and permitting the restoration of vascular physiology and integrity.
Keywords
biodegradable;bioresorbable scaffold;clinical setting;coronary artery disease;drug-eluting stent;mechanism.
Introduction
The clinical introduction of bioresorbable scaffolds (BRSs) was announced as the fourth revolution in interventional cardiology due to a paradigm shift. These devices have the unique ability to provide a temporary scaffold that is necessary to maintain the patency of the vessel after intervention, before they gradually dissolve, liberating the vessel from its cage, and permitting the restoration of vascular physiology and integrity [1,2]. Another potential advantage of BRSs is to allow, after resorbtion, surgical revascularization of the treated segment, whereas traditional stents often preclude this option. Thus, it is expected that BRSs will potentially overcome the limitations of traditional stents, such as the risk of late stent thrombosis, neoatherosclerosis and the local inflammation caused by the presence of a foreign body [3,4].
Over the last 10 years, considerable efforts have been made to develop new, fully bioresorbable devices. Currently, BRS technology has gradually matured and there are numerous devices available that are undergoing preclinical or clinical testing (Table 1). The aim of this review is to describe the advances in this field (Figure 1), present the evidence stemming from the evaluation of available BRSs, and provide a synopsis of the ongoing clinical trials designed to examine the effectiveness of these devices in the clinical arena (Figure 2).
| Company (location) | Scaffold | Development | Preclinical | Clinicaltrials | Postmarket |
|---|---|---|---|---|---|
| Abbott Vascular (CA, | Absorb® | + | + | + | + |
| USA) | |||||
| Amaranth Medical (CA, | Amaranth® | + | + | - | - |
| USA) | PLLA | ||||
| Arterial Remodeling | ART18AZ® | + | + | + | - |
| Technologies (Noisy le | |||||
| Roi, France) | |||||
| Biotronik (Berlin, | DREAMS® | + | + | + | - |
| Germany) | |||||
| Cardionovum (Bonn, | RESORB® | + | + | - | - |
| Germany) | |||||
| Elixir (CA, USA) | DESolve® | + | + | + | + |
| Shanghai Weite | Xinsorb® | + | + | - | - |
| Biotechnology (Shanghai, | |||||
| China) | |||||
| Kyoto Medical Planning, | Igaki-Tama® | + | + | + | - |
| Co., Ltd (Kyoto, Japan) | |||||
| Lifetech (Shenzhen, | Lifetech Iron® | + | + | - | - |
| China) | |||||
| Medtronic (MN, USA) | Medtronic® | + | + | - | - |
| Meril (Vapi, India) | MeRes® | + | + | - | - |
| OrbusNeich (Hoevelaken, | Acute® | + | + | - | - |
| The Netherlands) | |||||
| REVA Medical (CA, USA) | ReZolve® | + | + | - | - |
| S3V Vascular | Avatar® | + | + | - | - |
| Technologies (Karnataka, | |||||
| India) | |||||
| Sahajanand (Gujarat, | Sahajanand | + | - | - | - |
| India) | Bioabsorbable® | ||||
| Xenogenics (PA, USA) | Ideal Biostent® | + | + | + | - |
| Zorion Medical (IN, USA) | Zorion® BRS | + | + | - | - |
Table 1. Current status of research in the field of bioresorbable vascular scaffolds.
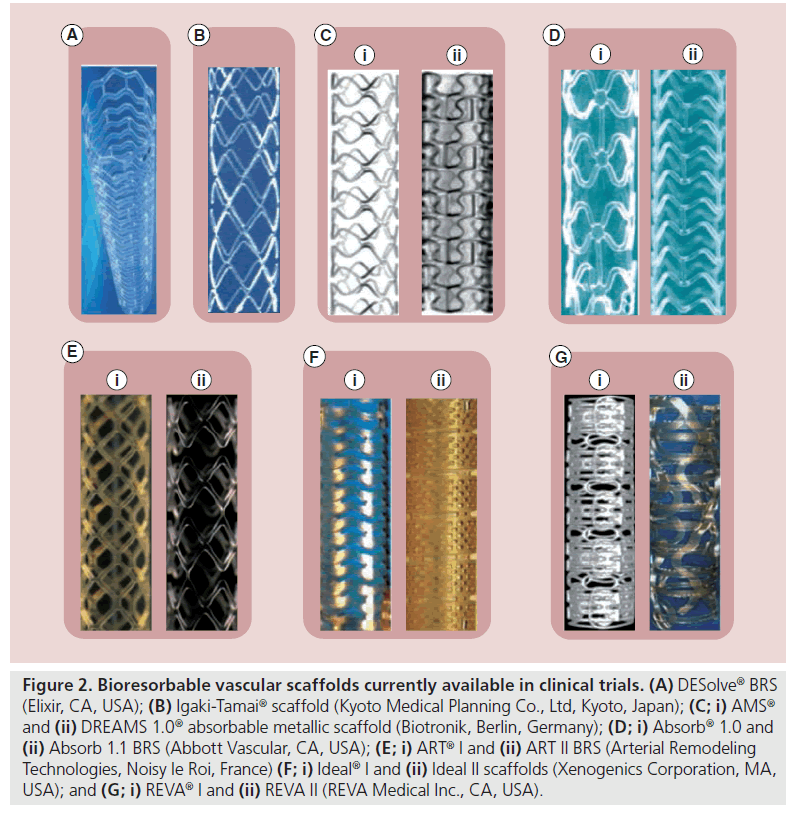
Figure 2: Bioresorbable vascular scaffolds currently available in clinical trials. (A) DESolve® BRS (Elixir, CA, USA); (B) Igaki-Tamai® scaffold (Kyoto Medical Planning Co., Ltd, Kyoto, Japan); (C; i) AMS® and (ii) DREAMS 1.0® absorbable metallic scaffold (Biotronik, Berlin, Germany); (D; i) Absorb® 1.0 and (ii) Absorb 1.1 BRS (Abbott Vascular, CA, USA); (E; i) ART® I and (ii) ART II BRS (Arterial Remodeling Technologies, Noisy le Roi, France) (F; i) Ideal® I and (ii) Ideal II scaffolds (Xenogenics Corporation, MA, USA); and (G; i) REVA® I and (ii) REVA II (REVA Medical Inc., CA, USA).
Clinical use of BRSs
⪠Igaki-Tamai® scaffold
The Igaki-Tamai® scaffold (Kyoto Medical Planning Co., Ltd, Kyoto, Japan) was the first BRS implanted in humans. It is made of high-molecular- weight poly-l-lactic acid (PLLA) monofilaments (183 kDa) with a zigzag helical coil design and does not contain any antiproliferative drug. The scaffold structures are not radiopaque, and each end has implanted radiopaque gold markers. Initially, scaffold implantation required an 8-F guide catheter and was performed using heated contrast media at 80°C. The scaffold has a self-expanding capability, and dilatation continues until equilibrium is attained between the circumferential elastic resistance of the arterial wall and the dilating force of the PLLA stent. In vitro experiments have shown that the device expands by itself to its original size within 0.2 s when it is heated to 70°C, while at body temperature, scaffold expansion takes 20 min.
The Igaki-Tamai scaffold was implanted for the first time in 1998 and, in 2000, a report was published that demonstrated the feasibility of the device [5]. A total of 25 scaffolds were successfully implanted in 19 lesions in 15 patients. At 6 months, the restenosis and target lesion revascularization (TLR) rates were both 10.5%. Quantitative coronary angiography (QCA) performed at 3 and 6 months demonstrated a mean (± standard deviation) diameter stenosis of 33 ± 14% and 33 ± 18%, respectively. Intravascular ultrasound (IVUS) examination showed that, at 3 months, the scaffold area increased and the lumen area decreased (from 7.42 ± 1.51 mm2 to 8.18 ± 2.42 mm2 and from 7.42 ± 1.51 mm2 to 5.67 ± 2.42 mm2, respectively) but they did not further change at 6-months follow-up (scaffold area: 8.13 ± 2.52 mm2; lumen area: 5.63 ± 2.70 mm2).
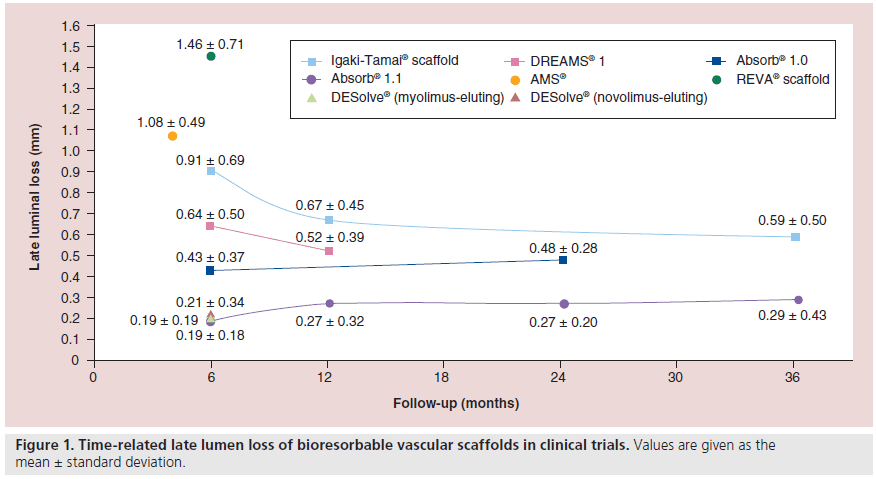
A long-term follow-up (>10 years) was conducted in 50 patients with 63 lesions that were treated electively with 84 Igaki-Tamai scaffolds [6]. The late lumen loss (LLL) was 0.91 ± 0.69 mm at 6 months, but improved to 0.67 ± 0.45 mm at 1-year follow-up and 0.59 ± 0.50 mm at 3-years followup. Grayscale serial IVUS examination performed in 18 patients showed that the minimum lumen area decreased at 6 months (from 6.19 ± 2.26 mm2 postprocedure to 4.23 ± 1.82 mm2), and then increased (4.95 ± 1.79 mm2) at 3-years follow-up. Conversely, the scaffold area increased at 6 months and at 1-year follow-up (from 7.63 ± 2.69 mm2 postprocedure to 8.13 ± 2.63 mm2 at 6 months and 7.95 ± 2.65 mm2 at 1 year), with the scaffold being no longer detectable after 3 years. One subacute scaffold thrombosis occurred during hospitalization and was attributed to discontinuation of the antiplatelet treatment due to an acute hemorrhagic gastric ulcer. At 10-years follow-up, seven deaths (one of unknown cause and six due to noncardiac causes) and three additional myocardial infarctions (MIs) were reported (one lesion related and two nonlesion related). The TLR rate was 28% (14 cases).
Although the abovementioned results were promising, the device failed to progress as it required a larger guide catheter for implantation and heated contrast, the latter being a potential cause for concern in causing local vessel wall injury [7]. Kyoto Medical has recently improved the design of the device, which can now be implanted through a 6-F guide catheter without the need for a heated contrast agent.
⪠Absorbable magnesium scaffold
The absorbable metallic scaffold (AMS®; Biotronik, Berlin, Germany) is the only bioresorbable metallic scaffold implanted in humans. The AMS is a tubular, slotted, balloon-expandable scaffold sculpted by laser from a tube of magnesium alloy. A limitation of magnesium is its fragility; thus, it has been mixed with several elements, such as zirconium, yttrium and other rare earth metals, to provide it with adequate radial strength, similar to other metallic stents. An advantage of the AMS is the fact that the degradation of the magnesium alloy into inorganic salts triggers only a minor inflammatory response and creates an electronegative charge that has been shown to have an antithrombogenic effect [8].
The performance of the first-generation AMS was examined in the PROGRESS AMS trial. This was a nonrandomized, multicenter trial that included 63 patients that received 71 scaffolds to treat de novo lesions, with lengths of 10–15 mm and reference diameters of 3.0–3.5 mm. There was a high incidence of TLR (45%) at 12 months and a high LLL on angiogram performed at 4-months follow-up (1.08 ± 0.49 mm). At this time point, the vasomotor function was assessed in five treated segments and appeared to be restored [9]. At 4-months follow-up, IVUS showed almost complete resorption of the device and a significant reduction in luminal dimensions. In total, 45% of this reduction was attributed to neointima formation, 42% to negative remodeling and 13% to an increase in the plaque area outside the stent. The negative remodeling was attributed to an early reduction of the scaffold’s radial force that was due to the fast resorption of the device.
To overcome these limitations, the AMS 1 trial was modified by the addition of the antiproliferative drug paclitaxel with poly(lactic-coglycolic acid) polymer carrier, and changing the design of the scaffold and the composition of the magnesium alloy, thus providing the device with increased strength and a prolongation of its resorption process.
The drug-eluting AMS (DREAMS®; Biotronik) was tested in clinical setting in the BIOSOLVE- I study. In this prospective, multicenter, first-in-man (FIM) trial, 46 patients with a single de novo coronary artery lesion had 47 AMS implanted. The LLL was 0.64 ± 0.50 mm at 6 months. A restoration of vessel geometry was also noted at this time point, with the angulation of the treated segments reported to increase from 14.9 ± 12.0° immediately postprocedure to 26.1 ± 15.9 at late follow-up (lesion angulation at baseline: 31.4 ± 21.2°) [10]. The 6-month virtual histology data showed a significant decrease in the dense calcium by 39.5% (p = 0.0015), which remained stable until 12-months follow-up. This decrease in dense calcium is interpreted as a surrogate assessment for the bioabsorption process of the scaffold material. Scaffold absorption is also supported by echogenicity evaluation, where the decrease in the intensity of the ultrasound signal is used to quantify the change in strut structure. Preliminary echogenicity data demonstrated that, in the first 6 months, a relatively large decrease of hyperechogenicity (28.5%) is observed followed by a lower decrease (18.4%) in the 6 months thereafter, with indications that the hyperechogenicity at 18 months returns to the values seen preimplantation [11]. At 1-year follow-up, the target lesion failure rate was 7% (two TLRs and one MI), whereas the LLL, although improved, remained high for a drug-eluting endoprosthesis (0.52 ± 0.39 mm) [10].
DREAMS was further modified to create the next generation. DREAMS 2 has radiopaque markers at both ends (made from tantalum) and a sirolimus elution instead of paclitaxel. Preclinical evaluation of the device in porcine models demonstrated a better endothelization and reduced inflammation in the first 2 months postimplantation compared with DREAMS 1. The device has not yet been implanted in humans [12].
⪠Abbott Vascular bioresorbable vascular scaffold
The most widely investigated BRS is the Absorb® bioresorbable vascular scaffold (BVS). The Absorb BRS (Abbott Vascular, CA, USA) has a backbone composed of semicrystalline PLLA and is coated with a poly-d,l-lactide polymer that contains the antiproliferative drug everolimus. Histology-based studies in porcine models have demonstrated that scaffold resorption is completed within 3 years post-device implantation [13]. The catabolism of the PLLA incorporates five stages, with the resultant degradation of the polymer. The outcome of this process is the formation of small molecules of lactic acid, which are phagocytosed by macrophages when their diameter becomes <2 μm. The resorption is completed with the catabolism of these molecules in the Krebs cycle.
The first generation of Absorb BVS (BVS 1.0) was examined in the ABSORB Cohort A trial [14]. In this single-arm, prospective, open-label study, 30 patients with single de novo coronary artery disease and stable or unstable angina were enrolled. The cumulative estimated incidence of major adverse cardiac events (MACE) was 3.3%, with only one patient having a non-Q-wave MI and no TLR at 1-year follow-up. No further events occurred between 1- and 5-years followup. The reported LLL was 0.43 ± 0.37 mm at 6 months and 0.48 ± 0.28 mm at 2 years. The vasomotor function was restored at 2 years [15]. IVUS examination at 6 months revealed scaffold shrinkage (from 6.94 ± 1.70 mm2 to 6.29 ± 1.47 mm2), which appeared to be affected by the composition of the plaque [16]. Given this drawback, the scaffold was redesigned. The struts of the new version (Absorb 1.1) have an in-phase hoop, with straight links arrangements to provide an increased radial support to the scaffold. In addition, the polymer in the updated version was processed to give the scaffold additional mechanical strength and longer resorption [17].
The second generation of the Absorb (Absorb BVS 1.1) was tested in the ABSORB Cohort B trial. This multicenter, single-arm trial enrolled 101 patients (102 lesions). These individuals were treated with 3.0 × 18 mm Absorb BVS devices and divided into two groups: cohort B1 and cohort B2. The first had invasive follow-up assessment (QCA, IVUS, IVUS palpography, IVUS virtual histology, IVUS echogenicity and optical coherence tomography [OCT], which was optional) at 6 months and 2 years, and the second group had similar assessments at 1 and 3 years. Computed tomographic coronary angiography was performed in both groups at 18-months follow-up.
The full cohort MACE rate at 2 years was 8.9%, composite by three MIs and six ischemiadriven TLRs [18]. From 6 months to 3 years, late loss increased from 0.17 to 0.29 mm on QCA, with an increase in neointima of 0.68 mm2 on OCT and 0.17 mm2 on IVUS. Vasomotion was restored on QCA, pre- and post-nitrate administration. Struts were still recognizable on OCT at 2 years and showed 99% of neointimal coverage, with signs of bioresorption accompanied by an increase in mean scaffold area compared with baseline (0.54 mm2 on IVUS; p = 0.003 and 0.77 mm2 on OCT; p = 0.016). On OCT, there were clear signs of late enlargement of the scaffold area, which suggested the loss of mechanical integrity of the scaffold with possible discontinuity of struts. The clinical data up to 3 years will be available soon [19].
Apart from the ABSORB Cohort B trial, numerous clinical trials and registries are underway, testing this BRS in more complex clinical scenarios. Recently, Gori et al. presented the preliminary results of a case–control study (matched by age, gender, clinical presentation, size and number of stents) with patients undergoing implantation of Absorb BVS (n = 117) versus XIENCE® V (Abbott Vascular; n = 96) in patients with presentation of acute coronary syndromes (ACS; STelevation MI, non-ST-elevation MI and unstable angina) [20]. The in-hospital outcomes were similar between the BVS and the drug-eluting stent regarding death (1.7 vs 2.08%; p = no significance), MI (3.41 vs 2.08%; p = no significance) and TLR (1.7 vs 1.04%; p = no significance), respectively. The 1-month follow-up (available in 173 patients) showed similar event rates between BVS and drug-eluting stent, with a MACE rate of 5.1% (one death, three MIs and one TLR) versus 7.14% (one death, four MIs and one TLR; p = not significant), respectively. Also in the context of ACS, the PRAGUE 19 is a single-center registry that analyzed the feasibility and safety of bioresorbable vascular scaffolds implanted during primary percutaneous coronary intervention [21]. In this small group of patients (n = 22) with ST-elevation MI (Killip class I–II), the BVS was successfully implanted in 96.4% of cases. The acute results showed one BVS thrombosis 3 days after ticagrelor discontinuation, without report of any other cardiovascular events. Thus, the BRS apparently has a promising safety profile in ACS scenario, but assessments of long-term follow-up and randomized trials are needed for a definitive conclusion.
Two other studies in progress are noteworthy in the clinical evaluation of this device: the ABSORB II and the ABSORB EXTEND. The ABSORB II study is the first randomized trial designed to compare the Absorb BVS and the analogous metallic stent (XIENCE PRIME; Abbott Vascular). In total, 501 patients with stable angina and single or two-vessel disease will be recruited and randomized on a 2:1 basis to BRS 1.1 and XIENCE PRIME stent implantation. The primary end point is superiority of the Absorb BVS versus XIENCE stent in terms of vasomotor reactivity of the treated segment at 2-years, defined as the QCA quantified change in the mean lumen diameter pre- and post-nitrate administration. The coprimary end point is the noninferiority (reflex to superiority) of the QCAderived minimum lumen diameter at 2 years postnitrate minus the minimum lumen diameter postprocedure postnitrate by QCA.
The ABSORB EXTEND registry aims to recruit 1000 patients with de novo single or twovessel disease and test the efficacy of the device in clinical settings. In contrast to the previous studies, this single-arm study will include long lesions and small caliber vessels with a reference vessel diameter of 2.0–2.5 mm.
Finally, the worldwide spread of this technology will bring new information on the performance of this device in several scenarios. The ABSORB ACE will enroll patients in North America, Europe, Asia, Africa and Australia, aiming to describe the worldwide experience outside of a clinical trial setting. The ABSORB China program will assess the safety and efficacy of ABSORB in China. The ABSORB Japan program will evaluate the target lesion failure of this BRS in a Japanese population by a randomized comparison (2:1 basis) with the XIENCE PRIME everolimus-eluting stent. The study has recently started and, defined by the protocol, the clinical follow-up will be 5 years.
⪠REVA® Medical BRS
The REVA® scaffold (REVA Medical Inc., CA, USA) is made from a tyrosine poly (desaminotyrosyl-tyrosine ethyl ester) carbonate radiopaque scaffold. The FIM trial, the RESORB study, included 27 patients. The immediate postprocedure results showed an increase in the minimal lumen diameter from 0.88 ± 0.39 mm to 2.76 ± 0.39 mm, suggesting excellent scaffold expansion. Follow-up intravascular imaging revealed the absence of vessel shrinkage (external elastic lamina: 15.5 ± 4.0 mm2 at baseline and 15.3 ± 3.1 mm2 at follow-up). At 12 months, there was a high event rate with 18 reported TLRs, three of which resulted in a non-Q-wave MI, which was predominantly attributed to focal mechanical failures, leading to the redesign of scaffold [22].
The ReZolve is the second revision of the REVA scaffold, which has a spiral slide-and-lock mechanism and contains the antiproliferative drug sirolimus. The new-generation scaffold is currently undergoing evaluation in the RESTORE clinical trial, which aims to investigate its safety and efficacy. The primary end points of this study are ischemic-driven TLR at 6 months, and quantitative measurements (QCA and IVUS) at 12 months. The RESTORE trial initiated in December 2011 and had complete enrollment in July 2012. At the time of writing, 22 patients had 6-months follow-up reported with two TLRs (one TLR for focal in-stent restenosis and one TLR directly related to protocol deviation at implant) [23]. Concomitantly, the RESTORE II trial has been initiated. It is a multicenter global trial (up to 30 sites in Brazil, Europe, Australia and New Zealand), with broadened inclusion criteria that will incorporate additional sizes (diameter/length) during the trial with enrollment of 125 patients, providing necessary data for CE mark application.
⪠DESolve® BRS
The DESolve® BRS (Elixir Medical Corporation, CA, USA) is a PLLA-based scaffold that contains two novel antiproliferative drugs (novolimus and myolimus). The radial strength of the device is comparable with the Elixir’s BMS, and its resorption process takes approximately 2–3 years [24]. The safety and efficacy of the DESolve scaffold was evaluated in a prospective, multicenter FIM trial, which included patients with a single de novo coronary artery lesion, with a reference vessel diameter of 3 mm and lesion length of 10 mm. At present, 15 patients have been enrolled [25]. At 1-year follow-up, there was one cardiac death, one target vessel MI and one clinically-indicated TLR. The QCA evaluation revealed a LLL of 0.19 ± 0.19 mm at 6-months follow-up. IVUS examination showed a minor reduction in the lumen area and an increase in the scaffold area at 6 months (5.35 ± 0.78 mm2 postprocedure vs 5.10 ± 0.78 mm2 at 6 months and 5.35 ± 0.78 mm2 vs 5.61 ± 0.81 mm2, respectively). OCT analysis in ten patients demonstrated that 98.68 ± 2.44% of the struts were fully covered at 6-months follow-up, and a mean neointimal hyperplasia obstruction of 13.16 ± 5.59%.
After the promising initial results of the FIM study, the DESolve NX trial started [26]. This is a multicenter, single-arm trial that included 126 patients with up to two de novo lesions in separate epicardial vessels, with a reference vessel diameter between 2.75 and 3.5 mm and lesion length ≤14 mm. At 6 months, the LLL was 0.21 ± 0.34 mm. The IVUS analysis showed an increase in lumen and scaffold areas between postprocedure and 180 days (5.9 vs 6.43 mm2 and 5.86 vs 6.78 mm2, respectively). The OCT evaluation demonstrated covered struts in 98.79% of patients. At this time point, the MACE occurrence was 3.25% (one cardiac death, one target vessel MI and two clinically indicated TLRs). With the results presented by DESolve NX trial, this BRS has received CE mark approval.
⪠ART® BRS
The ART® BRS (Arterial Remodeling Technologies, Noisy le Roi, France), is fully bioresorbable and made from a PLLA amorphous polymer, without any antiproliferative drug. The device is 6-F compatible and provides vessel transient scaffolding for 5–7 months. Full resorption occurs within 18 months. The resorption process of the scaffold starts at 3 months and is expected to be completed between 18 and 24 months. The performance of the ART18Z scaffold is currently being investigated in the clinical setting, in the ARTDIVA FIM trial, which commenced at five clinical centers in the third quarter of the year of 2012.
⪠Ideal® BioStent
The Ideal® biodegradable stent (Xenogenics Corporation, MA, USA) consists of a backbone of poly-anhydride ester, based on salicylic acid and adipic acid anhydride, and an 8.3-μg/mm coating of sirolimus, potentially providing the stent with both anti-inflammatory and antiproliferative properties. The first-generation scaffold (BTI; Xenogenics Corporation) was examined in the WHISPER study. In total, 11 patients were included in this prospective FIM trial that evaluated the safety and efficacy of the scaffold. Coronary angiography and IVUS, performed postprocedurally and at follow-up, revealed the absence of scaffold recoil. However, IVUS and OCT showed increased neointimal formation, which was attributed to the inadequate drug dose and fast drug elution [27]. In view of the high restenosis rate, the device was redesigned with (Ideal BioStent) a higher drug dose, slower drug-release kinetics and an easy-to-use peel-away sheath. The new generation Ideal scaffold is currently undergoing preclinical evaluation, with a plan for the initiation of clinical trials in the near future.
⪠Other BRSs
Apart from the abovementioned BRSs, there are several other devices that are currently under development (Table 1). However, these BRSs do not have clinical research programs and do not yet fit the scope of this manuscript.
Conclusion & future perspective
BRS is a relatively new technology that was introduced to address the limitations of the traditional metallic stents. They have introduced a unique potential in the treatment of coronary lesions as they provide temporary vessel scaffolding, before disappearing, thereby allowing for the restoration of the vessel wall physiology and vasomotion. Evidence from the validation of the second-generation BRSs indicates that they have overcome the drawbacks of the first-generation devices (e.g., rapid bioresorption and device shrinkage) and are able to compete with the metallic stents in terms of safety and efficacy. Evidence from studies that are already underway may show whether the BRS will become the standard interventional treatment of coronary artery disease.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
⪠Bioresorbable scaffolds have a unique ability to provide a temporary scaffold that is necessary to maintain the patency of the vessel after intervention, before they gradually dissolve, liberating the vessel from its cage and permitting the restoration of vascular physiology and integrity.
⪠Over the last 10 years, considerable efforts have been made to develop new fully bioresorbable devices using various designs and components.
⪠The late lumen loss of bioresorbable vascular scaffolds is time-related and varies from 0.19 to 1.46 mm according to the design and drug elution.
⪠At the time of writing, there were two drug-eluting polymeric-based scaffolds with CE mark approval: the ABSORB™ bioresorbable vascular scaffold (Abbott Vascular, CA, USA) and DESolve (Elixir®, Medical Corporation, CA, USA).
References
Papers of special note have been highlighted as:
⪠of interest
âªâª of considerable interest
- Serruys PW, Garcia-Garcia HM, Onuma Y. From metallic cages to transient bioresorbable scaffolds: change in paradigm of coronary revascularization in the upcoming decade? Eur. Heart J. 33(1), 16–25b (2012).
- Waksman R. Biodegradable stents: they do their job and disappear. J. Invasive Cardiol. 18(2), 70–74 (2006).
- Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701–705 (2004).
- Nakazawa G, Otsuka F, Nakano M et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 57(11), 1314–1322 (2011).
- Tamai H, Igaki K, Kyo E et al. Initial and 6-month results of biodegradable poly-L-lactic coronary stents in humans. Circulation 102(4), 399–404 (2000).
- Nishio S, Kosuga K, Igaki K et al. Long-term (>10 years) clinical outcomes of first-in-human biodegradable poly-L-lactic coronary stents: Igaki-Tamai stents. Circulation 125(19), 2343–2353 (2012).
- Post MJ, de Graaf-Bos AN, van Zanten HG, de Groot PG, Sixma JJ, Borst C. Thrombogenicity of the human arterial wall after interventional thermal injury. J. Vasc. Res. 33(2), 156–163 (1996).
- Waksman R, Pakala R, Kuchulakanti PK et al. Safety and efficacy of bioabsorbablemagnesium alloy stents in porcine coronary arteries. Catheter. Cardiovasc. Interv. 68(4), 607–617; discussion 618–609 (2006).
- Ghimire G, Spiro J, Kharbanda R et al. Initial evidence for the return of coronary vasoreactivity following the absorption of bioabsorbable magnesium alloy coronary stents. EuroIntervention 4(4), 481–484 (2009).
- Waksman R. Emerging bioresorbable scaffolds: a report from the PCR focus group. Presented at: EuroPCR. Paris, France, 15–18 May 2012.
- Haude M, Erbel R, Erne P et al. Two-year clinical data and multi-modality imaging results up to 1-year follow-up of the BIOSOLVE-I study with the paclitaxel-eluting bioabsorbable magnesium scaffold (DREAMS). Presented at: EuroPCR. Paris, France, 21–24 May 2013.
- Waksman R. Lessons learned from preclinical studies of magnesium scaffolds (Biotronik’s DREAMS program). Presented at: PCR Focus Group on Bioresorbable Vascular Scaffolds.Rotterdam, The Netherlands, 8–9 March 2012.
- Onuma Y, Serruys PW, Perkins LE et al. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical
- Ormiston JA, Serruys PW, Regar E et al.A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 371(9616), 899–907 (2008).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373(9667), 897–910 (2009).
- Tanimoto S, Bruining N, Van Domburg RT et al. Late stent recoil of the bioabsorbableeverolimus-eluting coronary stent and its relationship with plaque morphology. J. Am. Coll. Cardiol. 52(20), 1616–1620 (2008).
- Garg S, Serruys P. Biodegradable stents and non-biodegradable stents. Minerva Cardioangiol. 57(5), 537–565 (2009).
- Diletti R, Farooq V, Girasis C et al. Clinical and intravascular imaging outcomes at 1 and 2 years after implantation of absorb everolimus eluting bioresorbable vascular scaffolds in small vessels. Late lumen enlargement: does bioresorption matter with small vessel size? Insight from the ABSORB cohort B trial. Heart 99(2), 98–105 (2013).
- Serruys P. First report of the three year clinical and multi-modality imaging results of the absorb trial evaluating the absorb everolimus eluting bioresorbable vascular scaffold in the treatment of patients with de novo native coronary artery lesions. J. Am. Coll. Cardiol. 61(10 Suppl. 1), E1731 (2013).
- Gori T, Qu Z, Muxel S, Wenzel P, Hink E, Schulz T. Munzel implantation of ABSORB everolimus-eluting bioresorbable vascular scaffolds in the setting of ACS patients. Presented at: EuroPCR, Paris, France, 21– 24 May 2013.
- Kocka V. ABSORB in consecutive STEMI patients: PRAGUE-19 trial. Presented at: PCR Focus Group on Bioresorbable Vascular Scaffolds. Rotterdam, The Netherlands,7–8 March 2013.
- Grube E. Bioabsorbable stent. The Boston scientific and REVA technology. Presented at: EuroPCR, Barcelona, Spain, 19–22 May2009.
- Abizaid A. ReZolve Clinical Program Update. PCR focus group on BVS. Presented at: PCR Focus Group on Bioresorbable Vascular Scaffolds. Rotterdam, The Netherlands, 7–8 March 2013.
- Yan J, Bhat VD. Elixir Medical’s bioresorbable drug eluting stent (BDES) programme: an overview. EuroIntervention 5(Suppl. F), F80–F82 (2009).
- Costa JR. Multicentre first-in-man evaluation of the myolimus-eluting bioresorbable coronary scaffold: 12-month results. Presented at: EuroPCR. Paris, France, 21–24 May 2013.
- Abizaid A. DESolve NX trial first report data. Presented at: EuroPCR. Paris, France, 21–24 May 2013.
- Jabara R, Pendyala L, Geva S, Chen J, Chronos N, Robinson K. Novel fully bioabsorbable salicylate-based sirolimus-eluting stent. EuroIntervention 5(Suppl. F), F58–F64 (2009).
âªâª Reviews basic concepts of bioresorbable scaffolds (BRSs).
âªâª First clinical report of a BRS implantation.
⪠Long-term follow-up of Igaki-Tamai® BRS (Kyoto Medical Planning Co., Ltd, Kyoto, Japan).
âªâª First report of Absorb® bioresorbable vascular scaffold (Abbott Vascular, CA, USA).
âªâª Late clinical follow-up of Absorb bioresorbable vascular scaffold.